Abstract
In clinical practice, the effect of a high vault on corneal endothelial cells after implantable collamer lens (ICL) implantation remains unclear. Many clinicians theoretically assume that a high postoperative vault leads to rapid endothelial damage, but no study has yet proven this hypothesis. We conducted a paired-eye study to compare changes in corneal endothelial cell density (ECD) between high and low postoperative vault groups. This retrospective study included 150 eyes of 75 patients with bilateral postoperative vault levels differing by more than 200 μm after ICL implantation. Patients were followed up for 7 years with ECD measurements, and changes in ECD were assessed between 6 months and 7 years post-surgery. Over the 7-year follow-up period, the percentage of ECD loss was 15.04% and 14.45% in the high- and low-vault groups, respectively. The bilateral paired-eye comparison revealed a significant reduction in ECD in the high-vault group at 3, 5, and 7 years postoperatively (P-value < 0.001). In this paired-eye comparison of long-term observations, a higher vault was associated with greater ECD loss. Our study confirms that a high vault level may be an important risk factor for ECD loss following ICL implantation.
Similar content being viewed by others
Background
The prevalence of myopia has recently surged globally1. Consequently, refractive surgeries have been increasingly utilized to rectify impaired vision resulting from uncorrected refractive errors2. Phakic intraocular lens (IOL) implantation has emerged as a safe and reliable approach for addressing refractive errors, offering predictable visual outcomes. This procedure is not only prevalent among highly myopic individuals but also among those deemed unsuitable for laser vision correction. A newly introduced Implantable Collamer Lens (ICL; STAAR Surgical, Monrovia, CA, USA) has exhibited favorable visual outcomes and safety profiles3, demonstrating corneal endothelial cell stability during short-term follow-up periods4.
One lingering concern regarding ICL surgery is the gradual loss of corneal endothelial cells during long-term follow-up5. Many clinicians theoretically assume that a high postoperative vault leads to rapid endothelial damage, but no study has yet proven this hypothesis. Although a decrease in corneal endothelial cell density (ECD) is a recognized complication of all phakic IOL surgeries, only a limited number of studies have explored the factors associated with ECD loss specifically in ICL patients. A prior study involving 48 ICL-treated eyes suggested a significant correlation between postoperative vault and ECD reduction6. Another study emphasized the role of the vault and the distance from the corneal endothelium to the central ICL in ECD loss among patients with shallow anterior chamber depth (ACD)7. However, assessing patients over extended periods in real-world clinical settings, amidst varying anterior segment conditions, presents challenges. Consequently, the extent to which elevated vault levels contribute to corneal endothelial cell loss post-ICL implantation remains uncertain.
This study investigated the hypothesis that high vault directly affects rapid ECD loss. To analyze this, paired-eye data was collected and ECD changes were studied by comparing eyes that showed different vaults under the same conditions. This study aimed to evaluate whether a relationship exists between postoperative vault and ECD loss following ICL surgery over a 7-year observation period, utilizing paired-eye comparison. To mitigate the impact of natural ECD loss due to aging and confounding variables arising from inter-patient differences8, we compared ECD loss between high- and low-vault eyes within the same individuals, ensuring precise matching of all covariates.
Methods
Aim, design, and setting of the study
The purpose of this study is to determine whether higher postoperative vault values affect endothelial cell density compared to lower values during a 7-year follow-up. This retrospective, paired-eye study included 150 eyes of 75 patients with bilateral postoperative vault levels differing by more than 200 μm after ICL implantation.
Participants
This retrospective observational study involved paired-eye contralateral analysis. We recruited patients who underwent ICL implantation between 2012 and 2014 and were followed up for at least 7 years. Ultimately, we included 75 patients who received bilateral ICL implantation, and we retrospectively gathered preoperative and postoperative ocular measurement data from the B&VIIT Eye Center in Seoul, South Korea. We conducted a thorough review of all medical record databases using conditional statements to identify patients who met the study criteria, without performing any additional sampling. Approval for this study was obtained from the Institutional Review Board of the Korean National Institute for Bioethics Policy. Informed consent was waived due to the retrospective nature of the study. All research procedures were conducted in accordance with the principles of the Declaration of Helsinki and KNIBP guidelines.
The inclusion criteria for ICL implantation were as follows: age between 19 and 40 years old, stable refraction, myopia ranging from 0 to − 18.0 diopters (D) with astigmatism of 6.5 D or less, anterior chamber depth of ≥ 2.8 mm, and endothelial cell density of ≥ 2400 cells/mm². Exclusion criteria included a history of ocular diseases, prior ocular surgery, trauma, and Fuchs’ corneal dystrophy. The selection of the ICL size was based on clinical judgment derived from a comprehensive evaluation by the surgeon. All surgeries were performed by board-certified ophthalmologists.
Examinations
The anterior segment was measured preoperatively using optical coherence tomography (OCT; Visante OCT; Carl Zeiss Meditec, Inc., Dublin, CA, USA). Vault measurements were conducted by expert examiners using Visante OCT before 2015 and CASIA2 (Tomey Co., Nagoya, Japan) after 2015. Since the initial postoperative vault values were incompletely collected, the vault values used to determine the groups in this study were those measured with the CASIA2 at the last visit. We categorized bilateral eyes into high- and low-vault groups for paired-eye comparison. Inter-eye vault differences were calculated based on the differences observed at the last visit. To ensure the inclusion of eyes with a significant difference between the two vaults, only eyes with a difference of 200 μm were included. This threshold was set empirically, taking into account the average inter-device vault error value of 210 μm reported in previous literature9.
Before 2017, central corneal ECD was manually measured using SP-8000 specular microscopy (Konan Medical, Hyogo, Japan). Since 2017, it has been measured automatically using EM-4000 (Tomey, Nagoya, Japan). Because endothelial cell measurements can show significant bias depending on the device, the results must be carefully separated and interpreted with respect to the specific device used10. To eliminate the influence of surgery, the change in ECD was calculated as the difference between the value measured with the SP-8000 six months after surgery and the value measured with the EM-4000 seven years after surgery.
All patients underwent routine preoperative examinations, including slit-lamp examination, manifest refraction, corrected distance visual acuity, and fundus examination. Additionally, corneal thickness, anterior chamber depth, and corneal white-to-white distance were measured. Postoperative examinations are recommended annually, and routine postoperative assessments include manifest refraction, uncorrected distance visual acuity (UDVA), slit-lamp examination, and fundus examination.
Surgical technique
The surgeons conducted ICL implantation following a standardized protocol. Patients undergoing surgery with an ICL without a central hole (Model V4) received peripheral iridectomy. Conversely, for patients undergoing surgery with an ICL featuring a central hole (Model V4c), iridectomy or iridotomy was omitted. In all cases, topical anesthetic eye drops were administered prior to surgery, followed by implantation of the IOL into the posterior chamber through a 3.0 mm superior clear corneal incision. Postoperatively, patients were prescribed antibiotics and steroid eye drops, to be administered four times daily for one week, followed by a reduced dosage of twice daily for an additional two weeks.
Statistical analysis
We primarily assessed bilateral differences in ECD changes based on high or low vault groups. Additionally, we examined the relationship between bilateral differences in vaults and ECD loss disparities. Non-paired eye data was analyzed to evaluate the relationship between vaults and ECD changes. Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS version 23.0; IBM, Chicago, Illinois, USA). Paired t-tests were utilized for bilateral comparisons between high- and low-vault groups. We used the Kolmogorov-Smirnov test to check whether the data followed a normal distribution. The test confirmed that all continuous variables followed a normal distribution (P > 0.05), allowing the use of parametric statistical tests such as paired t-tests. Pearson’s correlation coefficients were computed to assess the correlation between postoperative vault and ECD loss, as well as between bilateral differences in vaults and differences in ECD loss. We performed a two-way repeated measures ANOVA to assess the influence of vault group on ECD over the 7-year follow-up period. All statistical tests were conducted in a two-sided manner, with significance set at p < 0.05.
The minimum sample size was estimated based on the distribution of vault and ECD data from a previous study6, though the data were not from a paired-eye design. The sample size calculation was performed with an alpha level of 0.05 and a beta of 0.20 (80% power). In the previous study, the ECD change was reported as 97.35 ± 62.42 in the lower vault group and 162.86 ± 45.09 in the higher vault group (Supplementary Fig. 1). Using the mean and the larger variance, the required sample size was calculated to be 30 subjects in total, with 15 per group. This study secured a sample size that exceeds this requirement.
Results
The demographics and ocular measurements are shown in Table 1. The mean age of the included patients was 26.21 ± 5.01 years. Fifty patients were female (66.7%). Among the 75 patients, 48 had bilateral ICL without a central hole (V4), and 27 had bilateral ICL with a central hole (V4c). The mean vault values of the high and low vault groups were 295.7 ± 148.5 μm and 611.1 ± 161.3 μm, respectively. There were no significant differences between the high- and low-vault groups in terms of anterior chamber depth (P-value = 0.883), preoperative manifest sphere (P-value = 0.328), manifest cylinder (P-value = 0.380), preoperative uncorrected distance visual acuity (P-value = 0.242), or preoperative central ECD (P-value = 0.789).
Figure 1 displays the clinical outcomes of ICL implantation in the eyes included in this study. The preoperative mean corrected distance visual acuity was − 0.005 ± 0.634 logMAR. At 7 years postoperatively, the mean uncorrected distance visual acuity was 0.005 ± 0.656 logMAR. A scatter plot of attempted versus achieved correction illustrates myopic changes 7 years postoperatively. Only 76 eyes (51%) were within ± 0.50 D of attempted spherical equivalent refraction, and 117 eyes (78%) were within ± 1.00 D. A stability plot demonstrates myopic regression during the 7 years of postoperative follow-up.
Clinical outcomes of ICL implantation in eyes included in this study (n = 150). (A) Postoperative uncorrected visual acuity (UDVA) at 7 years compared to preoperative corrected distance visual acuity (CDVA). (B) Changes in CDVA from preoperative to 7 years postoperative. (C) Attempted versus achieved spherical equivalent values. (D) Spherical equivalent refractive accuracy results at 7 years postoperative. (E) Refractive astigmatism results at 7 years postoperative. (F) Stability of spherical equivalent refraction over time.
Contrary to expectations, when all 150 eyes were analyzed without pairing, no relationship was observed between vault and ECD changes seven years after surgery. Figure 2 depicts the distribution of ECD changes according to vault levels. As shown in Fig. 2a, correlation analysis revealed no significant relationship between postoperative vault levels and ECD changes (Pearson’s correlation coefficient = 0.085; P-value = 0.305). After categorizing the vault levels, as shown in Fig. 2b, the ECD changes according to the vault level groups showed no linear trend (P-value for linear trend = 0.333).
Figure 3 illustrates the analysis using bilateral differences (75 pairs of bilateral eyes) in postoperative vault levels and ECD changes 7 years postoperatively. As depicted in Fig. 3a, bilateral differences in ECD were significantly correlated with bilateral vault differences (Pearson’s correlation coefficient = 0.348, P-value = 0.002). When we analyzed the bilateral vault difference by dividing it into four groups, as shown in Fig. 3b, the largest difference in ECD reduction occurred in the group with the largest vault difference (140 cells/mm² of the difference in ECD reduction in the vault difference ≥ 400 groups). There was a significant linear trend between the vault difference and the mean difference in the ECD change (P-value for linear trend = 0.006).
Paired-eye analysis of endothelial cell density (ECD) changes between postoperative 6 months and 7 years in paired eyes (n = 75 pairs). (A) Distribution of differences in ECD changes and postoperative vault level difference. (B) Difference in ECD changes categorized by vault level difference. The differences in ECD changes were calculated as the ECD change of the high vault group minus the ECD change of the low vault group.
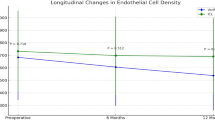
Table 2 presents the changes in ECD and the percentage of endothelial cell loss according to the follow-up period. Figure 4 illustrates the changes in ECD values according to group. At 6 months after surgery, the mean ECD values of the high and low vault groups were 2944.19 ± 296.32 cells/mm² and 2923 ± 312.01 cells/mm², respectively. At 7 years postoperatively, the mean ECD values of the high and low vault groups were 2460.49 ± 311.54 cells/mm² and 2401.32 ± 300.27 cells/mm², respectively. During the 7-year follow-up period, the percentage of ECD changes was 15.04% and 14.45% in the high- and low-vault groups, respectively. The bilateral paired-eye comparison showed a significant ECD reduction in the high vault at 3, 5, and 7 years postoperatively (P-value < 0.001). A two-way repeated measures ANOVA showed a significant effect of time on ECD (P < 0.001) and a significant interaction between vault group and time (P < 0.001), indicating greater ECD loss in the high vault group over time. However, the main effect of vault group alone was not significant (P = 0.344).
Figure 5 shows the ocular measurements in a representative case. The right eye with the higher vault showed greater ECD loss at 7 years postoperatively than the left eye with the lower vault.
Discussion
Clinically, there is consensus that high postoperative vault values can induce a rapid decrease in corneal endothelial cells, but there has been little research data on this to date. This study evaluated postoperative vault levels and ECD changes in patients who underwent ICL implantation. In this paired-eye comparison with long-term observations, the higher vault group was associated with greater ECD loss than the lower vault group. There was no difference in ECD between the two groups at 6 months after surgery, but a significant difference was observed after 3 years. Therefore, this study recommends avoiding vaults that are too high to prevent endothelial cell damage. This can be achieved by appropriately determining the ICL size through the nomogram11. To the best of our knowledge, this is the first study to directly analyze the paired-eye comparison of ECD and vault levels in patients who underwent ICL implantation.
Our study does not suggest that the difference in vault between the two eyes causes a decrease in endothelial cells, but rather shows a pattern in which a large vault value causes a relative decrease in endothelial cells. Because everyone’s guidance environment is different, we attempted to prove this with data through a paired-eye research design. To date, studies have not directly examined the hypothesis that postoperative ICL vault values cause endothelial cell loss. Because there are several conflicting results related to ECD changes during ICL implantation, it is not yet clear which factors are responsible for endothelial cell loss6,12. Our study confirms that a high vault level may be an important risk factor for ECD loss after ICL implantation. Recently, it has become possible to predict the postoperative vault accurately using data-based methods and machine learning13,14. Therefore, to prevent long-term damage to corneal endothelial cells, it is necessary to use these prediction tools to select an ICL size such that the postoperative vault is not too large.
Corneal endothelial cell loss after phakic IOL implantation has been reported to be the main cause of implanted IOL removal15. Several hypotheses have been proposed to explain endothelial cell loss after ICL implantation. The implanted ICL elevates the iris toward the cornea and narrows the anterior chamber angle16. Direct contact between the iris and the corneal endothelial cells can induce mechanical endothelial cell damage. In addition, physical friction between both ends of the ICL in the sulcus or ciliary body can cause persistent subclinical inflammatory reactions17, leading to corneal endothelial damage. Additionally, endothelial cell damage can result from wall shear stress on the corneal endothelium due to altered aqueous humor flow caused by the ICL18. Previous studies reported that the wall shear stress of the corneal endothelium is independent of the presence or absence of a central hole in the ICL19. However, determining the clinical variables for these hypotheses is difficult. A higher vault or arch owing to a large ICL size may cause a narrow cornea-iris angle, and tightness in the sulcus may cause stress between the IOL haptics and surrounding structures.
In this study, the paired-eye design demonstrated that a high postoperative vault significantly affected corneal endothelial cell loss after ICL implantation. Both eyes were sourced from the same individual, ensuring meticulous matching and correction for various factors. A previous 4-year retrospective study analyzing 48 eyes found a significant association between postoperative vault and endothelial cell loss6. However, due to the limited number of eyes, this study couldn’t fully eliminate the possibility of selection bias. Another study, conducted in cases of shallow anterior chambers, suggested that corneal endothelial cell aging and decline, attributed to factors beyond the ICL, might have influenced the outcomes7. However, the short 24-month follow-up period of this study hindered a comprehensive evaluation of progressive endothelial cell damage. Unlike previous studies, the data from this study revealed no correlation between vault and ECD changes in a non-paired design, where various inter-individual factors remained uncorrected.
Although the difference in endothelial cell loss between the high and low vault groups appears to be relatively small (15.04% vs. 14.45%), this study provides important insights into the potential long-term risks associated with high vault levels. Even minor reductions in ECD can become clinically relevant over time, particularly in younger patients or those who may require future intraocular surgeries, such as cataract extraction. Small cumulative losses in endothelial cells could increase the likelihood of corneal decompensation in these patients, especially if they experience other risk factors such as pre-existing endothelial dysfunction or additional surgeries20. Given that the corneal endothelium does not regenerate, even small differences in cell loss could have long-term implications for corneal health. Future studies with longer follow-up periods may be necessary to determine the true clinical impact of this difference.
The strengths of this study lie in its extensive 7-year follow-up period and the paired-eye design, enabling meticulous examination of inter-eye differences. Although most studies have reported the long-term stability of ICLs in corneal endothelial cells21, few have delved into factors influencing endothelial cell decline in long-term studies (> 5 years). The paired-eye analysis minimized the influence of variables other than vault and ECD changes, facilitating a clearer elucidation of their relationship by ensuring precise matching of risk profiles between higher and lower vault groups. This paired-eye design has elucidated various disease factors in previous literature22,23. In the non-paired eye comparison analysis, the vault showed no association with ECD change. However, the paired-eye comparison revealed that a higher vault was significantly associated with greater ECD loss compared to a lower vault.
However, this study has several limitations. First, the ECD could not be measured using a single device. It is possible that consistent endothelial cell measurements were not achieved due to equipment replacement during follow-up. Second, the vault could not be measured immediately after surgery. It is possible that the vault changed due to the aging of the crystalline lens during the follow-up observation period. Third, due to issues with research medical record data, parameters such as hexagonality and the coefficient of variation in endothelial cell size could not be collected. These variables may indicate the state of endothelial cells, along with the number of endothelial cells, and should be included in future research endeavors. Fourth, we analyzed ICLs with a central hole and ICLs without a hole together. Because the two IOLs had almost identical specifications in terms of size and vault, they were analyzed together. In this study, the secondary hydrodynamic effects caused by the hole were not analyzed. Since this was a paired-eye study using the same type of ICL in both eyes in all patients, we believe that these influences would not have had a significant impact on the results. Single-center data is another drawback of this study, as surgical techniques may vary by institution and measurement bias may exist24.
Conclusions
Despite the reported safety of ICL implantation, it is imperative to identify factors contributing to endothelial cell damage and enhance safety measures. Through this paired-eye comparison of long-term observations, we observed a correlation between higher vault and increased ECD loss. Our study underscores the significance of high vault levels as a potential risk factor for ECD loss following ICL implantation. Surgeons should therefore carefully consider ICL size selection, mindful that excessively high vault levels may exacerbate endothelial loss based on the findings of this study.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to personal privacy concerns but are available from the corresponding author on reasonable request.
Abbreviations
- ACD:
-
Anterior chamber depth
- ECD:
-
Andothelial cell density
- ICL:
-
Implantable Collamer Lens
- IOL:
-
Intraocular lens
- KNIBP:
-
Korean National Institute for Bioethics Policy
- OCT:
-
Optical coherence tomography
References
Holden, B. A. et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 123, 1036–1042 (2016).
Kim, T. I., Barrio, D., Wilkins, J. L. A., Cochener, M., Ang, M. & B. & Refractive surgery. Lancet. 393, 2085–2098 (2019).
Gonzalez-Lopez, F. et al. Long-term assessment of crystalline lens transparency in eyes implanted with a central-hole phakic collamer lens developing low postoperative vault. J. Cataract Refract. Surg. https://doi.org/10.1097/j.jcrs.0000000000000425 (2020).
Choi, H., Ryu, I. H., Lee, I. S., Kim, J. K. & Yoo, T. K. Comparison of implantation of posterior chamber phakic IOL implantation and laser vision correction in terms of corneal endothelial cells: 3-year observational paired-eye study. J. Cataract Refractive Surg. 49, 936 (2023).
Moya, T. et al. Implantable Collamer Lens for Myopia: Assessment 12 years after implantation. J. Refract. Surg. 31, 548–556 (2015).
Yang, W. et al. Four-year observation of the changes in corneal endothelium cell density and correlated factors after Implantable Collamer Lens V4c implantation. Br. J. Ophthalmol. 105, 625–630 (2021).
Qian, T. et al. Vault-correlated efficacy and Safety of Implantable Collamer Lens V4c Implantation for Myopia in patients with shallow Anterior Chamber depth. Ophthalmic Res. 66, 445–456 (2023).
Glynn, R. J. & Rosner, B. Methods to quantify the relation between Disease Progression in Paired eyes. Am. J. Epidemiol. 151, 965–974 (2000).
Almorín-Fernández-Vigo, I. et al. Agreement between optical coherence and Scheimpflug tomography: Vault measurements and reproducibility after implantable collamer lens implantation. J. Fr. Ophtalmol. 44, 1370–1380 (2021).
Choi, H., Ryu, I. H., Lee, I. S., Kim, J. K. & Yoo, T. K. Comparison of automated corneal endothelial cell analysis in healthy and postoperative eyes with phakic intraocular lens: a cross-sectional study and literature review. BMC Ophthalmol. 24, 318 (2024).
Zhao, H. et al. Development and Validation of Data-Level Innovation Data-Balancing Machine Learning models for Predicting Optimal Implantable Collamer Lens size and postoperative Vault. Ophthalmol. Ther. https://doi.org/10.1007/s40123-023-00841-7 (2023).
Kamiya, K. et al. Eight-year outcomes of implantation of posterior Chamber Phakic intraocular Lens with a Central Port for Moderate to High Ametropia. Front. Med. 8, (2021).
Kim, T. et al. Development of an implantable collamer lens sizing model: a retrospective study using ANTERION swept-source optical coherence tomography and a literature review. BMC Ophthalmol. 23, 59 (2023).
Kang, E. M. et al. Development of a web-based ensemble machine learning application to select the optimal size of posterior Chamber Phakic Intraocular Lens. Trans. Vis. Sci. Tech. 10, 5–5 (2021).
Zhang, H., Gong, R., Zhang, X. & Deng, Y. Analysis of perioperative problems related to intraocular Implantable Collamer Lens (ICL) implantation. Int. Ophthalmol. 42, 3625–3641 (2022).
Choi, H. et al. Predicting Postoperative Anterior Chamber Angle for Phakic Intraocular Lens Implantation using Preoperative Anterior Segment Metrics. Translational Vis. Sci. Technol. 12, 10 (2023).
Gros-Otero, J. et al. Atomic force microscopy comparative analysis of the surface roughness of two posterior chamber phakic intraocular lens models: ICL versus IPCL. BMC Ophthalmol. 21, 280 (2021).
Fernández-Vigo, J. I. et al. Computational simulation of aqueous humour dynamics in the presence of a posterior-chamber versus iris-fixed phakic intraocular lens. PLOS ONE. 13, e0202128 (2018).
Sun, Y., Chen, J., Zhou, S. & Jiang, Y. Numerical simulation of aqueous flow in a novel posterior chamber phakic intraocular lens versus its counterparts. Phys. Fluids. 35, 053608 (2023).
Price, M. O., Mehta, J. S., Jurkunas, U. V. & Price, F. W. Corneal endothelial dysfunction: evolving understanding and treatment options. Prog. Retin. Eye Res. 82, 100904 (2021).
Kisiel, F. B. & Gurumurthy, G. J. Endothelial cell loss post–implantable collamer lens V4c: meta-analysis. J. Cataract Refractive Surg. 50, 420 (2024).
Wang, J. J. et al. Risk of Age-related Macular Degeneration 3 years after cataract surgery: Paired Eye comparisons. Ophthalmology. 119, 2298–2303 (2012).
Lee, S. J., Lee, C. S. & Koh, H. J. Posterior vitreomacular adhesion and risk of exudative age-related macular degeneration: paired eye study. Am. J. Ophthalmol. 147, 621–626e1 (2009).
Shin, D. et al. Code-Free Machine Learning Approach for EVO-ICL Vault Prediction: a retrospective two-Center Study. Translational Vis. Sci. Technol. 13, 4 (2024).
Author information
Authors and Affiliations
Contributions
HC and TKY analyzed data, and drafted the manuscript. BYL and HJC acquired the data and interpreted the results. HC suggested the original study idea, interpreted the results, contributed to writing. SYL and TKY analyzed data and contributed to data interpretation and manuscript editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Approval for this study was obtained from the Institutional Review Board of the Korean National Institute for Bioethics Policy. Informed consent was waived due to the retrospective nature of the study. All research procedures were conducted in accordance with the principles of the Declaration of Helsinki and KNIBP guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, H., Lee, S.Y., Lee, B.Y. et al. Paired-eye comparison of endothelial cell density and vault height after implantable collamer lens implantation. Sci Rep 14, 27643 (2024). https://doi.org/10.1038/s41598-024-79613-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79613-7