Abstract
Chemical process intensification has attracted extensive interest of scholars. Distillation, as a high energy consumption industry, is in urgent need of energy saving and CO2 emissions reduction by means of process intensification. In this paper, isobutanol and p-xylene azeotrope was separated by pressure swing distillation (PSD). Combined with process integration method, four heat integration PSD processes including partial heat integration PSD (PHI-PSD), enhanced partial heat integration PSD (EPHI-PSD), full heat integration PSD (FHI-PSD), and enhanced full heat integration PSD (EFHI-PSD) were designed and optimized through sequential iterative procedure. The total annual cost (TAC), CO2 emissions, and thermodynamic efficiency were calculated and compared. By comparing relevant data, it is found that EPHI-PSD process has obvious advantages in terms of economic, thermodynamic efficiency, and environmental benefits. Specifically, comparing with the basic PSD process, thermodynamic efficiency of EPHI-PSD process is improved by 97.8% while TAC and CO2 emissions are decreased by 37.0% and 50.1%, respectively.
Similar content being viewed by others
Introduction
Chemical process intensification is a long-term goal for chemical engineering scholars, which has attracted people’s attention in recent years1,2,3,4. The so-called process intensification is to improve the process flow and equipment efficiency through technological innovation, so as to make the plant layout more compact, unit energy consumption lower and wastes less. Process integration is an important aspect of process intensification, including material integration and energy integration. Material integration utilizes the flow of materials between different units to optimize products and maximize resource utilization while energy integration is a beneficial method to heighten energy efficiency and lessen energy consumption. Distillation, as an important separation method, accounts more than 95% energy consumption for chemical separation processes5. Meanwhile, the CO2 emissions of distillation process are related with its energy consumption6. With the global climate change and the proposal of China’s carbon peak and carbon neutral target, how to reduce CO2 emissions has become the main focus. As a result, it has a very important sense to reduce the energy expenditure and CO2 emission of distillation process by process integration.
Under constant pressure, some liquid mixtures of two or more components can boil with azeotropes formation, which means that their compositions and boiling points remain unchanged, and the steam produced by boiling has the identical composition as the liquid. There is no chance to separate azeotropes by conventional distillation or fractionation. There are two types of common methods for separating azeotropes, one is the separation method of introducing other components, such as extractive distillation7,8,9, azeotropic distillation10,11,12, liquid-liquid extraction13,14; the other is the separation method without introducing other components, such as pressure swing distillation (PSD)15, membrane separation16,17,18. PSD is a particular distillation approach that employs distillation columns featuring diverse operating pressures to accomplish azeotropic separation and its characteristic is that the azeotropic composition changes significantly with pressure changes. For instance, Wang et al.19 studied the economy of PSD process with different feed compositions when separating tetrahydrofuran and ethanol azeotrope and found that the excellent PSD process with minimum total annual cost (TAC) is related with the column sequence. Zhang et al.20 investigated the dynamic control performance for the PSD scheme of ethylenediamine/water azeotrope and the results indicated that when the feed composition is disturbed, the pure proportional cascade temperature controller can weaken the violent oscillation effect of product components. Yang et al.21 separated tetrahydrofuran/ethanol/water azeotrope with PSD scheme by using three columns operated at different pressures and proposed the control strategy for the process flowsheet with minimum TAC.
With the emphasis on process intensification and environmental protection, many scholars began to design the PSD process from two aspects of improving equipment efficiency and realizing process integration22,23,24. Cao et al.25 found that the PSD with varied-diameter columns can save 3.2% TAC compared with ordinary columns taking the separation of methanol-chloroform azeotropic mixture as a case in point. Zhang et al.26 compared the untraditional PSD and the internally heat-integrated untraditional PSD for separating benzene/isobutanol mixture from the viewpoint of TAC and CO2 emission and found the latter can decrease TAC by 44.96% and CO2 emission by 57.48%. Li et al.27 found PSD process with heat pump can save more than 50% operating cost compared with ordinary PSD in separating ethanol/acetonitrile binary azeotrope. Mao et al.28 applied four modified PSD processes to separate 1,2-butanediol and ethylene glycol binary mixture, and found the full heat integration PSD (FHI-PSD) can save 35% TAC while the bottom flash PSD can save 95% CO2 emissions compared with basic PSD process. You et al.29 adopted heat integration PSD and heat pump PSD in separating the azeotrope of diisopropyl ether and isopropyl alcohol and the results implied that heat pump PSD can save 31.7% lifetime total cost and 69.9% CO2 emissions in contrast with ordinary PSD process.
As reported in literature, in the production of aromatics from isobutanol, the main products are aromatic substances and C1-C4 substances, whose proportions vary with different reaction conditions such as catalysts30,31,32. The segregation of products in the manufacture of aromatics from isobutanol is of great importance for enhancing the production efficacy of the process, which has already captured the attention of some academics33,34. In this study, for the convenience of simulation calculations, p-xylene was used to represent the aromatic substances in the products, and isobutanol was used to represent the C1-C4 substances in the products. The separation of p-xylene and isobutanol mixture is employed as an exemplar to provide guidance and reference for the product separation process of producing aromatics from isobutanol. However, isobutanol and p-xylene can form a binary azeotrope, ordinary distillation method can hardly achieve effective separation. In accordance with the outcomes of literature research, there are only a few reports on the separation of isobutanol and p-xylene. Recently, Wang et al.33 screened three imidazole-based ionic liquids that can be employed to separate isobutanol and p-xylene mixtures through experimental research and in combination with the calculation results of the conductor-like screening model. Additionally, the separation mechanism was analyzed from a microscopic molecular perspective. Nevertheless, in this literature, the process flowchart for segregating isobutanol and p-xylene using ionic liquids was not devised and optimized adequately, and the incorporation of ionic liquids demands subsequent segregation and recovery, which may also bring certain environmental issues.
Fortunately, isobutanol and p-xylene azeotrope belongs to pressure sensitive azeotrope, and PSD can be applied to achieve the effective separation of isobutanol and p-xylene. Compared with other separation processes such as extractive distillation, azeotropic distillation, and reactive distillation, PSD does not require the introduction of new constituent, thus avoiding subsequent separation and environmental issues. At the same time, due to the diverse column pressures, heat integration can be accomplished between the reboilers and condensers of different columns.
To obtain high purity products of isobutanol and p-xylene from their mixture, conventional PSD process was designed and optimized within this study. Considering the requirement of process integration in separation industry, four different heat integration PSD processes including partial heat integration PSD (PHI-PSD), enhanced partial heat integration PSD (EPHI-PSD), FHI-PSD, and enhanced full heat integration PSD (EFHI-PSD) were also investigated and optimized. The optimal separation process was selected from the perspective of maximum thermodynamic efficiency, minimum TAC and CO2 emissions.
Methods
Determination of property method
Non-random two liquid (NRTL) model is a thermodynamic model which can be used to describe the interactions between molecules in the liquid mixture35,36,37. In this case, NRTL model was also used to elaborate the thermodynamic behavior of isobutanol and p-xylene because the regression results of the NRTL model are more consistent with the literature data38 and the built-in binary interactive parameters from NRTL model was listed in Table 1.
Cost evaluation
TAC, as an important indicator for estimating the economic efficiency of special distillation processes, mainly covers the capital cost and energy consumption cost of the process39,40,41. The cost of manufacturing columns and heat exchangers constitutes the capital cost of the process, and the costs of other devices such as pumps, valves and pipelines are relatively small, so it is generally ignored. The cost of cooling water consumed by the condensers and the cost of steam consumed by the reboilers constitute the energy consumption cost of the process. In light of the fact that the cost of the vacuum pump and the electricity cost consumed by the vacuum system constitute a minor proportion of the TAC42,43, the expenditure of the vacuum system was not considered in this design. The calculation formula of these costs can refer to the methods mentioned in the published articles44, as listed in Table 2. The payback period of capital investment is three years, and the operation time of the plant is set as 8000 h per year.
Calculation of CO2 emissions
With the development of the world economy, more and more people are paying attention to environmental issues, especially the emissions of greenhouse gas CO245. Nevertheless, it is quite difficult to accurately calculate the CO2 emissions of the process. In order to simplify the calculation, scholars proposed to calculate CO2 emissions by using the steam source required by the reboiler. In this design, it is assumed that the steam is obtained by a boiler heated with heavy fuel oil. According to the calculation method provided by Gadalla et al.6, the calculation formulas of CO2 emissions are shown as follows:
where \(\:{F}_{{CO}_{2}}\)is the CO2 emissions, kg/h; \(\:{Q}_{fuel}\:\)is the fuel combustion quantity, kW; \(\:{N}_{HV}\:\)represents the net calorific value of heavy fuel oil, which can be set as 39,771 J/g;\(\:\:{\omega\:}_{C\:}\)is the carbon content of heavy fuel oil, and can be set to 86.5%; α is 3.67, which means the ratio of relative molecular weight of CO2 to C; r and \(\:{h}_{s}\) are the latent heat and enthalpy of vaporization, respectively, J/g; T0 represents the ordinary temperature, assumed as 298.15 K; TF and TS represent the flame temperature and stack temperature respectively, usually specified as 2073.15 K and 433.15 K.
Calculation of thermodynamic efficiency
The thermodynamic efficiency (η) of distillation is the ratio of the minimum work requested for separation to the actual work needed. This efficiency reflects the effective conversion potency in the distillation process, that is, the proportion of the input energy converted into the effective portion of product dissociation. In this study, the thermodynamic efficiencies of various thermal integration processes were calculated by employing the calculation method put forward by Seader et al.46, as shown below:
It should be noted here that Wmin stands for the minimum work needed for the separation process, measured in kJ/h; LW represents the work loss in the separation process, measured in kJ/h; n denotes the molar flow rate of process streams, in kmol/s; h signifies the enthalpy of process streams, measured in kJ/kmol; s indicates the entropy of process streams, measured in kJ/kmol·K; Tam represents the ambient temperature, measured in K; Ex stands for the exergy use of the process, in kW; QR and TR represent the heat load and temperature of the reboilers, measured in kW and K respectively; QC and TC represent the heat load and temperature of the condensers, with units of kW and K, respectively; Ws stands for the shaft work, measured in kW.
Process design and optimization
The separation process of isobutanol and p-xylene by PSD was designed and simulated through Aspen Plus V10 software. This design employs an equilibrium-based model, and the computation of the equilibrium-based model is founded on the supposition of equilibrium stage, meaning that the vapor-liquid phase departing each theoretical stage thoroughly attains equilibrium. Numerous scholars have utilized equilibrium-based models in simulating and devising special distillation procedures for separating mixtures11,47. The equilibrium-based model can well represent the vapor-liquid phase dispersion and temperature dissemination inside the distillation column. Additionally, some published literature also imply that the optimized number of stages acquired is the theoretical number of stages48,49 (with a default column efficiency of 1). This work also utilizes theoretical stage numbers to calculate the TAC of different thermal integration PSD processes.
The feed rate of the PSD process is assumed to be 100 kmol/h. Due to the lack of large-scale industrial application of producing aromatics with isobutanol, it is difficult to accurately determine the composition of its industrial reaction products. In this design, the molar ratio of isobutanol and p-xylene in the feed is equal, both of which are 50 mol%, and this feed composition setting is relatively close to that reported in a recently published patent34. The feed temperature is set as 25 °C. The product purity of isobutanol and p-xylene is 99.9 mol%.
Basic PSD without heat integration
The basic PSD process for separation of binary azeotrope mainly includes two columns, which operate at different pressures. Generally speaking, by changing the pressure, the composition ratio of isobutanol and p-xylene in the azeotrope is changed, thereby achieving the separation of these two components. Meanwhile, considering the economic factors, the pressure setting of the high-pressure column should ensure that the low-pressure steam can be made use of to heat the reboiler, so as to avoid the high cost of using the high-pressure steam. In this design, the boiling point of p-xylene under 1 atm is about 410 K, while the low-pressure steam temperature is 433 K, and the temperature difference just satisfies the design conditions of heat exchange. Therefore, the pressure of high-pressure column is set as 1 atm. In addition, for industrial production, atmospheric pressure operation is easy to achieve and is relatively safe with better controllability, and many scholars set one of the column pressures in PSD as atmospheric pressure15. The pressure setting of the low-pressure column should ensure that ordinary cooling water can be used to remove the heat from the low-pressure column condenser, so as to avoid the use of expensive frozen water. Through the simulation, it is found that when the low-pressure column pressure is chosen as 0.1 atm or above, the condenser of the low-pressure column can select cheap cooling water to remove the heat. In Fig. 1, TAC values of PSD process under different pressures of low-pressure column are presented. It is observable from the figure that when the pressure of the low-pressure column exceeds 0.1 atm, the TAC of the process also ascends with the rising of pressure. If the pressure continues to decrease, the cooling medium needs to be frozen water, and TAC has restarted to increase. So the low-pressure column pressure is determined as 0.1 atm. The data shown in Fig. 2 indicates that the azeotropic composition varies 17.5 mol% when pressure varies from 1 atm to 0.1 atm, which means the pressure setting is appropriate.
Meanwhile, the economy of the PSD process can also be affected by the distillation sequence. Distillation sequence refers to the issue that the raw material should first enter the atmospheric pressure column or the low-pressure column. It has been found that the feed composition of 50 mol% isobutanol is much the same as the azeotropic composition at 0.1 atm, while the vapor-phase line and liquid-phase line are very close to each other in the range of azeotropic composition at 0.1 atm. Therefore, the feed flow rate with composition of 50 mol% isobutanol cannot be separated with the purity requirements when first entering the low-pressure column, and the TAC is high, so this situation has no value of detailed optimization.
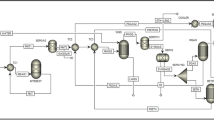
For the purpose of making the economy of PSD process more reasonable, the sequential iterative program was used to optimize the process so as to obtain the minimum TAC. The optimization procedure mainly includes seven variables, which are the theoretical stage number (NT1), reflux ratio (RR1) and feed location (NF1) of atmospheric pressure column, the theoretical stage number (NT2), reflux ratio (RR2) and feed location (NF2) of low-pressure column, and the feed location of recycling materials (NFR). The detailed optimization program adopts methods reported in literature50,51, as illustrated in Fig. 3. In simple terms, the initial values of NT1, NT2, RR1, and RR2 were given by a simple algorithm through preliminary simulation. The bottom flow rates of the two columns were altered to fulfill the design requirements of 99.9 mol% of product purity. Then, NT1 and NT2 were taken as the external circulation, NF1, NF2, NFR, RR1, and RR2 were taken as the internal circulation in the optimization procedures. The optimal results for the seven design variables are displayed in Fig. 4. By analyzing Fig. 4, it can be concluded that the PSD process owes the minimum TAC of 1.534 × 106 $/y when NT1=23, NT2=34, RR1 = 1.2, RR2 = 1.2, NF1=20, NF2=17, and NFR=21. Detailed information of optimization results for basic PSD process is shown in Fig. 5. CO2 emissions is calculated according to the formulas provided in above content and the result is 1658.97 kg/h. The calculated value of thermodynamic efficiency is 5.94%. Table 3 lists the TAC, CO2 emissions, thermodynamic efficiency, and other information about the basic PSD process.
PHI-PSD
For PSD process, cooling water is needed as cooling medium for overhead steam, while heating steam is needed for bottom reboiler. Therefore, it can be considered to use the top steam of the atmospheric pressure column as the heat source of the reboiler for the low-pressure column with the aim of realizing the process integration of PSD process and saving energy consumption and process investment. As shown in Fig. 5, the heat duty of overhead condenser for atmospheric column is 2708.8 kW with a temperature of 379.8 K, while the heat load of the bottom reboiler in low-pressure column is 1250.5 kW and the temperature is 329.6 K. Comparing the heat loads of these two heat exchangers, it can be seen that the heat that needs to be removed from the condenser is greater than the heat that needs to be provided in the reboiler, which indicates that auxiliary condenser needs to be installed for atmospheric pressure column, and the heat duty of auxiliary condenser is 1458.3 kW. The details of PHI-PSD are shown in Fig. 6. By using partial heat integration, the economic and environmental performance can be improved compared with basic PSD process with TAC of 1.242 × 106 $/y and CO2 emissions of 1222.19 kg/h. Moreover, the thermodynamic efficiency has risen by 35.0%.
EPHI-PSD
It can be seen from the above analysis of PHI-PSD that condenser heat load of atmospheric pressure column is much larger than that of low-pressure column reboiler, so additional cooling water is still needed for cooling. Meanwhile, the feed temperature of raw material is 298.15 K. After entering the atmospheric pressure column from the feed location, the raw material will be heated to the specified temperature. Also, the recycling material between the low-pressure column and atmospheric pressure column needs to be heated to the specified temperature because of its lower temperature. They both will increase the heat duty of the atmospheric pressure column reboiler and raise the consumption of heating stream, thus resulting in a decrease in the economic efficiency of the entire process and an increase in CO2 emissions. Therefore, from the viewpoint of fully utilizing energy, part overhead steam of atmospheric pressure column undergoes heat exchange with the feed material and recycling material to increase the temperature of the latter. Compared to ordinary PHI-PSD, two additional heat exchangers are required. Moreover, the feed condition of the atmospheric pressure column has been changed, so the reflux ratios for the two columns need to be optimized again, and Fig. 7 presents the optimization results. The information can be obtained in the figure that the optimal RR1 and RR2 for two columns are 0.70 and 1.20, respectively. Figure 8 shows the process flowsheet, material and equipment information of EPHI-PSD. The results show that the heat load of the reboiler situated at the base of the atmospheric pressure column is reduced by 32.3% compared with ordinary PHI-PSD process after adopting the EPHI-PSD process. The TAC of the whole process is reduced to 0.966 × 106 $/y and the CO2 emissions is reduced to 827.84 kg/h. The thermodynamic efficiency of EPHI-PSD is computed to be 11.75%.
FHI-PSD
Based on the analysis of the basic PSD process, another idea was put forward, that is, whether the heat load of the overhead condenser of atmospheric pressure column can be equal to that of low-pressure column reboiler by adjusting the reflux ratios of the two columns, so that the auxiliary condenser and extra cooling medium are not needed. Then, the “flowsheet design spec” function within Aspen Plus was exploited to attain FHI-PSD. When RR1 is adjusted, there is always a corresponding RR2 value. The RR1 and RR2 should be optimized again for FHI-PSD and the results are shown in Fig. 9. The optimal RR1 and RR2 for FHI-PSD are 0.56 and 1.8, respectively. Through full heat integration, TAC of PSD process can be decreased to 1.185 × 106 $/y and the CO2 emissions is decreased to 1060.72 kg/h, while the thermodynamic efficiency is found to be 9.21%. The specific information about FHI-PSD is displayed in Fig. 10.
EFHI-PSD
Through the analysis of PSD process, it can be found that the bottom flow of atmospheric pressure column is p-xylene, and its temperature is 411.1 K, so it needs to be cooled to obtain p-xylene product. Simultaneously, the overhead flow of atmospheric pressure column is a mixture of isobutanol and p-xylene, and its temperature is 379.8 K, which is higher than the feed location temperature of low-pressure column, so it is necessary to be cooled in low pressure column. If the heat of the two streams can be used to preheat the raw materials, the heat load of atmospheric pressure column reboiler can be decreased, thus reducing the energy cost. Therefore, based on the above analysis, the traditional FHI-PSD process is strengthened. Owing to the change in the feed temperature, the reflux ratios of the two columns needs to be optimized again, and Fig. 11 contains the optimization results. The optimal RR1 and RR2 of the two columns are 0.63 and 1.8, respectively. Figure 12 depicts the detailed process result regarding EFHI-PSD. The figure reveals that the reboiler heat load of the atmospheric pressure column is decreased to 2681.7 kW. TAC of the whole process is decreased to 1.096 × 106 $/y and the CO2 emissions is decreased to 936.63 kg/h. The thermodynamic efficiency of EFHI-PSD has increased to 10.53%.
Comparison of different PSD processes
Figure 13 reveals the temperature and liquid phase composition curves of atmospheric pressure column for different PSD processes. The temperature distribution curves of atmospheric pressure column for basic PSD and four heat integration PSD processes are very similar. In the range of 1–18 stages, the atmospheric pressure column temperature basically remains unchanged, and at this time, the content of isobutanol in the column is decreasing smoothly, while the content of p-xylene is increasing smoothly. In the range of 18–23 stages, the atmospheric pressure column temperature begins to increase slowly and forms a sharp increase trend in the later stage, and the contents of isobutanol and p-xylene are changing sharply. It can be seen from the composition cueves that the content of isobutanol for basic PSD (PHI-PSD) is the highest on the same stage before the feed location stage compared with EPHI-PSD, FHI-PSD, and EFHI-PSD. Because in basic PSD (PHI-PSD) process, the reflux flowrate is the largest, and the composition of isobutanol in the reflux liquid is about 80 mol%.
As shown in Fig. 14, regarding the low-pressure column, the temperature distribution curves of the two FHI-PSD processes coincide, and their liquid phase composition curves are also consistent. At the same stage location, the basic PSD (PHI-PSD) process has the highest temperature because its reflux flowrate is the smallest, and the liquid phase composition curve shows that the isobutanol content is also higher than that of other PSD processes. With the increase of the stage number, for the basic PSD (PHI-PSD) and EPHI PSD processes, the isobutanol content on the stage has experienced three procedures: rapid increase, slow growth, and rapid increase, until the specified purity of 99.9 mol% is reached. For two FHI-PSD processes, the isobutanol content on the stage has experienced four procedures: slow change, rapid increase, slow growth and rapid increase. The results demonstrate that the rise of heat duty and reflux flowrate of low-pressure column in two FHI-PSD processes has an obvious effect on the liquid phase composition distribution.
The detailed variables information about different PSD processes is listed in Table 3 to make a comparison. As shown in the table, in comparison with the basic PSD processes devoid of heat integration, TAC and CO2 emissions of the four heat integration PSD processes have been reduced to varying degrees, while the thermodynamic efficiency has risen to different extents. For the ordinary PHI-PSD process, the operation cost is decreased by 26.4% in contrast to the PSD process devoid of heat integration, while the capital cost is slightly increased. The main reason is that the temperature difference becomes smaller when the atmospheric pressure column top steam is used to heat the low-pressure column reboiler, triggering the growth of heat exchange area, which induces the rise of capital cost of heat exchanger. For the EPHI-PSD process, although the heat exchangers quantity is increased in contrast to the basic PSD process, the overall heat exchange area is reduced by 10.8%, so the overall capital cost of heat exchangers is declined. The overall column equipment cost also becomes lower, because the diameter of atmospheric pressured column is reduced. In contrast to the basic PSD process, TAC and CO2 emissions of the FHI-PSD process are decreased by 22.8% and 36.1%, respectively. Operating cost is decreased by 36.2%, while capital cost is increased by 12.5%. The rise of capital cost is primarily attributed to two reasons. First, the increase of condenser heat duty and reboiler heat duty of low -pressure column and the decrease of heat exchange temperature difference of reboiler result in the raise of total heat exchanger area by 9.40% after adopting full heat integration. Then, the diameter of the low-pressure column is increased by 40.2%, so the capital cost of the low-pressure column shell is also increased. TAC and CO2 emissions of the EFHI-PSD process are decreased by 28.6% and 43.5% respectively in contrast to those of the basic PSD process devoid of heat integration. Moreover, the percentage of increase in thermodynamic efficiency is 77.3%. For TAC, the operating cost is decreased by 43.6%, while the capital cost is increased by 10.6%. There are two reasons for the increase of capital cost. On the one hand, the total heat exchange area of heat exchanger has been increased by 3.87%, which is primarily attributed to the raise of heat load for low pressure column condenser and reboiler, the reduction of heat exchange temperature difference of reboiler, and the need of heat exchange area for feed material preheater. On the other hand, as a result of the expansion of low-pressure column diameter, the cost of the column shell is also increased by 14.6%. In general, TAC and CO2 emissions of the EPHI-PSD process are the lowest, which are decreased by 37.0% and 50.1% respectively in contrast to the basic PSD process, while the thermodynamic efficiency reaches the highest level, showing an increase of 97.8% compared with the basic process. The comparison results imply that EPHI-PSD process is an optimal choice for separating isobutanol and p-xylene azeotropes from both the perspectives of economy, thermodynamic efficiency, and environmental factors.
Conclusions
By analyzing t-xy phase diagram under different pressures, the viability of PSD was determined. In this work, the basic PSD and four different heat integration PSD processes including PHI-PSD, EPHI-PSD, FHI-PSD, and EFHI-PSD for separating azeotrope of isobutanol and p-xylene were designed and optimized by sequential iterative procedure. TAC, CO2 emissions, and thermodynamic efficiency of these processes were accurately calculated and comprehensively compared. The findings suggest that all the four heat integration PSD processes are able to reduce TAC and CO2 emissions compared with the basic PSD. Simultaneously, they are also capable of enhancing thermodynamic efficiency. The EPHI-PSD and EFHI-PSD processes have more advantages in economy, thermodynamic efficiency, and environmental protection than ordinary PHI-PSD and FHI-PSD. Although the capital cost of FHI-PSD and EFHI-PSD processes is increased in contrast to the basic PSD, the energy costs have obvious reduction, implying these two processes can have good cost savings in the long term. The EPHI-PSD process displays the optimum economy and environmental behavior with 37.0% TAC reduction, 50.1% CO2 emissions reduction, and 97.8% rise in thermodynamic efficiency.
However, we need to admit that our design has some limitations. Firstly, when designing these processes, we did not consider the actual tray efficiency, which is an important indicator for evaluating the separation capacity and performance of distillation column. Furthermore, for the purpose of making a more accurate estimation of process TAC, it is necessary to consider the expenditure of the vacuum system. In future studies, we will take these factors into account so as to make the process results more reasonable and closer to actual industry.
Data availability
All data generated or analysed during this study are included in this published article.
References
Tan, J. et al. Process intensification in reactive extraction by phase inversion in gas/liquid/liquid microdispersion system. Chem. Eng. Sci. 268, 118295 (2023).
Goyal, H., Chen, T., Chen, W. & Vlachos, D. G. A review of microwave-assisted process intensified multiphase reactors. Chem. Eng. J. 430, 133183 (2022).
Choubtashani, S. & Rashidi, H. CO2 capture process intensification of water-lean methyl diethanolamine-piperazine solvent: experiments and response surface modeling. Energy. 267, 126447 (2023).
Poonguzhali, E., Kapoor, A. & Prabhakar, S. Membrane assisted process intensification and optimization for removal and recovery of phenol from industrial effluents. Sep. Purif. Technol. 319, 124026 (2023).
Mahdi, T., Ahmad, A., Nasef, M. M. & Ripin, A. State-of-the-art technologies for separation of azeotropic mixtures. Sep. Purif. Rev. 44, 308–330 (2015).
Gadalla, M. A., Olujic, Z., Jansens, P. J., Jobson, M. & Smith, R. Reducing CO2 emissions and energy consumption of heat-integrated distillation systems. Environ. Sci. Technol. 39, 6860–6870 (2005).
Cui, Y., Zhang, Z., Shi, X., Guang, C. & Gao, J. Triple-column side-stream extractive distillation optimization via simulated annealing for the benzene/isopropanol/water separation. Sep. Purif. Technol. 236, 116303 (2020).
Salman, M., Javed, N., Liu, X. & He, M. Azeotrope separation of ethyl propionate and ethanol by extractive distillation and pressure swing distillation method. Sep. Purif. Technol. 311, 123361 (2023).
Jiao, Y. et al. Economic, environmental, energy and exergy analysis and multi-objective optimization for efficient purification of a friendly gasoline additive by extractive distillation coupled with pervaporation. Fuel. 335, 127069 (2023).
Shan, B. et al. Control of the azeotropic distillation process for separation of acetonitrile and water with and without heat integration. Chem. Eng. Process. -Process Intens. 165, 108451 (2021).
Wang, M., Song, E., Li, L., Zhang, Y. & Wang, E. Application of dividing wall column in azeotropic distillation with intermediate boiling-point heteroazeotrope: Simulation and optimization. Chem. Eng. Res. Des. 189, 384–400 (2023).
Li, X. et al. Investigation of energy-efficient heat pump assisted heterogeneous azeotropic distillation for separating of acetonitrile/ethyl acetate/n-hexane mixture. Chin. J. Chem. Eng. 55, 20–33 (2023).
Yin, K. et al. Mechanism analysis of liquid-liquid extraction separation for dimethyl carbonate and methanol by choline-based DESs as extractants. Fluid Phase Equilib. 566, 113701 (2023).
Cumplido, M. P., Cháfer, A., Guayazan-Jaimes, J. I., de la Torre, J. & Badia, J. Potential of phosphonium-based ionic liquids to purify 1-propanol from water by liquid–liquid extraction at mild conditions. J. Chem. Thermodyn. 165, 106588 (2022).
Liang, S. et al. Insight into pressure-swing distillation from azeotropic phenomenon to dynamic control. Chem. Eng. Res. Des. 117, 318–335 (2017).
Castro-Muñoz, R. et al. Towards azeotropic MeOH-MTBE separation using pervaporation chitosan-based deep eutectic solvent membranes. Sep. Purif. Technol. 281, 119979 (2022).
Banjerdteerakul, K., Peng, H. & Li, K. Covalent organic frameworks based membranes for separation of azeotropic solvent mixtures by pervaporation. J. Membr. Sci. 678, 121679 (2023).
Sun, H. et al. Facile fabrication of a continuous ZIF-67 membrane for efficient azeotropic organic solvent mixture separation. Angew Chem. 135, e202300262 (2023).
Wang, Y., Cui, P., Ma, Y. & Zhang, Z. Extractive distillation and pressure-swing distillation for THF/ethanol separation. J. Chem. Technol. Biotechnol. 90, 1463–1472 (2015).
Zhang, Q., Li, C., Zeng, A., Ma, Y. & Yuan, X. Dynamic control analysis of partially heat-integrated pressure-swing distillation for separating a maximum-boiling azeotrope. Sep. Purif. Technol. 230, 115853 (2020).
Yang, A. et al. Design and control of pressure-swing distillation for separating ternary systems with three binary minimum azeotropes. AIChE J. 65, 1281–1293 (2019).
Zhai, J., Chen, X., Sun, X. & Xie, H. Economically and thermodynamically efficient pressure-swing distillation with heat integration and heat pump techniques. Appl. Therm. Eng. 218, 119389 (2023).
Liu, C., Yang, D., Zhang, Q., Zhang, Q. & Cui, C. Control of fully heat-integrated pressure-swing distillation with strict pressure manipulation: a case study of separating a maximum-boiling azeotrope with small pressure-induced shift. Sep. Purif. Technol. 323, 124455 (2023).
Neto, G. F., Brito, K. & Brito, R. Improving control of fully heat-integrated pressure swing distillation through partially flooded reboiler/condenser. Sep. Purif. Technol. 322, 124334 (2023).
Cao, Y. et al. Comparison of pressure-swing distillation and extractive distillation with varied-diameter column in economics and dynamic control. J. Process. Control. 49, 9–25 (2017).
Zhang, Z. et al. Energy-saving investigation of pressure-swing distillation strengthening configurations for benzene/isobutanol binary azeotrope. Sep. Purif. Technol. 296, 121381 (2022).
Li, X. et al. Thermodynamic efficiency enhancement of pressure-swing distillation process via heat integration and heat pump technology. Appl. Therm. Eng. 154, 519–529 (2019).
Mao, W. et al. Heat integrated technology assisted pressure-swing distillation for the mixture of ethylene glycol and 1, 2-butanediol. Sep. Purif. Technol. 241, 116740 (2020).
You, X., Ma, T. & Qiu, T. Design and optimization of sustainable pressure swing distillation for minimum-boiling azeotrope separation. Ind. Eng. Chem. Res. 58, 21659–21670 (2019).
Dedov, A. G., Loktev, A. S., Karavaev, A. A. & Moiseev I. I. A novel direct catalytic production of p-xylene from isobutanol. Mendeleev Commun. 28, 352–353 (2018).
Yu, L. et al. Transformation of isobutyl alcohol to aromatics over zeolite-based catalysts. ACS Catal. 2, 1203–1210 (2012).
Mo, Y., Choi, Y., Choi, H. & Park, S. Aromatization of iso-butanol with CO2 as an enhancer over ZSM-5 catalysts. Res. Chem. Intermed. 43, 7223–7239 (2017).
Wang, Y. et al. Phase equilibrium of p-xylene and isobutanol in ionic liquid separations: thermodynamic and mechanistic analysis. ACS Sustainable Chem. Eng. 12, 3364–3377 (2024).
Zhu, Z. et al. Liquid liquid extraction process for separating binary azeotropic mixtures of p-xylene/isobutanol using imidazole ionic liquids. CN 117430478 A, 01.23 (2024).
Yang, A. et al. Investigation of energy-efficient and sustainable reactive/pressure-swing distillation processes to recover tetrahydrofuran and ethanol from the industrial effluent. Sep. Purif. Technol. 250, 117210 (2020).
Guo, J. et al. Vapor-liquid equilibrium experiment and extractive distillation process design for the azeotrope ethyl propionate n-propanol using ionic liquid. J. Mol. Liq. 350, 118492 (2022).
Del-Mazo-Alvarado, O. & Bonilla-Petriciolet, A. Assessment of the simultaneous regression of liquid-liquid and vapor-liquid equilibria data of binary systems using NRTL and artificial neural networks. Fluid Phase Equilib. 561, 113537 (2022).
Lores, M., De la Torre, J., Burguet, M. & Monton, J. Vapor – liquid equilibria for the binary systems isobutanol with m-xylene, o-xylene and p-xylene at 101.3 kPa. J. Chem. Eng. Data. 44, 869–872 (1999).
Qin, Y. et al. Multi-objective optimization and comparison of the entrainer-assisted pressure-swing distillation and extractive distillation separation sequences for separating a pressure-insensitive binary azeotrope. Comput. Chem. Eng. 165, 107959 (2022).
Han, D., Chen, Y. & Dong, M. Process evaluation for the separation of acetonitrile-water using extractive distillation with deep eutectic solvents as entrainers. Comput. Chem. Eng. 164, 107865 (2022).
Akinciturk, T. & Kaymak, D. B. Design and control of an energy-efficient triple-column pressure swing distillation configuration for separation of acetone-methanol-hexane mixture. Comput. Chem. Eng. 160, 107731 (2022).
Chen, Y., Yu, B., Hsu, C. & Chien, I. Comparison of heteroazeotropic and extractive distillation for the dehydration of propylene glycol methyl ether. Chem. Eng. Res. Des. 111, 184–195 (2016).
You, X., Rodriguez-Donis, I. & Gerbaud, V. Low pressure design for reducing energy cost of extractive distillation for separating diisopropyl ether and isopropyl alcohol. Chem. Eng. Res. Des. 109, 540–552 (2016).
Douglas, M. Conceptual Design of Chemical Processes (McGrawHill, 1988).
Leng, J., Fan, S., Lu, C., Feng, Z. & Dong, L. Sustainable design and multi-objective optimization of heat pump assisted extractive distillation process for separating a ternary mixture of methyl acetate, tetrahydrofuran and methanol. J. Clean. Prod. 419, 138186 (2023).
Seader, J., Henley, E. & Roper, D. Separation Process Principles: Chemical and Biochemical Operations (John Wiley & Sons Inc, 2011).
Wang, M., Li, L. & Wang, E. Purification of methyl isobutyl ketone via dividing wall column with an internal boiling point of a heterogeneous azeotrope. Chem. Eng. Process. Process Intens. 179, 109053 (2022).
Yi, X., Kong, J., Song, Y., Wang, J. & Sun, L. Isobaric vapor–liquid equilibria and extractive distillation process design for separating ethanol and diethoxymethane. J. Chem. Eng. Data. 66, 4326–4334 (2021).
Duan, C. & Li, C. Novel energy-saving methods to improve the three-column extractive distillation process for separating ethyl acetate and ethanol using furfural. Sep. Purif. Technol. 272, 118887 (2021).
Wang, Y., Cui, P. & Zhang, Z. Heat-integrated pressure-swing-distillation process for separation of tetrahydrofuran/methanol with different feed compositions. Ind. Eng. Chem. Res. 53, 7186–7194 (2014).
Zhu, Z., Wang, L., Ma, Y., Wang, W. & Wang, Y. Separating an azeotropic mixture of toluene and ethanol via heat integration pressure swing distillation. Comput. Chem. Eng. 76, 137–149 (2015).
Acknowledgements
Support for the research of this work from the Science and Technology Research Program of Henan Province (Project 242102320206) is gratefully acknowledged.
Funding
The work was supported by the Science and Technology Research Program of Henan Province (Project 242102320206).
Author information
Authors and Affiliations
Contributions
Liang S.S.: Project administration, Investigation, Conceptualization, Visualization, Validation, Writing - original draft. Bu G.L.: Software, Supervision, Writing - review & editing.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, S., Bu, G. Comparison of different heat integration pressure swing distillation processes for separating isobutanol and p-xylene azeotrope. Sci Rep 14, 28472 (2024). https://doi.org/10.1038/s41598-024-79870-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79870-6
Keywords
This article is cited by
-
Integrated Assessment of Controllability and Exergy Efficiency in Extractive and Pressure Swing Distillation
Process Integration and Optimization for Sustainability (2025)