Abstract
Developing and testing alternate hypotheses about patterns, mechanisms, and consequences of movement in geographically-large, heterogeneous, natural systems can advance the scientific understanding of animal migration and benefit the conservation of most mobile species. Within organismal movement trajectories, different combinations of residence and movement are predicted from existing ecological theories (e.g. long distance migration, site fidelity, central place foraging, ideal free distribution, habitat shifts). However, testing these conceptually-based, spatially-explicit hypotheses about animal movement and migration in the field can be logistically challenging. Here our purpose is to introduce Resmo, a framework of metrics and analyses that integrate site-specific RESidence and across-site MOvements. We illustrate the ecological insights from this framework using the empirical example of coastal Striped Bass (Morone saxatilis) during their seasonal feeding migration. Our use of site-specific Resmo applied to empirical telemetry data enhanced the understanding of feeding behavior of migratory fish, suggested testable ecologically-meaningful hypotheses about foraging, and identified criteria on which to base the selection of future sampling locations. In summary, the Resmo approach provides a useful new direction for thinking about animal migration, animal movement, biological conservation, and future priorities for empirical field data collection related to understanding the distribution of mobile organisms.
Similar content being viewed by others
Introduction
Animal mobility is a critically important ecological and life-history trait that has consequences for individual performance, population trends, spatially-explicit community patterns, and responses to disturbances1,2,3. Long distance animal migration mesmerizes scientists and the public for good reasons4,5,6,7, but shorter-distance movements within the migratory track (i.e. stopover behavior, seasonal within-site feeding behavior) can also affect individuals, populations, communities, and ecosystems8,9,10. Fish exhibit a rich suite of ecologically-meaningful, long-distance migratory, and shorter-distance movement patterns11,12,13,14. Using telemetry data from migratory fish that are foraging, here our purpose is to illustrate how new ecological insights can emerge from integrating empirical patterns of residence (where and when an organism stays at a specific location) and movement (where and when an organism moves to or from a specific location).
Scientific understanding of migrating animals is rapidly advancing. As transmitters and receivers have become more technically advanced, researchers increasingly use biotelemetry to quantify patterns of fish in their natural environment15,16,17. These advances in tracking technology have dramatically increased the number of data-based fish telemetry case studies18,19,20, expanded the availability of telemetry field methods21,22,23, stimulated the development of geographically-extensive tracking networks24,25,26, and facilitated the development of more sophisticated statistical tools27,28. In general, movement ecology has aided fisheries management29,30,31, helped to facilitate an assessment of anthropogenic threats32, and has provided a promising direction for improved conservation33,34,35.
Gaps
Yet, at least three important gaps still exist in our knowledge of migratory fish and other mobile organisms. The first gap is that limited empirical data exist to connect longer-distance migration patterns and smaller-scale behavior within individual nodes of the migration cycle [spawning, overwintering, foraging36. As an example, increased growth has been hypothesized as one driver of long-distance migration37,38. This hypothesized trend can be interpreted as either that achieved increased growth is driving long-distance migration or that fish are searching for locales that offer increased growth. However, under either interpretation, patterns of seasonal, site-specific foraging behavior that are responsible for this increased growth are often assumed rather than empirically linked to migratory behavior.
A second gap is that integration of residence and movement data is limited even though staying (residence) and going (movement) are two equally important components of individual telemetry trajectories. Theories of mobile organism distribution that emphasize either residence [e.g. site fidelity, philopatry, habitat selection39,40,41 or movement [e.g. long-distance migration, habitat shifts38,42,43 require the testing of specific predictions about site-specific residence, site-specific movement, as well as, site-specific conditions. A third gap is that identifying appropriate field locations to test hypotheses about site-specific drivers of residence, movement, and distribution in large, heterogeneous systems is logistically challenging. Statistically-driven research sampling designs have advantages, but do not necessarily capture how mobile organisms encounter resource heterogeneity in the field. This third gap exists both because of the large number of possible sampling locations that exist across space and time in geographically-large systems and the inherent difficulties associated with adequately sampling heterogeneous habitats. We address these three gaps by examining how integrating measures of residence and movement can identify alternate distributional outcomes for field-based hypotheses related to the feeding node of a coastal fish migration.
Advantages that the Resmo approach can provide
We define Resmo as the measurement and integrated analysis of RESidence and MOvement data to inform spatially-explicit, empirical patterns of mobile organismal distribution. Our approach can be applied to both individual animals (animal-specific Resmo) or specific sites (site-specific Resmo). Resmo metrics and analyses have broad generality for understanding the distribution of mobile organisms (including but not limited to animal migration), and are especially useful in geographically-large, heterogeneous natural systems in which sampling is especially challenging for the reasons described earlier. Unless an animal does not often change geographic location through time (Fig. 1A) or rarely stops moving (Fig. 1B), the distribution of all mobile animals combines residence and movement. Empirical trajectories of mobile animals (discovered through telemetry) illustrate complex and ecologically-revealing combinations of residence and movement (Fig. 1C). Site-specific Resmo, which we develop here, allows tagged animals to tell researchers how individual field sites within the same system might differ relative to functional processes, what data are needed, and where to look next. Consequently, our Resmo approach can suggest nuanced, alternate, and testable outcomes for hypotheses about mobile organism distribution (Fig. 1–III). In our results section, we show data on alternate outcomes to a hypothesis about patterns of residence and movement. In our discussion section, we review several examples of testable hypotheses and related alternative outcomes that emerged from our use of the Resmo approach.
Why the Resmo approach can be useful is illustrated by graphics that show: (I) mobile organisms RESide and MOve; (II) data-driven trajectories show complex distribution patterns of RESidence and MOvement; and (III) testable hypotheses with multiple alternate outcomes emerge from the Resmo approach. (A,B) By looking at changes in location (Y axis) through time (X axis), simplified trajectories show organisms change location through time (A) rarely (high-residence low-movement), (B) frequently (low-residence high-movement), or in intermediate combinations. (C) An empirical organismal trajectory depicts a much more complex pattern of residence (multi-color dots) and movement (lines) across stationary receiver locations (red squares). Tagged Striped Bass #57,566 moves both south-north (Y axis) and west–east (X axis) in Plum Island Estuary, MA, from June–September (Z axis) with the third temporal dimension emerging from the water (or graphic). Our Resmo approach proposes that by quantifying patterns of site-specific residence (Y axis) and site-specific movement (X axis) within a system, testable alternative outcomes to new hypotheses emerge (D–I). For hypothesis 1 (i.e., whether Striped Bass exhibit similar or different patterns of residence and movement at individual sites within the same system), alternative outcomes include (D) low-residence low-movement sites only (H1a; filled circles); (E) low-residence high-movement sites only; (H1b; stars); (F) high-residence low-movement sites only (H1c; open squares); (G) high-residence high-movement sites only (H1d; thunderbolts); or (H) tagged organisms reside and move differently at different locations within a system (H1e). In (D–H), each symbol represents a site within a system, each panel represents a system, and symbols represent different patterns in different quadrats of Resmo space. (I) Once site function patterns are identified, a second testable hypothesis emerges (i.e., whether site-specific residence-movement patterns of Striped Bass are linked to specific foraging conditions). Specifically, in hypothesis 2, we ask are Resmo patterns related to eight site-specific foraging-related drivers (X axis in separate plots). The eight types of potential foraging-related drivers include: (1) location (edge, center of the estuary); (2) prey amount (biomass, number); (3) predictable timing of prey presence; (4) prey type (fish or invertebrate); (5) ease with which a Striped Bass can capture prey; (6) specific abiotic variables that increase foraging success (drop-offs, current velocity, other habitat characteristics); (7) other biotic variables that affect foraging (competitors); and (8) foraging behavior (learned or opportunistic).
Taxa and system
The empirical example that we use to illustrate the utility of the Resmo framework is the U.S. coastal Striped Bass (Morone saxatilis). We focus on the seasonal feeding migration of these fish from spawning and overwintering sites to northern estuaries along the east coast of the United States. Within the U.S., mid-Atlantic coastal migratory Striped Bass spawn primarily in the Chesapeake Bay, Delaware Bay, and Hudson River estuaries in the spring (Fig. 2A). Then subadults and adults can make a seasonal northward migration through the summer and early fall44,45. Striped Bass consume a variety of prey during this migration period46, can be seasonal residents in northern estuaries, and may even remain within a single New England estuary [e.g. Plum Island Estuary (PIE), MA, USA; Fig. 2B) for several months47,48,49. In late summer and fall, migrant Striped Bass return southward to overwintering and spawning areas50. Our objectives are to (1) illustrate the use of site-specific residence and site-specific movement metrics and analyses, and (2) show how Resmo can aid in the creation of testable hypotheses that advance research and conservation planning for migrating and other mobile animals because how mobile animals reside and move affects patterns, drivers, and consequences of organismal distribution including effective conservation. Not all mobile animals (or Striped Bass) make long distance migrations, but many non-migratory animals (including Striped Bass) are quite mobile. Thus, our Resmo concept has useful science and conservation applications for both migratory behavior and distributional patterns of non-migratory, mobile organisms.
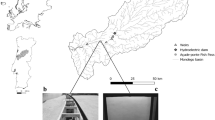
Our study site and receiver placement is shown. (A) U.S. range of migratory Striped Bass on the east coast of the United States. This is the geographic area relevant to this study. (Note that coastal Striped Bass also have been documented to spawn in Canada.) A black square outline indicates the location of PIE in northeastern Massachusetts, USA, the primary focus for fish tagging. (B) Also shown are physical details of PIE. Specific rivers, ocean connections, a central sound, and large island are described in the methods. (C) We used a 26-unit stationary telemetry array within PIE to track tagged fish. The numbers in this panel identify the site location identity of each receiver. These receiver number locations are frequently referenced in the text, are listed as axes or data points for several figures (Figs. 4, 5, 8), and are indicated on other maps (Fig. 7). The coastal map was created using Program R with packages ggplot2, maps, and mapdata. Sources of data for the PIE maps include USDA, USGS, AEX, GeoEye, Getmapping, Aerogrid, IGN, IGP, UPR-EGP, and the GIS User Community. The aerial photo of PIE was created in R with the open access program “Leaflet.” Data sources are Leaflet | Tiles Esri – Source: ESRI, I-cubed, USDA, USGS, AEX, GeoEye, Getmapping, Aerogrid, IGN, IGP, UPR-EGP, and the GIS User Community.
Results
Evidence that Striped Bass tagged in PIE were coastal migrants
We repeatedly detected Striped Bass tagged within PIE (447,972 detections for 59 individual tagged fish at 26-stationary receivers). This extensive dataset allowed us to pursue ecological objectives about site-specific residence and movement for coastal migrants during the feeding node of their long-distance migration along the U.S. East Coast. Fish internally tagged and released within PIE in June 2015 were detected moving throughout the estuary during the 1st week after tagging, demonstrating that they survived the tagging process (100% detected in the last week of June; Fig. 3A). Most tagged Striped Bass were detected in PIE at least once a month during the summer (July: 87%; August: 81%; Fig. 3A). In 2015, tagged fish spent an average of 69 days in PIE (Fig. 3B). Detections within PIE started to decrease in fall 2015 as fish started to migrate southward (September: 65%; October: 13%; Fig. 3A). All but one tagged fish (98%) were detected leaving PIE by November 1, 2015. After leaving PIE, most tagged Striped Bass were detected by coastal arrays outside of PIE. Coastal detections occurred in nearby locations [Merrimack River (n = 12), Massachusetts Bay, (n = 50)], and more distant spawning grounds [Hudson—East Rivers (n = 18); Chesapeake Bay-Coastal Delaware (n = 7); Fig. 3C)]. In summer 2016, 33 of the original 59 tagged Striped Bass returned to PIE (56%; Fig. 3D), and again spent an average of about two months in PIE (mean = 69 days; Fig. 3E). Of these second-year returnees, 18 individuals were detected weekly (30% of original tagged fish; 55% of the returning tagged fish; Fig. 3D). These detections confirm that our tagged Striped Bass were coastal migrants that fed in PIE during the summer, migrated primarily southward in the late summer-fall, and could repeatedly return to the same northern feeding area (PIE).
Detections of tagged Striped Bass within and outside PIE MA, USA (n = 59). (A) Proportion of 59 fish tagged in 2015 (Y axis) that were detected monthly (X axis). The first bar on the left indicates detections in the week following tagging. (B) Total days (Y axis) that individual tagged fish (X axis) were detected in PIE in 2015. An arrow and dotted line indicate the mean residence (in days) for all tagged fish detected. (C) Detections of tagged fish outside of PIE in 2015–2016 (open circle = detection location, location name, and number of fish detected). (D) Proportion of fish tagged in 2015 (Y axis) that were detected each month in PIE in summer 2016 (X axis). (E) Total days (Y axis) that individual tagged fish (X axis) were detected in PIE in 2016. An arrow and dotted line indicate the mean residence (in days) for all tagged fish detected. The coastal map was created using Program R with packages ggplot2, maps, and mapdata.
Residence and movement data
Individual residence and movement metrics by tagged Striped Bass within PIE were difficult to interpret (Fig. 4). Across the 26-receiver sites within PIE, mean residence times at specific receivers (hereafter referred to as receiver-specific residence) for the 50 tagged fish that stayed in PIE ≥ 30 days varied from high [(> 75%); very high: 3 receivers – numbers 9, 12, 13; high: 4 receivers – numbers 5, 7, 14, 17] to intermediate [(51–74%); 6 receivers – numbers 3, 4, 16, 18, 19, 24] to low [low (25–50%): 6 receivers – numbers 6, 8, 11, 15, 20, 23; very low (< 25%): 6 receivers – numbers 1, 2, 10, 21, 22, 25, 26; Fig. 4A]. Receiver-specific movements (i.e., movements at a specific receiver averaged across all 50 fish that stayed in PIE ≥ 30 days) ranged from high [(> 75%); very high: 4 receivers—numbers 5, 7, 9, 12; or high: 3 receivers – numbers 6, 8, 14] to intermediate [(51–74%); 6 receivers – numbers 11, 13, 15, 16, 17, 18) to low [low (26–50%): 6 receivers – numbers 3, 4, 19, 20, 24, 25; or very low (< 25%): 7 receivers – numbers 1, 2, 10, 21, 22, 23, 26; Fig. 4B].
Behavior of tagged Striped Bass within PIE. (A) Mean residence time in hours (Y axis) and (B) mean number of movements (Y axis) at each of the 26 individual receivers. These receiver-specific values are the average of all tagged Striped Bass, detected in PIE for ≥ 30 days (n = 50), that visited a specific receiver. Data are mean and standard error. The colored panels represent ranges including high (> 76%), intermediate (51–75%), low (26–50%), and very low (0–25%).
Site-specific residence and movement clusters
In contrast, receiver-specific statistical clusters of combined residence-movement metrics (site-specific Resmo) revealed intriguing patterns about how migratory Striped Bass used specific locations within PIE during the summer feeding node of their coastal migration. For tagged Striped Bass detected in PIE ≥ 30 days, three strong receiver-site groups emerged from a cluster analysis of nine receiver-specific residence and receiver-specific movement metrics (n = 26 receivers; n = 50 tagged Striped Bass). These clusters included 17, 6, 3 receivers in clusters 1, 2, 3, respectively, and had Jaccard’s bootstrap means of 0.95, 0.86, 0.92 (Fig. 5A). Box plots of five examples of the nine cluster metrics identified differences in how Striped Bass resided or moved at each of these three clusters of receiver sites. Cluster 1 (n = 17 receiver sites; Fig. 5A,B) were characterized by two metrics that showed low receiver-specific Striped Bass residence times (Fig. 6A,B), two metrics that displayed low numbers of receiver-specific Striped Bass movements (Fig. 6C,D), and one metric that revealed Striped Bass movements of short distance (Fig. 6E). Cluster 2 (n = 6 receiver sites; Fig. 5A,B) were characterized by two metrics that showed high receiver-specific residence times (Fig. 6A,B), two metrics that displayed high numbers of receiver-specific movements (Fig. 6C,D), and one metric that revealed moderate distance movements (Fig. 6E). Cluster 3 (n = 3 receiver sites; Fig. 5A,B) were characterized by two metrics that showed low receiver-specific residence times (Fig. 6A,B), two metrics that displayed moderate numbers of receiver-specific movements (Fig. 6C,D), and one metric that revealed movements of shorter distance (Fig. 6E).
Shown are the results for three clusters of receiver sites (n = 26 receivers) created by nine metrics. The nine metrics used in the receiver-specific cluster analysis quantified average patterns of tagged Striped Bass residence and movement at each receiver location within PIE, MA, USA. (A) Receiver number (Y axis; 1–26 refer to Fig. 2C) by silhouette width (X axis) shows three clusters of receivers with Jaccard bootstrap means > 0.86. (B) Principal component analysis (PCA) of the three clusters of receivers shows cohesion within and separation across clusters. Adjacent numbers identify location identity of each receiver (1–26; refer to Fig. 2C). Symbols identify the three clusters of receivers. Cluster 1 = low-use (solid black dot; n = 17 receivers); Cluster 2 = high-use (gray square; n = 6 receivers), and Cluster 3 = variable-use (gray triangle; n = 3 receivers). Dotted lines connect the clusters to qualitatively show spatial patterns. Fifty tagged Striped Bass that were detected in PIE ≥ 30 days could visit each of the 26 receivers.
Shown are cluster-specific values of five metrics that help identify similarities and differences in the three clusters of receivers (identified in Fig. 5) used to detect tagged Striped Bass in PIE, MA, USA. These metrics include (A) whether receiver-specific residence time of tagged Striped Bass was greater than mean residence time for all receivers, (B) standardized receiver-specific residence time, (C) whether receiver-specific movements were greater than the mean of movements across all receivers, (D) standardized number of movements, and (E) movement distance. Metrics are described in detail in the methods. The units that are clustered are the 26 receivers. Data for residence time and movement metrics are averaged across all tagged Striped Bass detected at each receiver. Clusters correspond to those identified in Fig. 5. Cluster 1 = low-use. Cluster 2 = high-use. Cluster 3 = variable-use.
Other insights
Relationships between receiver cluster number and geographic location (Fig. 7A) and relative Striped Bass numbers at each receiver site (Fig. 7B) emerged when the locations of each of the 26-receiver sites were mapped by cluster number. Residence-movement patterns at receiver sites at the edges of our study area were often similar, but use characteristics of geographically-central receivers varied widely. Some cluster 1 sites (low-residence low-movement) were at the extreme northern (receiver sites 1, 2) and southern (receiver sites 25, 26) edges of PIE (Fig. 7A) and functioned as entrances and exits that could be used by many individual fish (Fig. 7B). Other low-use cluster 1 sites were at the extreme eastern and western edges of our study area (receiver sites 10, 11, 22, 23; Fig. 7A), and were visited by few individual tagged Striped Bass (Fig. 7B). High-use cluster 2 sites (high-residence high-movement) were consistently located in the center of the estuary (Fig. 7A), and were regularly visited by moderate (receiver site 12) to high numbers of individual Striped Bass (receiver sites 5, 7, 9, 13, 14; Fig. 7B). Variable-use cluster 3 sites (low-residence moderate-movement) were also consistently in the central region of our study area (Fig. 7A) and could be visited by many or few individual tagged fish (receiver sites 6, 8, 16; Fig. 7B). However, tagged Striped Bass did not exhibit high residence times and/or high movements at all central receiver sites. A third group of low-use cluster 1 sites were interspersed throughout the estuary (Fig. 7A) and were inconsistently visited by both many (receiver sites 3, 4, 17–21, 24) and few individual tagged Striped Bass (receiver site 15; Fig. 7B).
(A) Spatial location of each cluster of receiver sites within PIE, MA, USA, and (B) percent of the tagged Striped Bass population that visited each of the receiver sites within PIE. Numbers represent the receiver identity in both panels A and B (refer to Fig. 2C for original map of receiver locations with numbers). Color and shape represent the receiver site clusters in both panels (A,B). Cluster 1 (solid black dot) = low-use. Cluster 2 (solid dark gray square) = high-use. Cluster 3 (solid light gray triangle = variable-use). In B, the size of the symbol reflects the proportion of unique individual tagged Striped Bass that visited each receiver. The legend explaining figure symbols is in the upper right corner of each panel. Fifty tagged Striped Bass that were detected in PIE ≥ 30 days could visit each of the 26 receivers. Aerial photos of PIE were created in R with the open access program “Leaflet.” Data sources are Leaflet | Tiles Esri – Source: ESRI, I-cubed, USDA, USGS, AEX, GeoEye, Getmapping, Aerogrid, IGN, IGP, UPR-EGP, and the GIS User Community.
Discussion
Take home messages
The take home messages that we review below add to the understanding of feeding behavior of migratory fish (gap 1), suggest ecologically-meaningful hypotheses about residence, movement, and distribution of mobile organisms that are testable in the field (gap 2), and identify sites with specific use patterns that can direct sampling in future studies (gap 3).
Migratory striped bass feeding (gap 1)
The Resmo approach added to our understanding of Striped Bass distribution during the feeding node of their coastal migration. We observed that some migrant Striped Bass stayed in specific areas of PIE for a prolonged time, but also that these Striped Bass made frequent movements within PIE. Hence, studying within-estuary site-specific residence and movement patterns (especially related to foraging success) are important for understanding the Striped Bass coastal migration. One example of how our Resmo approach can guide tractable data collection that can advance our understanding of drivers of long-distance fish migration follows. Ecological theory about animal migration37,38,51 proposes that the energetic payoff for feeding migrations may be related to increased growth that results from the spring-fall migration. However, this prediction is logistically challenging to test empirically in a geographically-large, heterogeneous natural system. Specifically, to quantify if a growth payoff results from long distance migration, the distribution of a wide range of sizes/ages of individual Striped Bass from all coastal estuaries (Chesapeake Bay to Canada) would need to be consistently tracked along the coast and within estuaries from May to October, then linked to differential prey consumption and individual Striped Bass growth at all relevant sites for migratory and nonmigratory fish. Collecting this level of empirical data is not realistic from a logistical perspective. Site-specific Resmo metrics can help us prioritize date- and estuary-specific Striped Bass distributional responses (residence, movement), across-site options (e.g. how fish use sites), and within-site conditions (many prey, few prey, variable prey, physical habitat that affects foraging).
Different types of site uses (gap 2)
We developed and tested one hypothesis related to what residence and movement patterns occurred at different sites within PIE for Striped Bass during the foraging node of their coastal migration. Foraging theory is an example of a conceptual framework on which much progress was made in the controlled conditions that occur in the laboratory52, but for which field testing of theoretical predictions has proved more difficult because of the size and complexity of natural systems. Hypotheses about site use emerge from our Resmo approach and open new opportunities to test foraging theory in natural habitats with complex patterns of resource heterogeneity.
The specific hypothesis that we tested (H1; Fig. 1D–H) was whether Striped Bass exhibit similar or different patterns of residence and movement at individual sites within the same system. As a first potential outcome to this first hypothesis, all sites within a system could experience low-residence low-movement behavior by Striped Bass (hypothesis 1, outcome 1, H1a; four filled circles in the lower left quadrant of Resmo space; Fig. 1D). As a second potential outcome, all sites within a system could experience low-residence high-movement behavior by Striped Bass (hypothesis 1, outcome 2, H1b; four stars in the lower right quadrant; Fig. 1E). As a third possible outcome, all sites within a system could experience high-residence low-movement behavior by Striped Bass (H1c, four open squares in the upper left quadrant; Fig. 1F). As a fourth possible outcome, all sites within a system could experience high-residence high-movement (H1d; four thunderbolts; Fig. 1G). As a final outcome, different sites within a single system may be characterized by different combinations of Striped Bass residence and movement (H1e; one of each symbol; Fig. 1I]. Our Resmo data and analysis showed that Striped Bass foraging in PIE spent minimal time and moved little at low-use sites in PIE (Cluster 1; black circles, dotted ellipse outline; Fig. 8), spent much time and moved frequently at high-use sites in PIE (Cluster 2; filled gray squares, dashed rectangle outline; Fig. 8), and spent little time but moved a moderate amount at variable-use sites in PIE (Cluster 3; gray filled triangles within a solid triangle outline; Fig. 8). Based on these results, we can reject four of the five alternate outcomes proposed for this first hypothesis (H1a-d; Fig. 1D–G) leaving only outcome H1e (Fig. 1H), which was that different patterns of residence and movements coexist in PIE.
How the receiver sites identified unique types of site use for tagged Striped Bass in PIE, MA. USA. Each data point is a receiver site. Numbers inside the symbols identify the site (refer to Fig. 2C) and are the same as those used in other maps. The X axis is the average number of movements made by all Striped Bass that visited each receiver. The Y axis is the average residence time in hours of all Striped Bass that visited each receiver. Cluster 1 (solid black dots with dotted black outline) = low-use. Cluster 2 (solid dark gray square with a dashed outline) = high-use. Cluster 3 (solid light gray triangle with a solid gray outline = variable-use. Fifty tagged Striped Bass that were detected in PIE ≥ 30 days could visit each of the 26 receivers. These straightforward metrics are not exactly the same as the metrics used in the cluster analysis, but they show the same types of patterns.
Locations to target for future data tests (gap 3)
The Resmo approach can help identify sites to sample that test alternate outcomes to emerging hypotheses. Many conceptually-based hypotheses that provide the foundation on which to base expectations and predictions are developed under simplified laboratory, mesocosm, or modeling conditions. However, creating ecologically-meaningful, spatially-explicit hypotheses about movement, residence, and distribution that are actually testable in the field is challenging because we often do not know where mobile organisms are located, when-why-how they move, and where to sample to distinguish among multiple outcomes. For example, alternate foraging theories predict that increased growth could occur when foragers trade off residence and movements to stay in a profitable site [theory: site fidelity39], move to and stay within profitable habitats [theory: habitat selection41], use specific spatial patterns [theory: central place foraging53], consistently adjust distribution to match prey [theory: resource tracking54], balance resources and competitors [theory: ideal free distribution55], or move to take advantage of seasonally abundant resources [theory: long distance migration37,38,51]. Researchers logistically cannot sample all aspects of foraging at all relevant locations at all possible times to test amongst these theories in the field, but the Resmo approach can help prioritize sites and times to collect relevant data.
A second hypothesis that emerged is whether site-specific residence-movement patterns of Striped Bass are linked to specific foraging conditions (H2; Fig. 1I). Below we propose four possible outcomes for this second hypothesis, then suggest how these alternatives might be tested in the future. First, do Striped Bass spend minimal time at low-use sites (Cluster 1; black circles, dotted ellipse outline; Fig. 8) because of either site-specific location, harsh physical habitat conditions that reduce foraging success, or low prey availability? Second, does Striped Bass nomadic exploration of variable-use sites (Cluster 3; gray filled triangles within a solid triangle outline; Fig. 8) reflect fluctuating or unpredictable foraging conditions? Third, was traditional site-fidelity (high-residence low-movement) absent in this analysis because spatially predictable and temporally-consistent, high-value feeding sites with sedentary prey were uncommon in a dynamic tidal estuary? Fourth, is the concentration of Striped Bass within the high-use sites (Cluster 2; filled gray squares, dashed rectangle outline; Fig. 8) related to foraging Striped Bass seeking out a limited number of locations that provide above-average energetic rewards (e.g. high prey numbers, high prey quality, or physical conditions or foraging behavior that result in high catchability of prey).
These four outcomes for this second hypothesis can be tested in the future by comparing eight types of commonly collected foraging-related data (Fig. 1I, X axis) across low-use, variable-use, and high-use sites; Fig. 1I, Y axis). The eight types of potential foraging-related drivers discussed below are (1) location (edge, center of the estuary); (2) prey amount (biomass, number); (3) predictable timing of prey presence; (4) prey type (fish or invertebrate); (5) ease with which a Striped Bass can capture prey; (6) specific abiotic variables that increase foraging success (drop-offs, current velocity, other habitat characteristics); (7) other biotic variables that affect foraging (competitors); and (8) foraging behavior (learned or opportunistic). Potential tests of the four outcomes for hypothesis two, described above, using one or more of these eight foraging-related drivers follow. First, compared to other sites, is the location of low-use sites (low-residence low-movement) more likely to be at the edge of the target ecosystem (north–south edge, east–west edge; foraging driver 1) compared to other site types? Second, compared to other sites, do variable-use sites (low-residence high-movement) have fewer prey (foraging driver 2) that are unpredictable in location and timing (foraging driver 3) leading to nomadic Striped Bass behavior? Third, was fidelity to a specific site (high-residence low-movement) not detected in this analysis because sedentary prey (invertebrates; foraging driver 4) could not be easily and regularly captured (foraging driver 5) at predictable locations in this dynamic ecosystem (foraging driver 3)? Fourth, compared to other sites, are high-use sites (high-residence high-movement) characterized by above average prey (quantity or quality; foraging drivers 2, 4); more physical habitat discontinuities that increase prey catchability (drop-offs, cul-de sacs, creek edges; foraging driver 6); reduced numbers of competitors (conspecific, heterospecific; foraging driver 7); or more locations where potentially successful foraging tactics can be learned (e.g. waiting at creek mouths to potentially catch invertebrates on an ebbing tide, foraging driver 8). Comparing these eight (and other) foraging-related drivers at these three site use types (low-use, variable use, high-use) can help tease apart alternate outcomes for this second emerging hypothesis.
As a third category of emerging hypotheses, our Resmo approach also raises the possibility of testing ideas that have been previously discussed only in conceptual frameworks and tested only in controlled experiments. An example of this third category of hypothesis is whether individual animals exhibit different personalities while foraging in complex environments56,57). Our Resmo methods for identifying low-use, variable-use, and high-use sites make the collection of data that could test these challenging questions tractable.
Multiple metrics
Quantifying multiple responses from a trajectory tells a more informative story about the function of sites than individual responses alone9,23. In our research, population metrics (response category 1: i.e. population proportion, detections, counts) were useful in assessing the effectiveness of our methodology and for defining the boundaries of our study area. However, in PIE, the population proportion of tagged Striped Bass did not contribute important ecological information about how sites were used, specifically if individual fish spent a lot of time at a site or if individual fish were just passing through on their way to somewhere else. Quantifying movement (response category 2: i.e. mean number of movements, movement distance per day, direction, duration, extent of movement) is critically important for understanding distribution, abundance, and conservation of mobile organisms. However, movement metrics can have multiple causes and consequences, and can be challenging to interpret accurately alone. Time–space metrics (response category 3: i.e., residence time, utilization distribution, home range) is important but residence also alone does not accurately describe complex distribution patterns for mobile and migratory fish. We would have missed important trends about foraging behavior of migratory Striped Bass if we had not interpreted all metrics together. For example, without using both resident and movement metrics, we could not have identified characteristics and locations of high-use sites. Thus, combining population, movement, and residence responses, as we do with our Resmo site-function examples, provides an innovative and generalizable guide for future organismal distribution research.
Generality
Our results have features specific to our study, but also provide general applicability. First, our focus was on the northern apex of the migratory cycle, at which our tagged fish were feeding. Others may be more interested in other locations [overwintering, spawning36], in connecting migration routes, or in migration patterns in general12. Combining patterns of residence and movement, as we do here, can be informative in specific locations (i.e., stopover patterns within and across migratory cohorts) and in general (along the migration corridor). Second, our telemetry detection design used a passive acoustic array because, for small and medium sized animals, stationary detection arrays are a major source of telemetry data. Resmo is applicable with any organism that moves (in the water or on land) and with any potential tracking methodology (many forms of telemetry, passive integrated transponder tags, other passive detection methods) provided the spatial data are at a fine enough scale within the study area. Third, we used site-specific Resmo to show that even in a geographically-large, heterogeneous natural study system, we can identify testable, nuanced outcomes for hypotheses. Others may also be interested in fish-specific Resmo, which can identify if groups of fish of the same species in the same geographic area use space similarly, move the same way, or have similar behaviors9,47. Both individual and site residence-movement metrics have utility for different questions. Finally, we only identified potential locations to collect data to test hypotheses about why Resmo patterns occur. However, our explicit insights about creating these potential hypotheses and testable outcomes have broad utility. Specifically, Resmo metrics provide a useful new direction to obtain empirical data to understand field distributions for mobile organisms in general and for migratory animals in particular.
Methods
Study site
Plum Island Estuary (PIE) is a temperate estuary in Massachusetts, USA (Fig. 2A) that consists of three major rivers (Parker, Rowley, Ipswich Rivers), a man-made connection to the coastal Merrimack River (Plum Island River), an open water embayment (Plum Island Sound;13.2 km long), and a large central island, Middle Ground Island58 (Fig. 2B). The southern entrance of Plum Island Sound connects directly to the Atlantic Ocean near the mouth of the Ipswich River. To the north, the Plum Island River connects indirectly to the ocean via the coastal Merrimack River. PIE (surface area of 12.8 km2 at low tide and 20.0 km2 at high tide) includes substantial heterogeneity in aquatic habitats that affect fish including confluences, depth variation, drop-offs, sand bars, and islands15,48.
General design
This research was conducted under the auspices of the Kansas State University (KSU) Institutional Animal Care and Use Committee (IACUC) Protocol #3576. In our study designed to assess natural variation in residence and movement by individual fish, the experimental units in our test group were both individual fish (n = 59 tagged Striped Bass) and individual receiver sites (n = 26). Because we were assessing the variation in natural populations, a trend that is unknown for the system in which we worked, we did not use a control group. Our fish sample size is similar to that used in other fish telemetry studies and in previous studies with the same organism19,47. Because we tagged all members of the captured population using identical procedures, no randomization or blinding was implemented. We eliminated possible confounding variables by tagging all fish using the same, very-detailed protocol. Our research objectives, overall design, methods, and other components of our protocol were prepared prior to data collection and are registered with the KSU IACUC.
Tagging
During the summer of 2015, 59 Striped Bass [mean fish size = 524 mm, range 434–623 mm; 1.46 kg, range: 0.79–2.85 kg) were tagged in PIE using Vemco V13 acoustic tags (length: 36–48 mm, weight in air: 11–13 g, weight in water: 6–6.5 g)59. Specifically, over 11 days in 2015, expert anglers captured wild Striped Bass using flies with barbless hooks. Using Aqui-S (30 mg-L), individual fish were anesthetized until they lost orientation (mean = 2 min 18 s). During tag insertion, an assisting non-surgeon kept the fish gills and intact body integument continuously irrigated with ambient water. With a surgical scalpel (size 12), a 15–30 mm lateral incision was made below the pectoral fin and a disinfected tag was inserted into the fish body cavity. Using 2–4 surgical sutures (Ethicon, braided, coated Vicryl, 3–0, FS-2, 19 mm 3/8c, reverse cutting; mean surgery time = 2 min 31 s), incisions were closed. Fish were carefully placed in individual recovery tanks filled with ambient water where they were continuously observed post-surgery. Tagged fish were quickly and carefully released near capture locations once they started swimming upright (mean recovery time = 6 min 15 s).
Receiver array
In our research, we used identical deployment protocols for identical receiver models at each receiver location in order to detect the population of Striped Bass that were implanted with identical V13 transmitters using identical surgical methods (described above). To create bases on which to anchor receivers, plastic dishpans (429 mm × 356 mm × 187 mm) were filled with concrete in which we embedded multiple eyebolts and a central threaded rod (half the height of the upright receiver). Upright receivers were attached to the central threaded rod and central eyebolts with cable ties and hose clamps. A bridle attached to the four corner eyebolts was connected to rope of a length that slightly exceeded water depth at high tide. Each receiver base was placed upright on the bottom of the estuary at designated sites and marked with a buoy ball float.
Our receivers were deployed throughout PIE (Fig. 2C) from June 24, 2015 – October 26, 2015 because our focus was on developing and testing the Resmo approach using 2015 data. Specifically, we placed receivers at select non-confluence sites (receiver numbers 3, 12, 13, 18, 19, 20); select locations in the confluences of four similar-sized tributaries [Grape Island (receivers 21, 22, & 23); Third Creek (receivers 14, 15, & 16); Rowley River (receivers 5, 6, & 8), West Creek (receivers 9, 10, & 11); and major exits (receiver numbers 1, 2, 25, 26). The range of Vemco tags and Vemco receivers has been tested in PIE multiple times60,61. For these range tests, as we increased the distance between test tag and test receiver, we paused approximately every 100 m to allow detections to be recorded (5–15 min). We repeated this process in multiple directions (typically four cardinal compass headings) for transects of approximately 1000 m (total length) or until we encountered the shoreline. In another system59, this method provided a similar estimate to the methodology suggested by the receiver manufacturer, which was not logistically feasible in PIE given physical and logistical constraints. In PIE in 2005, the average range of 10 VEMCO receivers that were not blocked by physical obstructions was 533 m at high tide60. In PIE in 2009, at 20 different locations, the maximum receiver range was 600 m (at approximate mid tide)61. We adopt an approximate receiver range of 533 m for our data collection that integrated diel, tidal, daily, and seasonal time periods.
Humane treatment of fish
Fish handling methods and research design were approved by the scientists, administrators, and veterinarians who served on the KSU IACUC. All methods were carried out in accordance with protocol guidelines and other existing regulations. The study methods follow ARRIVE guidelines, which are addressed throughout. To minimize fish pain, suffering, and distress, we used a quick capture technique (fly fishing with barbless fly hooks by experienced anglers). Fish bodies and gills were kept wet at all times. Fish were anesthetized before surgery. Tags were small (< 2% body weight). Fish recovery was carefully monitored, and tagged fish were released near the capture site as soon as they regained their ability to swim upright. Surgeons practiced detailed tagging procedures before surgery and a third non-surgeon oversaw the tagging process to make sure no unexpected events occurred to stress fish. In a post-tagging evaluation of the protocol described here, survival did not differ between tagged and untagged fish47,61. Although we did not need to implement protocol-determined humane endpoints, we would have ceased tagging if fish had developed labored breathing, appeared overly stressed, or bled excessively.
Response metrics
All tagged fish were detected the week following tagging (n = 59). Nine tagged fish were detected in PIE for < 30 days. These “short-timers” were removed from the cluster analysis metrics because our focus was on residence and movements of tagged Striped Bass in PIE. For the 50 fish that were detected in PIE ≥ 30 d, we did not remove data in the days immediately following tagging fish because these initial data were a small part of the total dataset.
Individual fish data were summarized as three metrics. The number of uniquely coded individual fish that visited a given receiver site defined the metric “unique individuals.” We calculated the population proportion as the number of unique individuals detected compared to the number of fish tagged. The number of times a fish arrived or left a receiver site defined the fish-specific metric “number of movements.” How much time each fish spent at each receiver defined the fish-specific metric “residence time” (VTrack software)62 and was calculated as the time when a tagged fish was detected twice until it was not detected anywhere for one hour or was detected at another receiver site. For the Resmo cluster analysis, the above fish-specific metrics were summarized for all fish at each of the 26 receivers. Indices of variation (mean and standard error) are reported for length, weight, and all outcome measures (residence, movements).
Cluster metrics
A single metric did not capture all useful residence and movement information, so we created multiple metrics for responses of interest. For each of our 26 receiver sites, we calculated nine metrics that revealed ecological and statistical information about fish residence and fish movements. For all metrics, the receiver-specific value was calculated as a summary of responses for all tagged Striped Bass that visited that receiver. Because of the complexity of residence and movement responses and differences in data distributions at and across receiver sites, choosing interpretable and ecologically-meaningful metrics with understandable statistical properties required thought and creativity. Each metric is described in detail below. All metrics were calculated with Program R63.
Metric 1, “Residence time > mean residence,” and Metric 2, “Movements > mean movements” were binomial variables that compared receiver-specific values (residence time or movement) to overall mean values at all receivers (residence time or movement). The rationale for creating these first two metrics was that the absolute value at a receiver may have little meaning for mobile animals when considered in isolation. Likely, how animals perceive the utility of a location depends on how each receiver location compares to other locations. Metrics 1 and 2 were calculated by subtracting the mean values (residence time or number of movements) for all fish at all locations from the mean values (residence time or number of movements) for all fish at each individual receiver site. A positive number was given the value of 1, and a negative number was given the value of 0. Comparing an individual receiver to an array-wide mean conveys the desired information in a simpler and more interpretable way than making comparisons of all pairs of receivers. Binomial scoring avoids concerns about extreme observations and differences in distributions across receivers.
Metric 3, “Standardized residence time,” and metric 4, “Standardized number of movements,” summed the total values for all fish at each receiver site (residence time or number of movements), divided this sum by the number of fish that visited the site, then scaled these values so that the mean and standard deviation for all receivers were zero and 1, respectively. Receiver-specific values greater than the mean were positive and values less than the mean were negative. These standardization values depict if the metric was high or low and the distance from the mean. Although metrics 3, 4 are similar to metrics 1, 2, metrics 3, 4 report the magnitude of differences across sites not just direction. Mean standardization puts variables of different units into the same scale. Metrics 3, 4 were mean standardized using the scale function in the base library with Program R.
Metric 5, “Residence rank”, and metric 6, “Movement rank,” quantified the rank of residence time or number of movements across all 26 receiver sites. These metrics provided another way of comparing sites in a way that does not account for magnitude of difference and is not affected by differences in receiver data distributions. These metrics were calculated using the rank function in the base package for Program R.
Metric 7, “Difference crosses zero,” quantifies if trends in residence and movements are the same or different at an individual site. This metric is a binomial variable that compares trends in metric 1, Residence time > mean residence, with trends in metric 3, Movements > mean movements. This metric was calculated by subtracting metric 1 from metric 3. If the difference in metrics 1 and 3 equaled zero then the value of metric 6 remained zero. If the difference between metrics 1 and 3 was 1 or -1, metric 6 was given a value of 1. Sites with zero indicate that the site had either a high residence and high movement or a low residence and low movement. Values of 1 indicate that a site had either a high residence metric or a high movement metric but not both.
Metric 8, “Difference in residence and movement,” is calculated as the difference in the standardized number of movements subtracted from the standardized residence time variable. Like metric 7, this metric compares residence and movements at a receiver site, but unlike metric 7, this metric shows the magnitude of the differences between residence and movement values at a site not just the direction.
Metric 9, “Mean Distance (km),” quantified if fish that visited a receiver site moved relatively long or short distances. This variable was calculated as the summed total distance between receiver locations for all fish at each site divided by the total number of fish that visited that site. These values were standardized by centering (subtracting the mean) and scaling (dividing by the standard deviation) using the scale function in R. The distance moved conveys different information than the previous metrics and scaling facilitates meaningful comparisons across receivers with potentially different distributions.
Similarities existed across the nine metrics. Metrics 1, 3, 5 separated differences across receivers in residence, metrics 2, 4, 6 separated differences in movements across receivers, metrics 7, 8 compared residence and movement trends at a receiver; and metric 9 quantified distance of movement at each receiver. For metrics that collected similar information, we used different statistical approaches [e.g. comparing directions of responses with simple binomial variables (metrics 1, 2, 7), comparing magnitude of responses using standardized variables (metrics 3, 4, 8); and comparing ranks (metrics 5, 6)]. Thus, each of the nine metrics contributed unique but often related, ecological-statistical information. All metrics were used in the cluster analysis, but for brevity, we show box plots to illustrate major trends only for metrics 1, 2, 3, 4, 9.
Cluster analysis
Cluster analysis was performed using Partitioning Around Medoids (PAM) on Gower distance matrices with the optimal number of clusters determined by average silhouette width64. Cluster stability was assessed using 100 bootstrap resamples. Principal Component Analysis was used to visualize clustering results. Boxplots compared select metrics across clusters. Cluster analysis was performed with the fpc and cluster packages using Program R63. The Gower distance matrix is a measure used to calculate the dissimilarity between observations in a dataset that contains both continuous and categorical variables. The Gower distance matrix is particularly useful in situations where mixed data types are present, unlike Euclidean distance, which only works with continuous variables. The Gower distance matrix was calculated with the vegan package using Program R.
Summary of metrics and analysis
With the above calculation and analysis details, all visualizations and analyses are replicable with our data and with other telemetry data as suggested in the ARRIVE guidelines. These nine metrics worked well for our data and fish-system-question. These metrics and calculations can be ecologically meaningful across organisms and systems. However, modifications of metrics may be needed for different animal behaviors. Definitions, justifications, and methods for metrics should be clearly identified in all future uses.
Data availability
https://zenodo.org/records/14166068?token=eyJhbGciOiJIUzUxMiJ9.eyJpZCI6IjVmMmZlZjRiLWI2ZTYtNDVmZC05YTMzLTRiYzdmNGI2MzM4YyIsImRhdGEiOnt9LCJyYW5kb20iOiJiNGJiMGM4ODk4ZGZmZjE0YzNhMmZlYjhjMzhjYTg2OSJ9.5R9P5LIslYNTM74gh59Ytd0rTdJ7mSLk-P7qscoHMobF26wEQiy5MPvPuauILOx6yXnIYtqsysT5V28cU0yrNQ or from the corresponding author.
References
Swingland, I. R. & Greenwood, P. J. The Ecology of Animal Movement (Clarendon Press, 1983).
Hansson, L. A. & Åkesson, S. Animal Movement Across Scales (Oxford University Press, 2014).
Nathan, R. et al. Big-data approaches lead to an increased understanding of the ecology of animal movement. Science 375, eabg1780 (2022).
Webster, M., Marra, P., Haig, S., Bensch, S. & Holmes, R. Links between worlds: unravelling migratory connectivity. Trends Ecol. Evol. 17, 76–83 (2002).
Wilcove, D. S. & Wikelski, M. Going, going, gone: Is animal migration disappearing?. PLoS Biol. 6(7), 1361–1364 (2008).
Joly, K. E. et al. Longest terrestrial migrations and movements around the world. Sci. Rep. 9, 15333 (2019).
Wright, R. M. et al. First direct evidence of adult European eels migrating to their breeding place in the Sargasso Sea. Sci. Rep. 12, 15362 (2022).
Ceriani, S. A., Weishampel, J. F., Ehrhart, L. M., Mansfield, K. L. & Wunder, M. B. Foraging and recruitment hotspot dynamics for the largest Atlantic loggerhead turtle rookery. Sci. Rep. 7, 16894 (2017).
Taylor, R. B., Mather, M. E., Smith, J. M. & Gerber, K. M. Confluences function as ecological hotspots: geomorphic and regional drivers can identify patterns of fish predator distribution within a seascape. Mar. Ecol. Prog. Ser. 629, 133–148 (2019).
Lathouwers, M. et al. Rush or relax: migration tactics of a nocturnal insectivore in response to ecological barriers. Sci. Rep. 12(1), 4964 (2022).
Donaldson, M. R. et al. Making connections in aquatic ecosystems with acoustic telemetry monitoring. Front. Ecol. Environ. 1210, 565–573 (2014).
Secor, D. H. Migration Ecology of Marine Fishes (Johns Hopkins University Press, 2015).
Ogburn, M. B. et al. Addressing challenges in the application of animal movement ecology to aquatic conservation and management. Front. Mar. Sci. https://doi.org/10.3389/fmars.2017.00070 (2017).
Goossens, J. et al. Elucidating the migrations of European seabass from the southern north sea using mark-recapture data, acoustic telemetry and data storage tags. Sci. Rep. 14(1), 13180 (2024).
Hussey, N. E. et al. Aquatic animal telemetry: A panoramic window into the underwater world. Science 348, 6240 (2015).
Lennox, R. J. et al. Envisioning the future of aquatic animal tracking: technology, science, and application. BioScience 6710, 884–896 (2017).
Crossin, G. T. et al. Acoustic telemetry and fisheries management. Ecol. Appl. 274, 1031–1049 (2017).
Frank, H. J., Mather, M. E., Smith, J. M., Muth, R. M. & Finn, J. T. Role of origin and release location in pre-spawning movements of anadromous alewives. Fish. Manag. Ecol. 18, 12–24 (2011).
Kennedy, C. G., Mather, M. E., Smith, J. M., Finn, J. T. & Deegan, L. A. Discontinuities concentrate mobile predators: Quantifying organism-environment interactions at a seascape scale. Ecosphere 7, e01226 (2015).
Gerber, K. M., Mather, M. E. & Smith, J. M. Multiple metrics provide context for the distribution of a highly mobile fish predator, the blue catfish. Ecol. Freshw. Fish 128, 141–155 (2019).
Smith, J. M. et al. Evaluation of a gastric radio tag insertion technique for anadromous river herring. N. Am. J. Fish. Manag. 29, 367–377 (2009).
Frank, H. J., Mather, M. E., Smith, J. M., Muth, R. M. & Finn, J. T. What is ‘fallback’?: metrics needed to assess telemetry tag effects on anadromous fish behavior. Hydrobiologia 35, 237–249 (2009).
Gerber, K. M., Mather, M. E. & Smith, J. M. A suite of standard post-tagging evaluation metrics can help assess tag retention for field-based fish telemetry research. Rev. Fish. Fish Biol. 273, 651–664 (2017).
Griffin, L. P. et al. Keeping up with the Silver King: Using cooperative acoustic telemetry networks to quantify the movements of Atlantic tarpon Megalops atlanticus in the coastal waters of the southeastern United States. Fish. Res. 205, 65–76 (2018).
Krueger, C. C. et al. Acoustic telemetry observation systems: challenges encountered and overcome in the Laurentian Great Lakes. Can. J. Fish. Aquat. Sci. 7510, 1755–1763 (2018).
Iverson, S. J. et al. The ocean tracking network: Advancing frontiers in aquatic science and management. Can. J. Fish. Aquat. Sci. 767, 1041–1051 (2018).
Hooten, M. B. et al. Animal Movement: Statistical Models for Telemetry Data (CRC Press, 2017).
Whoriskey, K. et al. Current and emerging statistical techniques for aquatic telemetry data: A guide to analysing spatially discrete animal detections. Methods Ecol. Evol. 107, 935–948 (2019).
Lennox, R. J., Blouin-Demers, G., Rous, A. M. & Cooke, S. J. Tracking invasive animals with electronic tags to assess risks and develop management strategies. Biol. Invasions 185, 1219–1233 (2016).
Taylor, M. D., Laffan, S. W., Fairfax, A. V. & Payne, N. L. Finding their way in the world: Using acoustic telemetry to evaluate relative movement patterns of hatchery-reared fish in the period following release. Fish. Res. 186, 538–543 (2017).
Brooks, J. L. et al. Biotelemetry informing management: case studies exploring successful integration of biotelemetry data into fisheries and habitat management. Can. J. Fish. Aquat. Sci. 767, 1238–1252 (2019).
Cooke, S. et al. Animal migration in the Anthropocene: threats and mitigation options. Biol. Rev. 99, 1242–1260 (2024).
Allen, A. M. & Singh, N. J. Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2015.00155 (2024).
Hays, G. C. et al. Translating marine animal tracking data into conservation policy and management. Trends Ecol. Evol. 345, 459–473 (2019).
Mather, M. E. et al. Merging scientific silos: Integrating specialized approaches for thinking about and using spatial data that can provide new directions for persistent fisheries problems. Fisheries 46, 485–494 (2021).
Harden Jones, F. R. Fish Migration (St Martin’s Press, 1968).
Gross, M. R., Coleman, R. & McDowall, R. M. Aquatic productivity and the evolution of diadromous fish migration. Science 239, 1291–1293 (1988).
Dingle, H. V. & Drake, A. What is migration?. BioScience 57, 113–121 (2007).
Switzer, P. V. Site fidelity in predictable and unpredictable habitats. Evolut. Ecol. 7(6), 533–555 (1993).
Dittman, A. & Quinn, T. P. Homing in Pacific salmon: mechanisms and ecological basis. J. Exp. Biol. 199(1), 83–91 (1996).
Van Moorter, B., Rolandsen, C. M., Basille, M. & Gaillard, J. Movement is the glue connecting home ranges and habitat selection. J. Anim. Ecol. 85, 21–31 (2016).
Dahlgren, C. P. & Eggleston, D. B. Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 81, 2227–2240 (2000).
Creel, S. et al. Habitat shifts in response to predation risk are constrained by competition within a grazing guild. Front. Ethol. https://doi.org/10.3389/fetho.2023.1231780 (2023).
Waldman, J. R., Dunning, D. J., Ross, Q. E. & Mattson, M. T. Range dynamics of Hudson River Striped Bass along the Atlantic coast. Trans. Am. Fish. Soc. 119, 910–919 (1990).
Mather, M. E., Finn, J. T., Kennedy, C. G., Deegan, L. A. & Smith, J. M. What happens in an estuary does not stay there: patterns of biotic connectivity resulting from long term ecological research. Oceanography 26, 168–179 (2013).
Ferry, K. H. & Mather, M. E. Spatial and temporal diet patterns of young adult and subadult Striped Bass feeding in Massachusetts estuaries: trends across scales. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 4, 30–45 (2012).
Pautzke, S. M., Mather, M. E., Finn, J. T., Deegan, L. A. & Muth, R. M. Seasonal use of a New England estuary by foraging contingents of migratory Striped Bass. Trans. Am. Fish. Soc. 139, 257–269 (2010).
Kennedy, C. G., Mather, M. E. & Smith, J. M. Quantifying integrated, spatially-explicit, ecologically-relevant, physical heterogeneity within an estuarine seascape. Estuaries Coasts 40, 1385–1397 (2017).
Taylor, R. B., Mather, M. E., Smith, J. M. & Gerber-Boles, K. M. Can identifying discrete behavioral groups with individual-based acoustic telemetry advance the understanding of fish distribution patterns?. Front. Mar. Sci. 8, 723025 (2021).
Mather, M. E. et al. Destinations, routes, and timing of adult Striped Bass on their southward fall migration: implications for coastal movements. J. Fish Biol. 77, 2326–2337 (2010).
Baker, R. R. The Evolutionary Ecology of Animal Migration (Holmes & Meier, 1978).
Stephens, D. W., Brown, J. S. & Ydenberg, R. C. Foraging: Behavior and Ecology (University of Chicago Press, USA, 2007).
Rosenberg, D. K. & McKelvey, K. S. Estimation of habitat selection for central-place foraging animals. J. Wildl. Manag. 63(3), 1028–1038 (1999).
Abrahms, B. et al. Emerging perspectives on resource tracking and animal movement ecology. Trends Ecol. Evol. 36(4), 308–320 (2021).
Kacelnik, A., Krebs, J. R. & Bernstein, C. The ideal free distribution and predator-prey populations. Trends Ecol. Evol. 7(2), 50–55 (1992).
Wolf, M. & Weissing, F. J. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27(8), 452–461 (2012).
Hirsch, P. E., Thorlacius, M., Brodin, T. & Burkhardt-Holm, P. An approach to incorporate individual personality in modeling fish dispersal across in-stream barriers. Ecol. Evol. 7(2), 720–732 (2017).
Deegan, L. A. & Garritt, R. H. Evidence for spatial variability in estuarine food webs. Mar. Ecol. Progr. Ser. 147, 31–47 (1997).
Gerber, K. M., Mather, M. E., Smith, J. M. & Peterson, Z. Evaluation of a field protocol for internally-tagging fish predators using difficult-to-tag ictalurid catfish as examples. Fish. Res. 209, 58–66 (2019).
Pautzke, S. M. Distribution Patterns of Migratory Striped Bass in Plum Island Estuary, Massachusetts. MS Thesis, University of Massachusetts (2008).
Kennedy, C. G. Habitat heterogeneity concentrates predators in the seascape: linking intermediate-scale estuarine habitat to striped bass distribution. M.S. Thesis, University of Massachusetts (2013).
Campbell, H. A., Watts, M. E., Dwyer, R. G. & Franklin, C. E. V-Track: software for analysing and visualising animal movement from acoustic telemetry detections. Mar. Freshw. Res. 63, 15–820 (2012).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2024). https://www.R-project.org/
Kaufman, L. & Rousseeuw, P. J. Partitioning around medoids (program pam). In Finding Groups in Data: An Introduction to Cluster Analysis (eds Kaufman, L. & Rousseeuw, P. J.) 68–125 (Wiley, 1990).
Acknowledgements
This project was administered by the Kansas Cooperative Fish and Wildlife Research Unit as a joint effort between Kansas State University, the U.S. Geological Survey, U.S. Fish and Wildlife Service, the Kansas Department of Wildlife and Parks, and the Wildlife Management Institute. The Plum Island Ecosystems LTER program (OCE-0423565, OCE-1058747, OCE-1238212) provided financial support, lodging and invaluable field, laboratory, and other assistance throughout the research. We thank the PIE LTER scientific community for support and feedback. Linda Deegan, Anne Giblin, Barry Clemson, Sam Kelsey, and Cristina Kennedy provided field, laboratory, and editorial assistance. We are also appreciative of the cooperative spirit and actions of fellow members of the Atlantic Cooperative Telemetry (ACT) network. Reviews by Anthony Overton, John Waldman, and an anonymous reviewers improved the manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. A review of scientific and common names was completed; tribe and geologic names do not apply to this manuscript. No AI technologies were used in the writing process. https://pie-lter.mbl.edu/data-catalog/. Publication of this article was funded in part by the Kansas State University Open Access Publishing Fund.
Author information
Authors and Affiliations
Contributions
MM: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources, Supervision; Validation; Visualization; Roles/Writing—original draft; and Writing—review & editing. RT: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Roles/Writing—original draft; and Writing—review & editing. KB: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Roles/Writing—original draft; and Writing—review & editing. JS: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Roles/Writing—original draft; and Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mather, M.E., Taylor, R.B., Smith, J.M. et al. Integrated patterns of residence and movement create testable hypotheses about fish feeding migrations. Sci Rep 15, 5951 (2025). https://doi.org/10.1038/s41598-024-79627-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79627-1