Abstract
This study aimed to analyze Optical Coherence Tomography (OCT) parameters and Macular Pigment Optical Density (MPOD) changes in patients affected by Retinitis pigmentosa (RP). Eighteen eyes of 18 patients suffering from early-stage RP were enrolled in our observational study. 18 eyes of 18 patients age and gender matched were enrolled as controls. Patients were analyzed at baseline by undergoing complete baseline ophthalmologic examination, Spectral-domain Optical Coherence Tomography (OCT), Electroretinogram (ERG) and Heterochromatic Flicker Photometry (HFP). Main outcome measures were Macular Pigment Optical Density (MPOD), Central macular thickness (CMT), Central Choroidal Thickness (CCT) and Choroidal Vascularity Index (CVI). Lower CCT (p = 0.006), CVI (p < 0.001) and MPOD levels (p = 0.038) were found in affected patients, whereas higher CMT was detected in cases compared to healthy controls. Correlation analysis revealed the presence of a negative correlation between BCVA and Age and CMT and BCVA and a positive correlation between CCT and MPOD and CVI and CCT. Retinal and choroidal variations occur in patients affected by early-stage RP regarding functional and anatomical changes.
Similar content being viewed by others
Introduction
Retinitis Pigmentosa (RP) is a relatively rare disease with a worldwide reported prevalence ranging from 1:1878 to 1:7000 characterized by photoreceptor loss and visual impairment1. The condition can be diagnosed basing on the presence of a familiar story of RP, photophobia, night blindness, central visual field constriction and the evidence of mid-peripheral bone spicule pigmentation, waxy optic disc pallor, attenuation of retinal arteries at the fundus oculi examination. Moreover, RP typically presents with reduced standard full-field electroretinogram, photoreceptors and retinal pigment epithelium cell loss at the Optical coherence tomography (OCT)2,3,4. resulting in significant impact in terms of patient quality of life. RP is one of the conditions most present in inherited retinal diseases (IRD), mostly related to rod-specific gene mutations, which subsequently cause alteration in Retinal Pigment Epithelium (RPE), ganglion cells and cone degeneration5. It can have an X-linked, recessive, dominant or rarely digenic or mitochondrial transmission and can be presented as isolated or syndromic. Up to 40–50% have been reported to be sporadic6. Supportive therapies such as Vitamin A and vitamin E supplementation have been demonstrated to be cover a role in RP course of disease, despite yet being highly controversial depending on the patient genotype7. However, Voretigene neparvovec (Luxturna) gene therapy is the only approved treatment for RP patients affected by RPE65 deficiency, a factor required for 11-cis-vitamin A production during the retinal visual cycle8. To date, no routine therapies are directed at treating the onset of disease, despite the possibility of managing RP-associated conditions as the presence of macular edema (ME), epiretinal membrane (ERM) and cataract; thus, an early and careful diagnosis is critical for offering the patient effective medical care5. It must be considered RP heterogeneity in the disease presentation, which makes identifying typical disease traits a fundamental point for diagnosis and patient care. Lutein and meso-zeaxanthin, forming the Macular pigment (MP), are substances located in the fovea which have been reported to cover a protective role in reducing oxidative stress by partially absorbing the high-energy blue light9,10. Consequently, it has been speculated about MP role in protecting against degenerative eye diseases, and its assessment study is necessary for investigating the role of carotenoids and their function11,12. Sandberg et al. investigated the relationship between macular pigment optical density (MPOD) and serum lutein and zeaxanthin by finding them to be independently related to serum total cholesterol, serum lutein, iris color, and central foveal retinal thickness in patients affected by RP13. Besides, Bayat et al. recently improved OCT knowledge on RP by analyzing choroidal structure in patients with IRD. They found significantly lower CVI in patients with IRD compared to age-matched controls14. A comprehensive study of patients affected by RP is fundamental in better describing and knowing the disease and improving patients care. Thus, our study aimed to analyze structural OCT parameters and MPOD in patients affected by RP.
Methods
Eighteen eyes of 18 patients affected by early-stage RP were enrolled in our observational study. Patients were enrolled at the Ophthalmology Clinic of University “G. d’Annunzio”, Chieti-Pescara, Italy, from January 2023 to January 2024. Inclusion criteria were: eyes with confirmed diagnosis of RP, patients aged over 18 years and Best Corrected Visual Acuity (BCVA) > 0.5 logarithm of the minimum angle of resolution (LogMAR) to ensure the correct execution of examinations. Early stage retinitis pigmentosa was defined as definite diagnosis of retinitis pigmentosa with preserved BCVA > 0.5 LogMAR, conserved foveal structure evident at OCT examination in the central ring of the ETDRS mapping on Spectralis HRA + OCT (Heidelberg Engineering; Heidelberg, Germany) and less than 2 years from clinical diagnosis to study enrollment. Exclusion criteria included: Previous intraocular surgery; Evidence of advanced RP (either extended macular atrophy or undetectable ERG)15; Ocular media opacities according to Lens Opacities Classification System III; Presence of systemic or local diseases other than RP, presence of central macular edema (CME). 18 eyes of 18 healthy patients, age and gender-matched, were enrolled as controls. The study adhered to the tenets of the Declaration of Helsinki and was approved by an Institutional Review Board of the University “G.d’Annunzio” of Chieti- Pescara, Italy. Specific informed consent was obtained from all participants. All patients underwent complete baseline ophthalmological examination, including BCVA evaluation, Goldmann applanation tonometry, slit lamp biomicroscopy, indirect fundus ophthalmoscopy, fundus autofluorescence (FAF) using Spectralis HRA + OCT (Heidelberg Engineering; Heidelberg, Germany) and Optos California device ( California Optomap, Optos plc, Dunfermline, Scotland, UK), Spectral Domain OCT (SD-OCT) using Spectralis HRA + OCT (Heidelberg Engineering; Heidelberg, Germany), Electroretinogram (ERG) (Retimax CSO, Florence, Italy) and macular pigment optical density (MPOD) quantification with heterochromatic flicker photometry (HFP) using the MP Screener II (Elektron Technology, Cambridge, United Kingdom). The main outcome measures were MPOD assessment, Central Macular Thickness (CMT), Central Choroidal Thickness (CCT) and Choroidal Vascularity Index (CVI).
BCVA evaluation
BCVA was assessed by testing patients with the Early Treatment Diabetic Retinopathy Study (ETDRS) Chart using the LogMAR system.
Electroretinogram
According to the International Society for Clinical Electrophysiology of Vision (ISCEV) protocols, full-field (ffERG) and multifocal (mfERG) ERG was recorded for each patients using Retimax CSO, Florence, Italy16,17.
OCT analysis
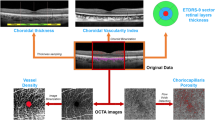
SD-OCT was performed using the Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). The acquisition protocol included a 49 horizontal rasters dense linear B-B-scans centered on the fovea and horizontal and vertical B-scans centered on the fovea acquired with enhanced depth imaging (EDI) mode for all patients. All images were acquired by a single trained ophthalmologist (AQ). Images with poor signal strength (< 25) were excluded and thus repeated. CMT was measured using the central 1 mm diameter circle of the ETDRS thickness map. CCT was measured vertically from the outer border of the RPE to the inner border of the sclera using the inbuilt manual caliper on EDI OCT scans18. CVI was calculated using a validated ImageJ application algorithm. EDI-OCT horizontal and vertical single-line scans centered on the fovea were exported, and then, once identified manually the choroid, which was defined as the area between the outer border of the RPE to the inner border of the sclera, the limits of the Region of Interest (ROI) were obtained. The total choroidal area (TCA) was calculated as the total area of the ROI. The images were binarized using Niblack’s auto-local threshold, and dark pixels were defined as the luminal choroidal area (LCA). In contrast, white pixels were defined as the stromal choroidal area (SCA). CVI was obtained as the ratio between LCA and TCA19. Two graders’ experts in the retinal field (AQ and MLR) analyzed images independently. In disagreement, a third retinal specialist was consulted (LT). (Fig. 1)
MPOD analysis
MPOD was measured using an MP Screener II macular densitometer (MPSII® Elektron Technology, Cambridge, United Kingdom). The device exploits the HFP method to assess the blue light wavelength absorption (465 nm) by MP (which is greatest in the foveal region) compared to the green wavelength light (530 nm). All measurements were performed by a single trained ophthalmologist (AQ) before pupil dilatation, and every test was repeated twice by a second ophthalmologist (MP) (with a 30-minute interval between measurements) to verify the instrument’s repeatability20. Rejected values were interpreted as poor-quality registrations, and patients were thus re-tested. If rejected values were found again, the result was interpreted and recorded as non-measurable (Fig. 1).
Statistical analysis
Descriptive statistics included frequencies and percentages for categorical variables and median [first; third] quartile for quantitative ones. Nonparametric methods were preferred according to the Wilk test. The differences between cases and controls were evaluated using a non-parametric unpaired t-test (Mann–Whitney test). Spearman rank correlation coefficient (rho) was used in cases to investigate the relationships between investigated parameters. All p-values were two-tailed, and a p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using the R environment (version 4.1; http://www.r-project.org/).
Results
In our observational study, 18 eyes of 18 patients affected by RP were enrolled as cases (G1). Ten eyes over 18 were from male patients (56%), whereas eight eyes over 18 were from male patients (44%). 18 eyes of 18 patients age and gender-matched were enrolled as controls (G2). Mean time from diagnosis to study enrollment was 1.55 years. The descriptive analysis is summarized in Table 1. The median age was 46 [20.0; 52.0] in G1 and 36.0 [32.0; 41.5] in G2. SD-OCT scans were acceptable for all eyes for qualitative and quantitative parameters. MPOD was evaluated in all cases. All evaluations were performed at baseline. BCVA was statistically significantly higher (p < 0.001) in controls (0.00 [0.00;0 .00)] when compared to cases 0.05 [0.00; 0.18] in the absence of confounding parameters (cataract, vitreoretinal interface diseases, glaucoma) other than RP due to inclusion criteria. CCT was significantly lower in cases (234.00 [196.00; 286.00]) when compared to controls (316 [270.00; 393.00], p = 0.006). Conversely, CMT was found to be higher in cases (205.00 [187.00; 248.00]) compared to controls (180.00 [163.000; 216.000], p = 0.058), although not significantly. CVI appeared significantly lower in cases than controls (0.66 [0.65; 0.68] in G1, 0.70 [0.69; 0.73] in G2 p < 0.001). MPOD was found to be significantly lower (p = 0.038) in cases (0.42 [0.30; 0.55]) compared to controls (0.55 [0.41; 0.65]).(Fig. 2) In cases, correlation analysis revealed the presence of a negative correlation between BCVA and Age and CMT and BCVA. Moreover, a positive correlation was found between CCT, MPOD, and CVI and CCT (Fig. 3).
Correlation analysis between parameters in cases using Spearman correlation coefficient (rho). Indeed, the p-value is recorded under the correlation coefficient. Accordingly to the study methodology, LogMAR BCVA has been used to record BCVA values. In cases, the correlation between BCVA and Age is rho = 0.47 (p = 0.050), indicating a moderate positive correlation. This suggests that BCVA values also tend to increase as age increases, meaning that older individuals tend to have worse visual acuity. The correlation between BCVA and CMT is rho = 0.62 (p = 0.006), which shows a strong positive correlation. This indicates that as central macular thickness (CMT) increases, BCVA values also increase, corresponding to a deterioration in visual acuity.
Discussion
The study of retinitis pigmentosa has always been affected by the wide heterogeneity of disease presentation and course. The possibility of identifying factors that could define the disease despite the wide range of possible symptoms has made the study of RP a topic to focus on due to the recent advancements in RP treatment and the upcoming knowledge in subretinal delivery therapies. Retinal and choroidal changes occurring in eyes with RP have been a matter of study for years. Toto et al. in their cross-sectional study found RP patients to have Ganglion Cell Complex (GCC) alterations, choroidal vessel, and retinal changes related to macular function. The authors speculate that the thinner GCC found in their RP patients could have been responsible for the reduced macular function measured by mfERG21. The importance of retinal ganglion cells (RGCs) has been demonstrated by different authors, who found decreased number of RGCs in RP patients, presenting reduced retinal function22. Different hypotheses have been proposed to explain this effect, including a lower blood flow to the inner retinal layers and reduced transsynaptic signal caused by photoreceptor cell degeneration23,24,25. Besides, Tong Tao et al. have underlined the possibility of studying the Muller cells as a biomarker of retinal degeneration progression. In fact, in their study, they found the increased GFAP and ERK expression to be related to retinal degeneration progression in RCS rat retinal cells, reinforcing the role of Muller cells activation in a multiplicity of physiopathological conditions and hypothesizing Muller cells activation to be secondary to retinal degeneration26. Different authors have thus proposed the potential therapeutic role of Muller glial cells in regenerating the retina27. Sanges et al., by transplanting in retinitis pigmentosa mice models treated cells to preactivate WNT signaling, demonstrated them to be able to hybridize into Muller glial cells and re-enter in the cell cycle, finally differentiating into photoreceptors, reducing the ongoing retinal degeneration28. Furthermore, Roesch et al. reported Muller glial cells to have a key role in retinal degeneration by undergoing gliosis, with glial fibrillary acidic protein (Gfap) upregulation29. Muller cells are glial cells that maintain the entire retinal structure (30). The location of Muller cells in the fovea is characterized by being the only type of glial cells present in that area31. Moreover, it has been demonstrated that the “Muller cell cone” (MMC), a tissue deriving from the vertically oriented muller cells over the foveal area, is the area of Macular pigment location with a centrifugally increased gradient decreasing from the center of the foveola up to the periphery as demonstrated by the distribution of macular autofluorescence in healthy eyes20,31,32,33,34. Macular pigment is believed to be protective against ultraviolet radiation damage by absorbing short-wavelength visible light and having an antioxidant effect due to its composition in xanthophylls35. Considering the overall ultrastructural alterations found in eyes affected by retinitis pigmentosa, we were interested in analyzing whether changes in MPOD could be found between affected and healthy eyes. Interestingly, changes in MPOD were found between the two groups with lower MPOD in eyes affected by retinitis pigmentosa when compared to healthy eyes. This finding can be explained by a different foveal cell distribution in RP eyes, which can be responsible for a different MPOD distribution over the foveal area. To our knowledge, only a few authors have previously analyzed occurring changes in MPOD in patients affected by RP. Sandberg et al., in their study, have analyzed the relationship of MPOD and serum lutein in retinitis pigmentosa by finding it to be independently related to serous lutein, serum total cholesterol, iris color and central foveal retinal thickness in affected eyes13. Consistent with our results, the authors found reduced MPOD in their cohort of retinitis pigmentosa patients without evidence of CME. Moreover, the authors speculated the increase in MPOD to be possible following lutein supplementation, which resulted in reduced oxidative damage over the long term and thus reduced photoreceptor degeneration. However, they acknowledged the necessity of future longitudinal studies. Nevertheless, it must be considered that due to the wide spectrum of clinical presentation related to different phenotype-genotype correlations in patients with retinitis pigmentosa, the studied parameter may present differences basing on the patients genotype. Future studies should be aimed at analyzing this parameter basing on different genotypes. Indeed, vascular changes are typically occurring in patients with RP. Vessel sclerosis, narrowing with subsequent lumen occlusion due to thickening of blood vessel wall are typical traits of RP23,36. Moreover, it has been demonstrated by Koniexzka et al. that RP patients may suffer from primary vascular dysregulation syndrome, with vessel predisposition to react to different stimuli37. Different studies have previously investigated the role of choroid and choriocapillaris in retinitis pigmentosa patients. Mastropasqua et al. found a reduced radial peripapillary network in RP patients38. Toto et al. showed alterations in both choroid and retina vessels in RP patients when compared to healthy subjects, with particular attention to the Superficial Capillary Plexus and Deep Capillary Plexus, which were found to be related to macular function21. Indeed, choroidal circulation was found to be altered in RP patients in several studies, associated with the presence of a reduced CCT39,40. Therefore, our interest in the present study was to investigate choroids by analyzing CCT and CVI. According to the existing literature, CCT was significantly reduced in eyes with RP. In their retrospective analysis, Sodi et al. found the choroid significantly reduced in the RP group compared to controls41. Similarly, Akai et al. found a reduction in CCT in patients with early RP41. A similar result was obtained by Aknin et al., who found reduced CCT in patients with RP and observed a relation with BCVA by interpreting the BCVA worsening as a sign of disease progression and the occurring reduced CCT as an index of worsening disease43. Our study observed reduced BCVA in patients with RP compared to healthy patients. However, the lack of a significant correlation between BCVA and CCT is probably due to the enrollment criteria for BCVA. Conversely, Chablani et al. did not find differences in CCT in affected patients, whereas Tan et al. observed an increase in CT compared to the control group44,45. Therefore, the necessity of identifying a choroidal parameter that could better assess choroid changes in observed patients has been highlighted. CVI is a well-established parameter which can evaluate choroidal vasculature without the influence of systemic or local factors. Different studies have made CVI a well-known and well-accepted parameter useful in the study of retinal and choroidal disease19,46. Our study found reduced CVI in affected patients, thus reflecting the vascular changes occurring in patients with RP. Similarly, Bayat et al., by investigating choroidal structure in patients with Inherited retinal disease, found CVI to be significantly lower in patients with IRD, assuming it to be related to changes in choroidal vessel lumen rather than stromal changes. In their study, patients with a diagnosis of RP, Leber congenital amaurosis and cone-rod dystrophy displayed the lowest CVI values when compared to healthy patients14. Accordingly, Shen et al. found changes in CVI by analyzing choroidal al choriocapillaris structure in patients with RP47. Besides, Iovino et al. reported lower CVI and higher CT in RP patients with CME, confirming the role of the choroid in CME genesis and suggesting CVI as a possible future damage biomarker48. In this light, our finding of higher CME in patients with RP compared to controls despite the absence of macular edema should be further elucidated. Although previous reports have highlighted the occurrence of structural layers alterations in patients with RP compared to controls which was evident at the OCT exam49, a higher CME may be attributable to incipient macular edema not resulting in visual impairment. In fact, our patients presented higher CT and lower CVI, which has previously been related to the presence of macular edema48. The perfect assessment of ultrastructural and functional modifications occurring in RP patients covers a key role of study due to the upcoming novelty in therapeutic tools that could change the course of genetic diseases. Choroidal and retinal biomarkers have demonstrated in different conditions to be useful in disease monitoring and characterization to identify the most effective and suitable treatment.
Conclusions
This is the first study analyzing structural and functional parameters in RP patients by focusing on occurring MPOD alterations. Although we acknowledge the necessity of multiple studies to confirm and enhance the role of this parameter as a possible tool to monitor the disease course and progression and focus on its possible implication in genotypic-phenotypic correlations, it is important to underline the necessity of characterizing genetic diseases to move forward with tailored treatment. Our study shows that choroidal and retinal changes occur in patients with early RP in terms of lower CCT, CVI and MPOD.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Abbreviations
- RP:
-
Retinitis pigmentosa
- OCT:
-
Optical coherence tomography
- ERG:
-
Electroretinogram
- HFP:
-
Heterocromatic flicker photometry
- MPOD:
-
Macular pigment optical density
- CMT:
-
Central macular thickness
- CCT:
-
Central choroidal thickness
- CVI:
-
Choroidal vascularity index
- BCVA:
-
Best corrected visual acuity
- IRD:
-
Inherited retinal diseases
- RPE:
-
Retinal pigment epithelium
- ME:
-
Macular edema
- ERM:
-
Epiretinal membrane
- MP:
-
Macular pigment
- LogMAR:
-
logarithm of the minimum angle of resolution
- CME:
-
Central macular edema
- FAF:
-
fundus autofluorescence
- SD-OCT:
-
Spectral domain OCT
- ETDRS:
-
Early treatment diabetic retinopathy study
- ffERG:
-
Full field electroretinogram
- mfERG:
-
Multifocal ERG
- ROI:
-
Region of Interest
- TCA:
-
Total choroidal area
- LCA:
-
Luminal choroidal area
- SCA:
-
Stromal choroidal area
- GCC:
-
Ganglion cell complex
- RGC:
-
Retinal ganglion cells
- Gfap:
-
Glial fibrillary acidic protein
- MMC:
-
Muller cell cone
References
Hartong, D. T., Berson, E. L. & Dryja, T. P. Retinitis pigmentosa. Lancet. 368 (9549), 1795–1809 (2006).
Poornachandra, B. et al. Quantifying microstructural changes in retinitis pigmentosa using spectral domain—optical coherence tomography. Eye Vis. ;6(1). (2019).
Okonkwo, O., Hassan, A., ChinezeThelma Agweye, Victor, U. & Akanbi, T. Clinical presentation and macular morphology in retinitis pigmentosa patients. Ann. Afr. Med. 22 (4), 451–455 (2023).
Giusti, C., Forte, R. & Vingolo, E. M. Clinical pathogenesis of macular holes in patients affected by retinitis pigmentosa. Eur. Rev. Med. Pharmacol. Sci. 6(2–3), 45–48. (2002).
Kamde, S. P. & Anil, A. Retinitis pigmentosa: Pathogenesis, diagnostic findings, and treatment. Cureus. (2023).
D’Esposito, F. et al. RP1 Dominant p.Ser740* pathogenic variant in 20 knowingly unrelated families affected by rod–cone dystrophy: Potential founder effect in Western Sicily. Medicina 60(2), 254 (2024).
Smirnov, V. M. et al. Large Benefit from simple things: High-dose vitamin A improves RBP4-related retinal dystrophy. Int. J. Mol. Sci. 23(12), 6590 (2022).
Wu, K. Y. et al. Retinitis pigmentosa: Novel therapeutic targets and drug development. Pharmaceutics 15(2), 685 (2023).
Algvere, P. V., Marshall, J. & Seregard, S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol. Scand. 84 (1), 4–15 (2006).
Sharpe, L. T., Stockman, A., Knau, H. & Jägle, H. Macular pigment densities derived from central and peripheral spectral sensitivity differences. Vis. Res. 38 (21), 3233–3239 (1998).
de Kinkelder, R. et al. Macular pigment optical density measurements: evaluation of a device using heterochromatic flicker photometry. Eye. 25 (1), 105–112 (2010).
Quarta, A. et al. Analysis of macular pigment optical density in macular holes with different border phenotypes. Ophthalmol. Ther. (2024).
Sandberg, M. A., Johnson, E. J. & Berson, E. L. The relationship of macular pigment optical density to serum lutein in retinitis pigmentosa. Investig. Opthalmol. Visual Sci. 51 (2), 1086 (2010).
Bayat, K. et al. Choroidal structure investigated by choroidal vascularity index in patients with inherited retinal diseases. Int. J. Retina Vitreous. 9(1). (2023).
Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 1, 40 (2006).
Hood, D. C. et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc. Ophthalmol. 124 (1), 1–13 (2011).
Bach, M. et al. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. 126 (1), 1–7 (2012).
Ruggeri, M. L. et al. Long term follow up of brolucizumab in macular neovascularization. Ophthalmic Res. (2023).
Toto, L. et al. Choroidal and retinal imaging biomarkers in different types of macular neovascularization. J. Clin. Med. 12 (3), 1140 (2023).
Cerino, L., Aharrh-Gnama, A., Ruggeri, M. L. & Carpineto, P. Macular pigment optical density assessed by heterochromatic flicker photometry in eyes affected by primary epiretinal membrane. Retina. (2021).
Toto, L. et al. Macular features in retinitis pigmentosa: correlations among ganglion cell complex thickness, capillary density, and macular function. Investig. Opthalmol. Visual Sci. 57 (14), 6360 (2016).
Vámos, R., Tátrai, E., Németh, J., Holder, D. E., DeBuc, D.C., Somfai, G.M. The structure and function of the macula in patients with advanced retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 52 (11), 8425–8425 (2011).
Li, Z. Y., Possin, D. E. & Milam, A. H. Histopathology of bone spicule pigmentation in retinitis pigmentosa. Ophthalmology. 102 (5), 805–816 (1995).
Jones, B. W. et al. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 150, 149–165 (2016).
Saha, S. et al. Changes in ganglion cells during retinal degeneration. Neuroscience. 329, 1–11 (2016).
Zhao, T. T., Tian, C. Y. & Zheng, Q.Y. Activation of Müller cells occurs during retinal degeneration in RCS rats. Adv. Exp. Med. Biol. 575–583 (2009).
Heravi, M. & Rasoulinejad, S. A. Potential of Müller glial cells in regeneration of retina; clinical and molecular approach. Int. J. Organ Transplant. Med. 13(1), 50–59 (2022).
Sanges, D. et al. Reprogramming Müller glia via in vivo cell fusion regenerates murine photoreceptors. J. Clin. Investig. 126 (8), 3104–3116 (2016).
Roesch, K., Stadler, M. B. & Cepko, C. L. Gene expression changes within Müller glial cells in retinitis pigmentosa. Mol. Vis. 18, 1197–1214 (2012).
Li, X., Liu, J., Hoh, J. & Liu, J. Müller cells in pathological retinal angiogenesis. Transl. Res. 207, 96–106 (2019).
Bringmann, A. et al. The primate fovea: Structure, function and development. Prog. Retin. Eye Res. 66, 49–84 (2018).
Syrbe, S. et al. Müller glial cells of the primate foveola: an electron microscopical study. Exp. Eye Res. 167, 110–117 (2018).
Bringmann, A. et al. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 25 (4), 397–424 (2006).
von Rückmann, A., Fitzke, F. W. & Bird, A. C. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br. J. Ophthalmol. 79 (5), 407–412 (1995).
Leung, I. Y. F. Macular pigment: New clinical methods of detection and the role of carotenoids in age-related macular degeneration. Optometry J. Am. Optom. Assoc.. 79 (5), 266–272 (2008).
Milam, A., Li, Z. & Fariss, R. Histopathology of the human retina in retinitis pigmentosa. Prog. Retin. Eye Res. 17 (2), 175–205 (1998).
Konieczka, K., Flammer, A. J., Todorova, M., Meyer, P. & Flammer, J. Retinitis pigmentosa and ocular blood flow. EPMA J. ;3(1). (2012).
Mastropasqua, R. et al. Radial peripapillary capillary network in patients with retinitis pigmentosa: an optical coherence tomography angiography study. Front. Neurol. ;8. (2017).
Ayton, L. N., Guymer, R. H. & Luu, C. D. Choroidal thickness profiles in retinitis pigmentosa. Clin. Exp. Ophthalmol. 41 (4), 396–403 (2012).
Dhoot, D. S. et al. Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br. J. Ophthalmol. 97 (1), 66–69 (2012).
Sodi, A. et al. EDI-OCT evaluation of choroidal thickness in retinitis pigmentosa. Eur. J. Ophthalmol. 28 (1), 52–57 (2017).
Akay, F., Akmaz, B. & Güven, Y. Z. Optical coherence tomography detection of changes in inner retinal and choroidal thicknesses in patients with early retinitis pigmentosa. Arquivos Brasileiros De Oftalmologia 83(5). (2020).
Aknin, I. & Pradat, P. Choroidal thickness in healthy eyes using enhanced depth imaging optical coherence tomography and comparison with cases of retinitis pigmentosa. J. Français d’Ophtalmologie. 41 (10), 933–938 (2018).
Chhablani, J., Jonnadula, G. B., Srinivasa Rao, P., Venkata, A. & Jalali, S. Choroidal thickness profile in Retinitis Pigmentosa—correlation with outer retinal structures. Saudi J. Ophthalmol. 30 (1), 9–13 (2016).
Tan, R. et al. Choroidal vascularity index in retinitis pigmentosa: an OCT study. Ophthalmic surgery. Lasers Imaging Retina. 49 (3), 191–197 (2018).
Toto, L. et al. Choroidal modifications assessed by means of choroidal vascularity index after oral eplerenone treatment in chronic central serous chorioretinopathy. Eye (2022).
Shen, C. et al. Choroidal vascular changes in retinitis pigmentosa patients detected by optical coherence tomography angiography. BMC Ophthalmol. ;20(1). (2020).
Iovino, C. et al. Evaluation of the choroid in eyes with retinitis pigmentosa and cystoid macular edema. Investig. Ophthalmol. Vis. Sci. 60 (15), 5000–5000 (2019).
Nagasaka, Y. et al. Increased aqueous flare is associated with thickening of inner retinal layers in eyes with retinitis pigmentosa. Sci. Rep. 6, 33921. https://doi.org/10.1038/srep33921 (2016).
Author information
Authors and Affiliations
Contributions
Conceptualization, R.M.; methodology, R.G.; software, A.Q.; validation, L.T. and L.S.; formal analysis, M.D.N. and A.P.; investigation, M.L.R.; resources, L.B.B. and F.F.; data curation, M.P. and C.L.; writing—original draft preparation, M.L.R.; writing—review and editing, L.T.; visualization, R.M.; supervision, R.M. and L.S.; project administration, R.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University “G. d’Annunzio”of Chieti- Pescara. Informed consent was obtained from all subjects involved in the study.
Consent for publication
Consent was obtained from all subjects involved in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ruggeri, M.L., Baroni, L.B., Passamonti, M. et al. OCT analysis and MPOD assessment in patients affected by retinitis pigmentosa. Sci Rep 14, 28830 (2024). https://doi.org/10.1038/s41598-024-79979-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79979-8