Abstract
Globally, the age when children start using smartphones has decreased. Concurrently, the increased use of smartphones among children in developmental stages has caused serious effects, such as depression. While neuroimaging studies have predicted a significant overlap between the neurobiological changes caused by depression and smartphone overuse, few have simultaneously examined them. Therefore, we examined resting-state functional connectivity (FC) changes due to smartphone overuse and depressive symptoms in 69 children. We observed that FC in the salience network and regions involved in visual (e.g., the lateral occipital cortex) and motivational processing (e.g., the putamen) increased with smartphone overuse and depressive symptoms. Additionally, FC partially mediated the relationship between depressive symptoms and smartphone overuse, suggesting that changes in FC may be involved in the link between depressive symptoms and smartphone overuse. Our findings indicate that increased depressive symptoms could be associated with alterations in the salience network FC, which may influence visual attention or reward processing of salient stimuli, potentially contributing to smartphone overuse.
Similar content being viewed by others
Introduction
Globally, the percentage of smartphone users has increased steadily over the years. Smartphones offer several advantages such as easy access to information, improved productivity, and remote communication. Recent years has seen a noticeable trend of smartphone usage among children of progressively younger ages. Approximately 45% of Korean children are first exposed to smartphones between the ages of 12 and 24 months1, and 87% of elementary school students currently own smartphones2.
This surge in smartphone use has also raised concerns about overuse, which is often referred to as smartphone addiction or problematic smartphone use. Smartphone overuse causes tolerance, salience, and cravings similar to other substance- or behavior-related addictions3,4. In addition, it has negative consequences including physical health problems5, sleep interference6, emotional problems such as depression and anxiety7,8,9, and loss of control10. Depression has been identified as a potent predictor of increased smartphone use11. The more the depression, the more the reliance on smartphones. Smartphone use is suggested to be a maladaptive coping process for depression. These coping processes include repetitive negative thoughts, boredom proneness. For example, people may overuse their smartphones to dampen feelings of isolation and negative emotions, or as a means of relieving boredom12,13. Depression is also associated with a fear of missing out, which can be conceptualized as the result of an unmet need for social connection. It has been suggested that depressed people rely on their smartphones to alleviate the lack of social connection13,14. This, in turn, can exacerbate depression by increasing the amount of time spent alone and fostering feelings of loneliness15.
This issue is especially alarming for children in developmental stages as it increases their vulnerability to smartphone usage16. This is because children in developmental stages are more susceptible to addiction as their self-regulation ability is still immature. During this time, synapses are actively created and removed, and the brain is sensitized by new experiences. The breadth and quality of experiences during this period significantly determine a child’s development, and smartphone overuse can stunt development in many areas, including brain17,18. In fact, children and adolescents are more vulnerable to smartphone addiction than adults19.
To address the adverse effects of smartphone overuse, research on neurobiological changes associated with it has recently gained momentum. Neuroimaging techniques, particularly functional magnetic resonance imaging (fMRI), are useful in investigating how smartphone overuse affects brain development in children. They help quantify the connection between spatially-separated but functionally-related brain regions, providing insight into the underlying mechanisms of smartphone overuse19,20.
A growing body of research has consistently reported that smartphone overuse is associated with alterations in functional connectivity (FC) in the brain19,20,21,22,23. The salience network (SN) is of particular interest in this context. It comprises the anterior insula (AI), anterior cingulate cortex (ACC), rostral prefrontal cortex (RPFC), and supramarginal gyrus (SMG) and has two main functions—detecting internal or external salient events and facilitating and switching attentional resources toward salient stimuli. In other words, it is a moderator of the brain, evaluating the relevance of internal and external stimuli and engaging in a dynamic switch between the default mode network (DMN), which is associated with internally-oriented and self-referential functions, and the central executive network (CEN), which focuses on external goals or tasks24.
Dysfunction of the SN is associated with several adverse effects. As the SN directs more attention to salient stimuli and prioritizes them, its dysfunction can lead to addiction. Several reports have shown that adults exhibiting problematic use of smartphones have altered FC in the ACC and AI, which are considered key hubs of the SN, compared with controls21,22,23. For instance, the ACC showed increased FC in several regions, including the posterior cingulate cortex, precuneus, lateral prefrontal, and orbitofrontal, with a similar pattern in the FC of the AI21. Moreover, the SN exhibits increased connectivity with the DMN and decreased connectivity with the CEN22,23. This reflects the dysfunction of the SN in shifting internally-oriented attention to task-related externally-oriented attention. Importantly, this dysfunction considerably overlaps with depression-related changes25,26,27. Hyperactivity of the SN makes it difficult to divert attention from negative stimuli and focus on internally generated negative emotions, which is potentially related to attentional bias in depression28,29.
Neuroimaging studies have increasingly showed a substantial overlap between the neurobiological changes induced by smartphone overuse and those associated with depression. Identifying changes in specific SN-based FC affected by depression and smartphone overuse during childhood could provide valuable insights for designing interventions to prevent both problems. However, to the best of our knowledge, few studies have simultaneously examined the effects of depression and smartphone overuse on the SN. Moreover, considerable gaps remain in the understanding of the psychological underpinnings and neural mechanisms associated with normal or mildly-elevated smartphone use and depressive symptoms. Furthermore, as children are sensitive to external stimuli and undergo rapid developmental changes, depressive symptoms and smartphone use, even at subclinical levels, are more likely to interact and progress quickly to pathological levels. In light of these considerations, this study aimed to investigate the psychological and neural correlates of smartphone overuse and depressive symptoms in the context of children’s mental health. Specifically, we examined shared alterations in FC centered on the SN for smartphone overuse and depressive symptoms in children.
Results
Demographic data and behavioral results
Participants’ demographic and questionnaire data are presented in Table 1. The CDI-2: SR score was converted to a T-score, aligning with a mean of 50 and a standard deviation of 10, factoring in variables such as the raw score, as well as the child’s sex and age. Raw scores were directly employed for the three smartphone use scales.
We observed positive correlations between the scores of the three scales (i.e., S, SO, and SM) and the CDI-2: SR (i.e., total score, emotional problems, negative mood, negative self-esteem, functional problems, ineffectiveness, and interpersonal problems) (Table 2).
Relationship between the salience network and smartphone overuse
We investigated changes in FC related to smartphone overuse and CDI-2: SR subscale scores while adjusting for age, sex, handedness, and number of siblings. Higher scores on each scale correlated with elevated SN-based FC. Specifically, increased scores on the S-scale showed positive associations with FC between the ACC and right postcentral gyrus (PostCG); between the right AI and left inferior lateral occipital cortex (LOC), right lingual gyrus (LG), PostCG, and cuneus (CUN); and between the left RPFC and right PostCG. Furthermore, as the SO-scale scores increased, connectivity increased with both seeds (i.e., the ACC and right AI) and the right angular gyrus (AG). Regarding the SM-scale, FC in the ACC was significantly associated with the right CUN, inferior LOC, superior LOC, and precuneus, while FC in the left SMG was related to the left putamen.
Relationship between the salience network and depressive symptoms
Elevated levels of depressive symptoms positively correlated with SN-centered FC. Higher total CDI-2: SR scores were associated with increased connectivity between the ACC and right superior LOC. Similarly, the right AI showed elevated FC with the bilateral superior LOC, right inferior LOC, and left RPFC with the right frontal pole. A significant association between the left SMG and left putamen was observed. In addition, children’s emotional problems due to depressive symptoms were positively linked with the connectivity between the right AI, left superior LOC, and superior parietal lobule. Finally, with the increase in functional problems, FC from the left AI to the left putamen and that from the right AI to the left superior LOC and left temporal occipital fusiform cortex increased. Table 3; Fig. 1 show the FC that reached FDR-corrected statistical significance for each of the effects of smartphone overuse and depressive symptoms.
(A) Smartphone overuse effects, showing increased FC with the increase in smartphone overuse scores. (B) Depressive symptoms effects, showing increased FC with the increase in depressive scores. Pink nodes represent seeds (i.e., ACC and bilateral AI, RPFC, SMG), while light blue nodes represent regions exhibiting heightened FC corresponding to the seeds. The effects of smartphone overuse and depressive symptoms are shown in green and purple, respectively. S-scale: smartphone addiction scale, SO-scale: smartphone overdependence scale, SM-scale: smart media addictive tendency scale, R: right hemisphere, L: left hemisphere, ACC: anterior cingulate cortex, AI: anterior insula, RPFC: rostral prefrontal cortex, SMG: supramarginal gyrus, PostCG: postcentral gyrus, iLOC: inferior lateral occipital cortex, sLOC: superior lateral occipital cortex, LG: lingual gyrus, CUN: cuneus, AG: angular gyrus, PUT: putamen, FP: frontal pole, TOFC: temporal occipital fusiform cortex, CA: caudate nucleus, MidFG: middle frontal gyrus.
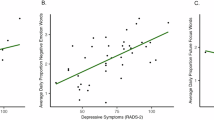
In general, higher smartphone overuse and depressive symptom scores correlated with heightened FC between the SN and various occipital regions, particularly the LOC. More importantly, the FC between the ACC and right superior LOC partially overlapped for the SM-scale and the CDI-2: SR total score. Likewise, the left RPFC–left putamen FC exhibited shared patterns with the SM-scale and the CDI-2: SR total and functional problems scores (Fig. 2).
(A) The FC shared by both smartphone overuse (SM-scale) and depressive symptoms (CDI-2: SR total score). The FC between anterior cingulate cortex and right superior lateral occipital cortex demonstrates a positive correlation with both (B) smartphone overuse and (C) depressive symptoms. Similarly, the FC between the left supramarginal gyrus and left putamen is positively associated with both (D) smartphone overuse and (E) depressive symptoms.
Mediation effects between FC, smartphone overuse, and depressive symptoms
The variables in the mediation analysis were determined based on the FC results. Specifically, the SM-scale and the total CDI-2: SR scores were selected because they showed a common increase in FC between the ACC and the right superior LOC (Fig. 2). Considering depressive symptom scores as causal variables, FC partially mediated the relationship between depressive symptoms and smartphone overuse (Fig. 3A; path a = .4487, p = .0001; path b = .2288, p = .0254; path c’ = .5474, p = .0000; path c = .6501, p = .0000). This suggests that an increase in depressive symptoms may alter ACC-right superior LOC FC, subsequently influencing smartphone overuse. In contrast, when smartphone overuse was the causal variable, the indirect pathway through which FC mediated the relationship between smartphone overuse and depressive symptoms was not significant (Fig. 3B; path a = .4991, p = .0000; path b = .1704, p = .1192; path c’ = .6119, p = .0000; path c = .6969, p = .0000).
Mediation results. (A) and (B) show the mediating effect of FC between ACC and right superior LOC, and (C) and (D) show the mediating effect of FC between left SMG and left putamen. In (A) and (C), depressive symptoms (CDI-2: SR total score) is the causal variable (X) and smartphone overuse (SM-scale) is the outcome variable (Y); the opposite is shown in (B) and (D). FC was included as a mediator (M) in all. Path a: X-M relationship; path b: M-Y relationship after adjusting for X; path ab: indirect effect of X on Y through M; path c’: direct effect of the X-Y relationship controlling for M; and path c: combined total direct and indirect effect. ACC: anterior cingulate cortex, R.sLOC: right superior lateral occipital cortex, L.SMG: left supramarginal gyrus, L.PUT: left putamen. *** p < .001, ** p < .01, * p < .05.
In contrast, for the left SMG, left putamen, and FC mediator, the indirect effects were significant when depressive symptom score was the causal variable and smartphone overuse score was the outcome variable (Fig. 3C; path a = .5472, p = .0000; path b = .3080, p = .0057; path c’ = .4815, p = .0000; path c = .6501, p = .0000) and vice versa (Fig. 3D; path a = .5890, p = .0000; path b = .2178, p = .0221; path c’ = .5368, p = .0000; path c = .6969, p = .0000). In Supplementary Table 1, we present the results of the mediation analysis for all possible combinations.
Discussion
The global increase in smartphone use has raised serious concerns, especially for children, as it can have devastating impacts on their brain development. This study has explored the functional alterations related to both depressive symptoms and smartphone overuse in children. Our results revealed that higher smartphone overuse and depressive symptom scores were associated with increased FC centered on the SN.
First, regions associated with attention and visual (e.g., LG, CUN, LOC, and AG), somatosensory (e.g., PostCG), and motivation (e.g., putamen) systems have been linked to the SN in smartphone overuse. These FC alterations potentially reflect an attentional bias characterized by increased sensory processing of salient stimuli. In a previous study, participants who were overweight/obese showed increased FC between the SN and visual regions compared with those who were lean. This suggests an elevated awareness of food in the fasting state, which is characterized by an extensive visual processing of salient stimuli30. Similarly, adolescents with internet gaming disorder (IGD) showed hyperconnectivity between the SN and regions involved in rapid motor responses to audiovisual stimuli (e.g., auditory and motor cortices) compared to those without it31. In contrast, underconnectivity between the SN and visual regions in the resting state has been observed in children with autism spectrum disorder, potentially suggesting a lack of selection of salient sensory information32.
Similarly, in this study, increased depressive symptoms were associated with heightened connectivity between the SN and visual regions (e.g., LOC and fusiform gyrus). In a previous research, LOC was a hub area separating adolescents with major depressive disorder from controls33. Depressive symptoms affect visual processing by causing unproductive top-down recruitment of attentional resources33,34. Furthermore, in our study, depressive symptoms were associated with regions that are important in emotional and cognitive regulation (e.g., the frontal pole and middle temporal gyrus), reward, and behavioral habit formation (e.g., the putamen and caudate). Several studies have suggested that functional abnormalities in these regions in individuals with depression may reflect increased sensitivity to and difficulty in disengaging from negative stimuli35,36,37,38.
In this study, heightened FCs between the ACC and right superior LOC as well as between the left SMG and left putamen were associated with increased scores for both smartphone overuse and depressive symptoms. Furthermore, we examined the mediating effects of these two types of FCs on depressive symptoms and smartphone overuse. First, FC in the ACC-right superior LOC partially mediated the relationship between depressive symptoms and smartphone overuse, suggesting that depressive symptoms may alter it, which, in turn, may cause smartphone overuse. It is plausible that the altered FC between the SN and visual processing regions in the current study reflects a modified attentional bias toward visual processing.
Second, depressive symptoms and smartphone overuse resulted in shared connectivity in the SN and left putamen. The mediation effect was significant in both pathways, from depressive symptoms to smartphone use, and vice versa. The putamen plays a pivotal role in updating reward-based values and selection behaviors, thereby facilitating responses to salient stimuli37,38.
The SN is connected to subcortical nodes in the striatal and limbic areas and its activation is accompanied by the subcortical activation of the cortico-striato-thalamic loop. In particular, the connections of the SN to the dorsal striatum, including the putamen and caudate nucleus, enable behavioral modulation in response to salient reward stimuli39. Imbalances in these loops can impair self-regulation of cognition, emotion, and behavior40. In one study, participants who were obese had greater FC between the SN and putamen than controls. Abnormal activation of the SN may contribute to overeating and obesity due to an imbalance in reward processing of salient stimuli (e.g., food)38. The putamen has recently been highlighted as a potential node for depression41. Increased connectivity between the SMG and the putamen is also associated with depression-induced anhedonia42. Compromise of the cortico-striatal circuits is well-studied in both affective and addictive disorders40,43.
Taken together, we explored the association between FC, depressive symptoms, and smartphone overuse in children, and found that increased depressive symptoms may be related to changes in SN-based FC. These changes can affect their visual attention or reward processing for salient stimuli, leading them to pay attention to negative or salient stimuli. In this study, we found that these changes could be linked to smartphone overuse. Depressed individuals rely on smartphones to alleviate fear of missing out13,14. In particular, early adolescents who habitually checked social media showed longitudinal increases in neural activation in salience and motivation networks44. Children are sensitive to and undergo changes in internal and external stimuli as they develop, suggesting that continued exposure to these rewarding stimuli may reinforce neural responses to these stimuli and functional abnormalities in the long term44. Therefore, future work could investigate changes in FC associated with maladaptive coping processes (e.g., fear of missing out) and cognitive-behavioral interventions aimed at addressing depression and smartphone overuse. This could include examining maladaptive coping processes and exploring the resulting neurodevelopmental changes.
On the one hand, changes in resting-state FC may serve as a bridge between metabolic changes in the brain and observable behavior45,46. What metabolic changes might be candidates to underlie the interaction between depression, smartphone overuse, and FC changes? For example, neural system imbalances between GABA, the major inhibitory neurotransmitter, and glutamate, the major excitatory neurotransmitter, may underlie FC abnormalities, which can lead to behavioral changes. GABA and glutamate have been implicated in both depression and addiction. Patients with depression often exhibit reduced GABA levels in the ACC and occipital regions47,48, whereas decreased glutamate levels in the ACC49,50 but elevated levels in the occipital region51. Similarly, addiction is associated with lower GABA and glutamate levels, especially in the occipital cortex and ACC. It is associated with high anxiety, impulsivity, cravings, impaired visual and attentional function and poor neuropsychological function45,52.
In contrast, one study measured GABA and glutamate in the brains of adolescents with smartphone/internet-addiction and those without it and found that those with the addiction had a higher level of GABA in the ACC. Cognitive behavioral therapy to reduce smartphone use normalizes neurotransmitter secretion levels in these individuals53. Thus, a neurochemical imbalance of GABA/glutamate may underlie FC abnormalities, which may contribute to depression and addiction, but the direction of this imbalance (i.e., high or low) remains unclear.
Other contributing factors include abnormalities in the serotonin system, a neurotransmitter that plays a key role in mood regulation, sleep, and impulse control. Serotonin deficiency can lead to increased depression and decreased impulse control, which may cause addiction. Numerous studies have reported that dysregulation of the serotonergic system may underlie mood disorders, leading to addiction54,55. In addition, a study using a mouse model confirmed that the SN is affected by depression and a combination of optogenetics and fMRI confirmed that serotonin is involved in the SN56. Future studies may need to jointly investigate the impact of abnormalities in these metabolic systems on depression, smartphone overuse, and FC changes.
Importantly, frequent comorbidities suggest that underlying neural mechanisms may overlap57. Further studies simultaneously examining depression and addiction are needed to understand the bidirectional relationship between the two. The vicious cycle of smartphone use and depression can impair not only the relationship between the SN and visual regions but also the functioning of other regions, such as the prefrontal cortex, which is responsible for attention, problem solving, and emotion regulation, later in life.
This study has some limitations. First, although we employed a mediation analysis, the cross-sectional nature of our study could not confirm the temporal sequence of our findings. Longitudinal studies tracking child brain development are needed to more precisely elucidate the causal relationships between depression, smartphone use, and FC alterations. Second, depressive symptoms and smartphone use scales were self-reported, which may have biased our results. Third, our study had a relatively small sample size, which made it challenging to differentiate between groups. This was particularly pronounced in distinguishing between individuals with normal and mild symptoms due to the lack of clear classification criteria. Finally, children’s smartphone usage is often supervised or managed by their caregivers, highlighting the importance of considering the influence of other factors, such as the quality of caregiver-child relationships, on the observed outcomes.
Methods
Participants
In total, 74 elementary school-aged children participated in the study. The exclusion criteria were as follows: (1) history of significant physical or neurological illnesses; (2) history of psychiatric diagnoses, such as major depression; (3) history of head trauma; (4) history of issues with medication and drug abuse; and (5) intelligence quotient scores below 70 (measured using the Korean Wechsler Intelligence Scale for Children-Fifth Edition). Additionally, five participants were excluded due to excessive head motion during the fMRI scanning, and finally, 69 were included (39 boys, 30 girls, mean age: 10.8 ± 0.8 years, range: 10–12 years). The Institutional Board of the Korea Brain Research Institute approved this study (KBRI-202103-HR-002) and conducted in accordance with the ethical standards of the Declaration of Helsinki. Informed consents were obtained for all participants and the children’s consents included the approval of their legal representative (parent and/or legal guardian).
Smartphone overuse
We measured children’s smartphone overuse using three scales: (1) the Smartphone Addiction scale (S-scale), (2) the Smartphone Overdependence scale (SO-scale), and (3) the Smart Media Addictive Tendency scale (SM-scale). The S-scale is a 15-item self-report measure that requires respondents to evaluate their smartphone-related behaviors58. The SO-scale consists of 10 items that assess self-control failure, salience, and the serious consequences of smartphone overuse59. Finally, the SM-scale consists of 15 items and assesses the problems arising from the excessive use of smart media60. All three scales are rated on a 4-point Likert scale (disagree completely = 1, agree completely = 4), with a higher total score indicating a higher smartphone addiction tendency. In our sample, Cronbach’s alpha coefficients for the S-, SO-, and SM-scales were 0.844, 0.909, and 0.893, respectively.
Depressive symptoms
We used the Korean Children’s Depression Inventory, Second Edition-Self-Report (CDI-2: SR)61,62 to assess depressive symptoms. It is a 28-item self-reported standardized scale to assess children’s emotions and behaviors.
The scale comprises two subscales: emotional and functional problems. The emotional problems subscale is divided into two components: (1) negative mood/physical symptoms and (2) negative self-esteem. Children experiencing negative moods/physical symptoms may exhibit sadness or irritability and physical symptoms, such as sleep disturbances, changes in appetite, and fatigue. Children with high levels of negative self-esteem may experience self-loathing and feelings of unloved. The functional problems subscale encompasses two aspects: (1) ineffectiveness and (2) interpersonal problems. Ineffectiveness refers to a child’s perception of their own competence in various activities, including schoolwork. Children experiencing interpersonal problems may struggle with peer interactions.
The children were presented with three statements for each item and asked to choose the one that best represented their experiences during the preceding two weeks. Higher scores indicated a greater intensity of depressive symptoms. The Cronbach’s alpha coefficient for the CDI-2: SR was 0.815 in our study.
Image acquisition and image processing
All MRI data were acquired using a 3T MR scanner (SIEMENS MAGNETOM Skyra, Erlangen, Germany) equipped with a 20-channel head coil at the K-MEDI Hub. A high-resolution 3D T1-weighted image was obtained with the following parameters: echo time (TE) = 2.3 ms; repetition time (TR) = 2400 ms; acquisition time (TA) = 6:28; flip angle = 8°; field of view (FOV) = 230 mm (x 0.906); 256 slices; and voxel size = 0.7 × 0.7 × 0.7 mm. The resting-state fMRI data were obtained with the following parameters: slice-thickness = 3.0 mm; TE = 28 ms; TR = 2000 ms; TA = 6:06; flip angle = 64°; FOV = 192; 180 measurements; 36 slices with full brain coverage; and voxel size = 3.0 × 3.0 × 3.0 mm. Participants were instructed to keep their eyes closed and remain at rest during the scanning, while ensuring that they did not fall asleep.
The fMRI data were preprocessed and analyzed using the Statistical Parametric Mapping (SPM) software (The Wellcome Centre for Human Neuroimaging, Department of Imaging Neuroscience, Institute of Neurology, University College London, UK) and CONN toolbox (https://www.nitrc.org/projects/conn) on MATLAB R2021a (MathWorks, Natick, MA). The default analysis pipeline within the CONN toolbox was used to preprocess the fMRI data. Preprocessing steps comprised the following: (1) exclusion of the initial five volumes to mitigate magnetic field inhomogeneity effects during the initial scan, (2) realignment to the mean image, (3) correction for slice timing, (4) removal of volumes based on ART criteria, specifically mean intensity > 5 standard deviations of the mean global signal or framewise displacement > 0.9 mm/repetition rate to address head movement effects, (5) application of anatomical component-based noise correction (aCompCor) to diminish physiological and other noise artifacts, (6) structural segmentation and normalization, (7) Gaussian smoothing with a 6 mm full-width at half-maximum, and (8) band-pass filtering of functional images (0.008–0.09 Hz).
Seed-based functional connectivity analysis
FC analysis, with the SN as the seed, was performed using the CONN toolbox. Seed-based analysis evaluates the temporal correlation between the blood-oxygen-level-dependent (BOLD) signal from a selected seed region and signals from all other voxels in the brain. In this study, the correlation coefficients were transformed into Z-scores using Fisher’s transformation to allow for a second-level analysis. It ensured that the data were normally distributed and suitable for statistical comparison. Initially, individual participant data were analyzed to obtain the first level seed-to-voxel connectivity maps. The CONN toolbox provides hierarchical cluster analysis-independent component analysis (HCA-ICA) network seeds originating from the ICA analysis of the Human Connectome Project (HCP) dataset63. Consequently, seven seeds corresponding to the SN were included: the ACC, right and left AI, RPFC, and SMG.
To investigate whether FC is altered by smartphone overuse and depressive symptoms, we regressed the main effects of three smartphone use scores (i.e., S-, SO-, and SM-scale) and CDI-2: SR total and subscale scores (i.e., emotional and functional problems) on FC. We fitted separate regression models for each scale, controlling for age, sex, handedness, and the number of siblings. The inclusion of the number of siblings as a control variable was informed by previous research indicating that individuals without siblings tend to experience higher feelings of loneliness, greater smartphone use for communication, and increased levels of depression64. Brain map threshold was p < .001 voxel-wise and corrected for multiple comparisons using a cluster-wise threshold of p-FDR corrected < 0.05.
Statistical analysis
Statistical analyses were conducted using SPSS 21 software (IBM-SPSS Inc, Chicago, IL, USA). A partial correlation analysis was conducted, controlling for age, sex, handedness, and number of siblings, to explore the association between smartphone overuse and depressive symptom scores.
To explore the specific relationship between smartphone overuse, depressive symptoms, and FC, we analyzed mediation effects using Model 4 of the PROCESS software65. The model uses 5000 bootstrapping resamples and assumes a significant mediating effect if the 95% confidence interval (CI) of the indirect effect does not include zero. In this mediation model, “path a” signifies the relationship between the independent variable (X) and the mediator variable (M), “path b” characterizes the connection between M and the dependent variable (Y), and the “indirect effect” is computed by multiplying path a and path b (indirect effect = ab). If the CI of the indirect path does not include zero, the result is considered statistically significant. “Path c’” represents the direct effect of X on the proposed M. Meanwhile, “path c” represents the total effect of the X-Y relationship without considering M (path c = indirect effect + direct effect). In the mediation analysis, the following control variables were considered: age, sex, handedness, and number of siblings.
Data availability
Data are available upon reasonable request to corresponding author (minyoung@kbri.re.kr).
References
Oh, J. & Park, Y. W. A study on pre-schoolers’ Smart Media Use and Parents’ perception. Korea Inst. Child. Care Educ. 13, 3–26 (2019).
Bae, S. R., Lee, C. & Lee, J. R. A Study on Youth’s Media Usage and Policy Measures Aimed at Different Target Groups I: Elementary School Students. (2020). https://kiss.kstudy.com/Detail/Ar?key=4050366
Kuss, D. J. & Griffiths, M. D. Social Networking Sites and Addiction: ten lessons learned. Int. J. Environ. Res. Public. Health 2017. 14, 311 (2017).
Van Rooij, A. J. & Prause, N. A critical review of internet addiction criteria with suggestions for the future. J. Behav. Addict. 3, 203–213 (2014).
V, B. M. K. & Walarine, M. T. Neck pain among smartphone users: an imminent public health issue during the pandemic time. J. Ideas Health. 3, 201–204 (2020).
Xie, X., Dong, Y. & Wang, J. Sleep quality as a mediator of problematic smartphone use and clinical health symptoms. J. Behav. Addict. 7, 466–472 (2018).
Alhassan, A. A. et al. The relationship between addiction to smartphone usage and depression among adults: a cross sectional study. BMC Psychiatry. 18, 1–8 (2018).
Elhai, J. D., Dvorak, R. D., Levine, J. C. & Hall, B. J. Problematic smartphone use: a conceptual overview and systematic review of relations with anxiety and depression psychopathology. J. Affect. Disord. 207, 251–259 (2017).
Gao, Y., Li, A., Zhu, T., Liu, X. & Liu, X. How smartphone usage correlates with social anxiety and loneliness. PeerJ e2197 (2016). (2016).
Chen, J., Liang, Y., Mai, C., Zhong, X. & Qu, C. General deficit in inhibitory control of excessive smartphone users: evidence from an event-related potential study. Front. Psychol. 7, 182702 (2016).
Lee, H. Exploration the Predicting variables of the addictive Mobile phone use of teenage: comparison 20 and 30 ages. Korean J. Youth Stud. 16, 117–153 (2009).
Jo, S. et al. Association of smartphone overuse with depression, anxiety, and other addictive behaviors: a nationwide community sample of Korean adults. Psychiatry Res. 304, 114133 (2021).
Elhai, J. D., Yang, H. & Montag, C. Cognitive- and emotion-related dysfunctional coping processes: transdiagnostic mechanisms explaining depression and anxiety’s relations with problematic smartphone use. Curr. Addict. Rep. 6, 410–417 (2019).
Elhai, J. D., Gallinari, E. F., Rozgonjuk, D. & Yang, H. Depression, anxiety and fear of missing out as correlates of social, non-social and problematic smartphone use. Addict. Behav. 105, 106335 (2020).
Shi, X., Wang, A. & Zhu, Y. Longitudinal associations among smartphone addiction, loneliness, and depressive symptoms in college students: disentangling between– and within–person associations. Addict. Behav. 142, 107676 (2023).
Park, J. H. & Park, M. Smartphone use patterns and problematic smartphone use among preschool children. PLoS One. 16, e0244276 (2021).
Mallawaarachchi, S. R., Anglim, J., Hooley, M. & Horwood, S. Associations of smartphone and tablet use in early childhood with psychosocial, cognitive and sleep factors: a systematic review and meta-analysis. Early Child. Res. Q. 60, 13–33 (2022).
Tome, J. M. S. & Lopez, M. A. P. Incidence of smartphones in the development of brain plasticity in children from 0 to 6 years old, in a context of high vulnerability. Principles Concepts Dev. Nowadays Soc. 1250–1260. https://doi.org/10.56238/PACFDNSV1-102 (2022).
Lin, H. M. et al. Structural and functional neural correlates in individuals with excessive smartphone use: a systematic review and Meta-analysis. Int. J. Environ. Res. Public. Health. 19, 16277 (2022).
Áfra, E. et al. Altered functional brain networks in problematic smartphone and social media use: resting-state fMRI study. Brain Imaging Behav. 1, 1–10 (2023).
Horvath, J. et al. Structural and functional correlates of smartphone addiction. Addict. Behav. 105, 106334 (2020).
Kwon, M., Jung, Y. C., Lee, D. & Lee, J. Altered resting-state functional connectivity of the dorsal anterior cingulate cortex with intrinsic brain networks in male problematic smartphone users. Front. Psychiatry. 13, 1008557 (2022).
Ahn, J., Lee, D., Namkoong, K. & Jung, Y. C. Altered functional connectivity of the Salience Network in problematic smartphone users. Front. Psychiatry. 12, 636730 (2021).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667 (2010).
Ai, H. et al. Brain Activation During Emotional Memory Processing Associated with Subsequent Course of Depression. Neuropsychopharmacology 2015 40:10 40, 2454–2463 (2015).
Jankowski, K. F. et al. Feeling left out: depressed adolescents may atypically recruit emotional salience and regulation networks during social exclusion. Soc. Cogn. Affect. Neurosci. 13, 863–876 (2018).
Yuen, G. S. et al. The salience network in the apathy of late-life depression. Int. J. Geriatr. Psychiatry. 29, 1116–1124 (2014).
Hilland, E., Landrø, N. I., Harmer, C. J., Maglanoc, L. A. & Jonassen, R. Within-network connectivity in the salience network after attention bias modification training in residual depression: report from a preregistered clinical trial. Front. Hum. Neurosci. 12, 429225 (2018).
Qiu, Y., Wu, X., Liu, B., Huang, R. & Wu, H. Neural substrates of affective temperaments: an intersubject representational similarity analysis to resting-state functional magnetic resonance imaging in nonclinical subjects. Hum. Brain Mapp. 45, e26696 (2024).
Kullmann, S. et al. Functional Network Connectivity Underlying Food Processing: disturbed salience and visual Processing in overweight and obese adults. Cereb. Cortex. 23, 1247–1256 (2013).
Han, D. H., Kim, S. M., Bae, S., Renshaw, P. F. & Anderson, J. S. Brain connectivity and psychiatric comorbidity in adolescents with internet gaming disorder. Addict. Biol. 22, 802–812 (2017).
Jao Keehn, R. J. et al. Underconnectivity between visual and salience networks and links with sensory abnormalities in Autism Spectrum disorders. J. Am. Acad. Child. Adolesc. Psychiatry. 60, 274–285 (2021).
Zhang, H. et al. Associations between childhood chronic stress and dynamic functional connectivity in drug-naïve, first-episode adolescent MDD. J. Affect. Disord. 299, 85–92 (2022).
Desseilles, M. et al. Depression alters top-down visual attention: a dynamic causal modeling comparison between depressed and healthy subjects. Neuroimage 54, 1662–1668 (2011).
Fournier, J. C. et al. Neural function during emotion regulation and future depressive symptoms in youth at risk for affective disorders. Neuropsychopharmacology 2021 46:7 46, 1340–1347 (2021).
Ma, C. et al. Resting-State Functional Connectivity Bias of Middle Temporal Gyrus and caudate with altered Gray Matter volume in Major Depression. PLoS One. 7, e45263 (2012).
Muranishi, M. et al. Inactivation of the putamen selectively impairs reward history-based action selection. Exp. Brain Res. 209, 235–246 (2011).
García-García, I. et al. Alterations of the salience network in obesity: a resting-state fMRI study. Hum. Brain Mapp. 34, 2786–2797 (2013).
Balleine, B. W., Delgado, M. R. & Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 27, 8161–8165 (2007).
Peters, S. K., Dunlop, K. & Downar, J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 10, 242701 (2016).
Talati, A. et al. Putamen structure and function in familial risk for Depression: a Multimodal Imaging Study. Biol. Psychiatry. 92, 932–941 (2022).
Gabbay, V. et al. Striatum-based circuitry of adolescent Depression and Anhedonia. J. Am. Acad. Child. Adolesc. Psychiatry. 52, 628–641e13 (2013).
Padula, C. B. et al. Targeting the Salience Network: a Mini-review on a Novel Neuromodulation Approach for Treating Alcohol Use Disorder. Front. Psychiatry. 13, 893833 (2022).
Maza, M. T. et al. Association of Habitual Checking Behaviors on Social Media with Longitudinal Functional Brain Development. JAMA Pediatr. 177, 160–167 (2023).
Moeller, S. J., London, E. D. & Northoff, G. Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: relationships to resting-state functional connectivity. Neurosci. Biobehav Rev. 61, 35–52 (2016).
Hyder, F., Fulbright, R. K., Shulman, R. G. & Rothman, D. L. Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J. Cereb. Blood Flow Metab. 33, 339–347 (2013).
Schür, R. R. et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of 1H-MRS studies. Hum. Brain Mapp. 37, 3337–3352 (2016).
Bhagwagar, Z. et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int. J. Neuropsychopharmacol. 11, 255–260 (2008).
Zhang, J. et al. Glutamate normalization with ECT treatment response in major depression. Molecular Psychiatry 2013 18:3 18, 268–270 (2012).
Benson, K. L. et al. 1H MRS Measurement of Cortical GABA and glutamate in primary insomnia and major depressive disorder: relationship to Sleep Quality and Depression Severity. J. Affect. Disord. 274, 624–631 (2020).
Sanacora, G. et al. Subtype-specific alterations of γ-Aminobutyric acid and glutamatein patients with Major Depression. Arch. Gen. Psychiatry. 61, 705–713 (2004).
Hayes, D. J. et al. Brain γ-aminobutyric acid: a neglected role in impulsivity. Eur. J. Neurosci. 39, 1921–1932 (2014).
Seo, H. S. et al. Changes of neurotransmitters in Youth with Internet and Smartphone Addiction: a comparison with healthy controls and changes after cognitive behavioral therapy. Am. J. Neuroradiol. 41, 1293–1301 (2020).
Kirby, L. G., Zeeb, F. D. & Winstanley, C. A. Contributions of serotonin in addiction vulnerability. Neuropharmacology 61, 421–432 (2011).
Müller, C. P. & Homberg, J. R. The role of serotonin in drug use and addiction. Behav. Brain. Res. 277, 146–192 (2015).
Mandino, F. et al. A triple-network organization for the mouse brain. Molecular Psychiatry 2021 27:2 27, 865–872 (2021).
Lüthi, A. & Lüscher, C. Pathological circuit function underlying addiction and anxiety disorders. Nature Neuroscience 2014 17:12 17, 1635–1643 (2014).
National Information Society Agency. Development and Validation of the Smartphone Addiction Inventory. (2011).
National Information Society Agency. The Survey on Internet Overdependence. (2016).
National Information Society Agency. Restructing the Smart Media Addiction Proneness Scale. (2014).
Kim, H. J., Lee, E. H., Hwang, S. T., Hong, S. H. & Kim, J. H. Psychometric Properties of the Children’s Depression Inventory-2 among a Community-Based Sample of Korean Children and Adolescents., Vol.37, No.2, pp.177–186 37, 177–186 (2018).
Kovacs, M. Children’s Depression Inventory (CDI and CDI 2). Encyclopedia Clin. Psychol. 1–5 https://doi.org/10.1002/9781118625392.WBECP419 (2015).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. https://home Liebertpub com/brain. 2, 125–141 (2012).
Lee, J., Lim, H., Allen, J., Choi, G. & Jung, J. Smartphone Addiction and depression among low-income boys since COVID-19: the moderating effect of being an only child. Healthc. 2021. 9, 1350 (2021).
Hayes, A. F. & PROCESS A Versatile Computational Tool for Observed Variable Mediation, Moderation, and Conditional Process Modeling 1.
Acknowledgements
This research was supported by the Korea Brain Research Institute (KBRI) Basic Research Program through the Korea Brain Research Institute (24-BR-05-01) and the National Research Foundation of Korea (NRF) grant (RS-2024-00397737) funded by the and was funded by the Ministry of Science and ICT Korea government.
Funding
This research was supported by the Korea Brain Research Institute (KBRI) Basic Research Program through the Korea Brain Research Institute (24-BR-05-01) and the National Research Foundation of Korea (NRF) grant (RS-2024-00397737) funded by the and was funded by the Ministry of Science and ICT Korea government.
Author information
Authors and Affiliations
Contributions
S.L. and M.J. designed the experiments; S.L., Y.C., J.R., J.B., and M.J. performed the experiments; S.L. and M.J. analyzed the data; and S.L., Y.C., J.R., J.B., and M.J. wrote the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Board of the Korea Brain Research Institute granted ethical approval for this study (KBRI-202103-HR-002). Written consent and verbal agreements were obtained from all participants, and their consent included approval from their legal representatives.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, S., Cheong, Y., Ro, J. et al. Alterations in functional connectivity in the salience network shared by depressive symptoms and smartphone overuse. Sci Rep 14, 28679 (2024). https://doi.org/10.1038/s41598-024-79951-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79951-6