Abstract
We aimed to identify prognostic risk factors for visual deterioration in eyes with epiretinal membrane (ERM) and open-angle glaucoma (OAG) through long-term follow-up. In this retrospective observational case series, we examined consecutive eyes with ERM and OAG over a minimum follow-up of 2 years. Visual outcomes and prognostic factors were analyzed in patients’ eyes undergoing ERM peeling with pars plana vitrectomy (PPV) (peeling group) and only observation (observation group). Eyes followed for less than 2 years after PPV were excluded. Among 100 eyes from 84 patients, 31 and 69 eyes were classified into peeling and observation groups, respectively. Over the follow-up period, best-corrected visual acuity (BCVA) in the observation group worsened from 0.03 ± 0.19 to 0.14 ± 0.33 (P = 0.0003), whereas that in the peeling group remained stable. Multivariate analysis revealed microcystic macular edema (MME) (odds ratio: 4.97; 95% confidence interval [CI]: 1.52 to 16.2; P = 0.008) and thin central foveal thickness (CFT) (odds ratio: 0.99; 95% CI: 0.98 to 1.00; P = 0.033) as risk factors for visual acuity decay. The presence of MME and thin CFT were risk factors for long-term visual deterioration in eyes with ERM and concomitant OAG. Vitrectomy with ERM peeling for eyes with OAG may be considered to preserve BCVA.
Similar content being viewed by others
Introduction
The epiretinal membrane (ERM) involves fibrocellular proliferation composed of glial cells, retinal pigment epithelial cells, or hyalocytes on the internal limiting membrane (ILM)1. Most ERMs are idiopathic, whereas intraocular surgery, retinal vascular disease, and uveitis are considered secondary risk factors. The prevalence of ERM varies among ethnic groups and increases with age2. Contraction of ERM leads to visual deterioration, metamorphopsia, or aniseikonia, and membrane peeling with pars plana vitrectomy (PPV) is the only treatment approach for visually impaired eyes3.
Glaucoma is one of the most common diseases leading to blindness, characterized by slow progressive degeneration of retinal ganglion cell axons at the lamina cribrosa4,5. Glaucoma can coexist in eyes with ERM6. Furthermore, eyes with pseudoexfoliation glaucoma are likely to develop ERM7. A previous study identified the presence of ERM as a potential risk factor for glaucoma progression in8. However, the surgical indication of ERM with glaucoma remains controversial, although it differs from that of idiopathic ERM.
Postoperative visual field (VF) deterioration after PPV for ERM with glaucoma has been reported9,10,11. Recent reports revealed that postoperative improvement of visual acuity was not evident12,13. Furthermore, some studies with several years of follow-up identified microcystic macular edema (MME), worse mean deviation (MD) of the VF test, thinner ganglion cell complex (GCC; retinal nerve fiber layer + ganglion cell layer + inner plexiform layer), advanced disc cupping, central VF defect, and longer axial length as poor prognostic factors for PPV for ERM with glaucoma9,11,12,13. Surgical procedures such as ILM removal or staining of ILM with indocyanine green (ICG) may also be risk factors for decreasing VF sensitivity10,14,15. This effect may be attributed to the dissociated optic nerve fiber layer (DONFL) caused by ILM removal, which may deteriorate postoperative visual function16,17,18. Distinguishing MME in ERM from macular edema, also referred to as retrograde maculopathy, which implies advanced optic neuropathy, is challenging19,20,21. Govetto et al. reported that MME is frequent in eyes with ERM concomitant with glaucoma and is less likely to resolve even following membrane peeling22.
However, the observation period in these studies was limited to a couple of years. Long-term safety after PPV for eyes with ERM concomitant with glaucoma is not guaranteed; however, decay in the glaucomatous VF progresses slowly. PPV with ILM peeling for eyes with ERM concomitant with glaucoma may accelerate glaucoma progression11. In eyes with ERM and glaucoma, surgical intervention can lead to critical deterioration of visual acuity. Moreover, an observation strategy may be better over the long term; however, the optimum strategy remains unclear. In this study, we aimed to identify risk factors for the deterioration of visual acuity in eyes with ERM concomitant with open-angle glaucoma (OAG) over a long follow-up, comparing eyes with or without surgical intervention.
Results
In total, 100 eyes from 84 patients were included in this study (31 eyes in the peeling group and 69 eyes in the observation group) (Table 1). The mean follow-up period was 65.9 ± 32.8 months (64.5 ± 27.6 months in the peeling group and 66.6 ± 35.0 months in the observation group; P = 0.764). Of note, 87 eyes from 73 patients were treated with topical intraocular pressure (IOP)-reducing agents (mean 1.6 ± 1.1 medications) at baseline. The mean IOP was 15.7 ± 4.6 mmHg at baseline. The peeling group had worse best-corrected visual acuity (BCVA), thicker central foveal thickness (CFT), more frequent ellipsoid zone (EZ) disruption, higher ERM stage, milder cup-to-disc (C/D) ratio, and better MD at baseline compared to the observation group. The mean BCVA in all eyes worsened from 0.10 ± 0.25 to 0.16 ± 0.32 (P = 0.067). Additional glaucoma surgery, including trabeculectomy (in 12 eyes) and trabeculotomy (in 10 eyes), was performed by glaucoma specialists when required. No significant intraoperative and postoperative complications were observed. Emery–Little cataract grades at baseline were grades 1, 2, and 3 in 6, 14, and 3 eyes in the peeling group and 24, 22, and 4 eyes in the observation group, respectively.
Optical coherence tomography (OCT) parameters
BCVA at baseline was significantly worse in higher ERM stages (P = 0.002), but it was not associated with the presence of MME (P = 0.8). Eyes with MME were more common in higher ERM stages at baseline (stages 1, 2, 3, and 4: eight, six, seven, and three eyes, respectively). MME at baseline was not related to the grade of disc cupping (P = 0.303) and defect of central VF (P = 0.88). The presence of MME was not associated with age either at baseline (P = 0.571) or at the final visit (P = 0.83). Additionally, in six of 24 (25%) eyes with MME at baseline, MME disappeared at the final visit, whereas eight of 76 (11%) eyes without MME at baseline developed MME at the final visit. EZ disruption was significantly associated with BCVA at baseline (P = 0.006), whereas no significant difference was observed at the final visit (P = 0.139). Pseudoexfoliation was not related to the ERM stage (P = 0.532).
Comparison between the peeling and observation groups
Over the follow-up period, BCVA in the observation group worsened from 0.03 ± 0.19 to 0.14 ± 0.33 (P = 0.0003), whereas it remained unchanged in the peeling group (0.25 ± 0.31 to 0.19 ± 0.29, P = 0.397) (Fig. 1). The change observed in the BCVA was better in the peeling group than in the observation group (P = 0.018). CFT (P < 0.001) and GCC (P < 0.001) decreased in the peeling group. MME improved in the peeling group (P = 0.04), whereas MME increased in the observation group (P = 0.01). No change was observed in EZ disruption in either group. The two representative cases are presented in Fig. 2.
The logarithm of the minimum angle of resolution (LogMAR), mean central foveal thickness (CFT) (µm), rate of microcystic macular edema (MME) (%), and mean ganglion cell complex (GCC) thickness (µm) throughout the follow-up period in the peeling (epiretinal membrane [ERM] removal with pars plana vitrectomy) and observation (without vitrectomy) groups. The asterisks represent a P value < 0.05.
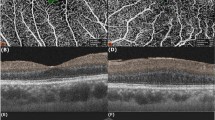
Representative cases with epiretinal membrane (ERM) and concurrent open-angle glaucoma in each group. In the peeling group, a 67-year-old male underwent ERM removal with a vitrectomy in the right eye. His visual acuity (VA) was increased from 0.2 to 0.6. His mean deviation (MD) worsened from − 9.80 dB to − 11.65 dB. The ERM and ILM were removed completely without recurrence, and microcystic macular edema (MME) improved. In the observation Group, a 64-year-old woman was observed without vitrectomy over the follow-up period. VA in her left eye worsened from 1.2 to 0.8, with an expansion of the central visual field defect. Her MD changed from − 15.72 dB to −15.33 dB. The ERM and MME were not resolved.
Risk factors in the peeling group
Twelve eyes exhibited worsened BCVA postoperatively in the peeling group (Table 2). The postoperative follow-up period was not associated with worsened BCVA (P = 0.166). Intentional ILM peeling (P = 0.948) and ICG usage (P = 0.841) were not risk factors for visual acuity deterioration. Furthermore, 23 of 24 phakic eyes received cataract surgery. Cataract extraction improved BCVA (P = 0.007). Postoperatively, the presence of DONFL and ERM recurrence were not related to visual acuity decay (P = 0.948 and P = 0.546, respectively). CFT thickness (P = 0.087), GCC thickness (P = 0.063), and the presence of MME (P = 0.376) at baseline were not related to worsened visual acuity. The presence of DONFL was not associated with postoperative visual acuity (P = 0.948).
Prognostic risk factors for visual acuity
Logistic regression analyses revealed a relationship between visual acuity decay and clinical factors (Table 3). MME (odds ratio [OR]: 4.97; 95% confidence interval [CI]: 1.52 to 16.2; P = 0.008) and thin CFT (OR: 0.99; 95% CI: 0.98 to 1.00; P = 0.033) were considered risk factors for visual acuity deterioration; however, cataract extraction and GCC at baseline were not identified as risk factors (P = 0.674 and P = 0.108, respectively). In the univariate analysis, VF at baseline and surgical factors such as ERM peeling, intentional ILM peeling, or ICG dying were not associated with BCVA worsening (P = 0.17, P = 0.214, P = 0.588, respectively). Further, within the observation group, the stage of ERM at baseline was not related to BCVA worsening (P = 0.545).
Discussion
In this retrospective study, we identified prognostic risk factors for visual acuity decline in 100 eyes with ERM concomitant with OAG over a long-term follow-up by comparing the peeling and observation groups. In the observation group, BCVA worsened considerably over the follow-up period; however, it did not deteriorate in the peeling group. Cataract surgery, or surgical procedures such as ILM peeling or ICG usage can have a profound effect on the visual outcomes. Accordingly, we utilized multivariate analysis, which revealed the presence of MME and thinner CFT as poor prognostic factors for visual acuity outcomes. The observation group included a higher proportion of early-stage ERM cases compared to that in the peeling group. This suggests that factors like cataract and glaucomatous visual field loss might have had a greater impact on visual outcomes than ERM itself. On the other hand, many eyes in the peeling group underwent cataract surgery, often for significant cataracts (E-2 and above). Approximately half of the eyes in the observation group had advanced cataracts (E-2 and above), but only 43% underwent surgery during the follow-up period. This suggests that cataract and glaucoma progression, rather than ERM, significantly affected visual acuity at the end of the two-year follow-up period. Since the comparison is not between equivalent stages of ERM, caution should be taken when interpreting the visual acuity results after 2 years.
MME was identified as an independent prognostic risk factor12,13and was reported in 2.8–7.9% of glaucoma cases21,23,24], 19.9–27.0% of idiopathic ERM without glaucoma cases25,26, and 57–61% of ERM and concomitant glaucoma cases12,13. Vitreoretinal traction, inflammatory reaction, and retrograde degeneration may cause MME22. The pathophysiology of MME remains unclear; however, in eyes with ERM concomitant with glaucoma, mechanical stresses exerted by ERM traction and transsynaptic (retrograde) degeneration of Müller cells caused by glaucoma seem to result in MME. Govetto et al. reported that MME was less likely to resolve following ERM removal in eyes with both ERM and glaucoma22. However, another study showed that MME in eyes with ERM concomitant with glaucoma decreased considerably postoperatively13. This difference may be related to the severity of glaucoma. Membrane peeling can release the tractional force of ERM; however, it cannot improve retrograde degeneration induced by glaucoma. In this long-term follow-up study, the number of eyes with MME decreased notably in the peeling group, whereas it increased notably in the observation group (Fig. 1), implying that the tractional factor was released in the peeling group but persisted in the observation group. However, MME at baseline and the final visit were not associated with postoperative BCVA in the peeling group (Table 2). MME is included in the diverse clinical spectrum of cystic macular edema (CME). Postoperative persistent CME after ERM removal may indicate inflammation and is often treated with steroids or nonsteroidal anti-inflammatory drugs27. However, in cases of ERM concomitant with glaucoma, postoperative persistence of CME can be related to retrograde maculopathy caused by glaucoma. However, it seems worthwhile to resolve MME by releasing the tractional factor. MME may not contraindicate PPV with ERM peeling in eyes with ERM and OAG.
A systematic review revealed a thicker CFT and thicker ganglion cell inner plexiform layer as prognostic risk factors in ERM surgery28. However, in this study, thinner CFT was a factor for poor prognosis. Macular thickness may be reduced in eyes with severe glaucoma29,30. Thinning of the macula has been associated with retinal nerve fiber layer thinning30. Asrani et al. showed that retinal thickness was lower in eyes with deeper cupping of the optic disc or worse, VF29. In cases of severe glaucoma, CFT thinning caused by glaucomatous retinal ganglion cell degeneration may overcome CFT thickening induced by ERM traction. Disproportionately preserved macular volume in severe glaucoma was associated with MME in some studies31. Thick macula in eyes with ERM concomitant with severe glaucoma may imply MME due to dysfunction of Müller cells or degeneration of bipolar cells. In the peeling group, CFT and GCC thickness at baseline were not related to postoperative worsening of BCVA. However, CFT and GCC thickness decreased significantly after membrane peeling (Fig. 1). Thinner GCC may indicate a poor prognosis of VF in eyes with ERM concomitant with glaucoma11. Thinning of the macular volume may imply fragility of the macular structure; however, the evidence remains inconclusive. Surgical intervention may need to be avoided in eyes with lower CFT. In cases of ERM, CFT generally increases, so thinner CFT (while still thicker than normal) might indicate milder ERM that has less impact on vision. Additionally, this result could be driven by the greater influence of glaucoma or cataract progression on vision rather than ERM severity. Thus, caution should be exercised when considering CFT as a prognostic factor for surgical outcomes in cases of ERM with concurrent glaucoma.
ILM removal prevents ERM recurrence32. However, most of the ERM recurrences are not clinically notable, and the long-term effects of ILM peeling remain unclear32. ILM removal can result in DONFL appearance, which reflects damage to Müller cells16,18, and Müller cell debris adhered to peeled ILM in the DONFL area18. Decreased retinal sensitivity after ILM removal was observed15. VF defects after ILM peeling may be related to ICG toxicity14. Moreover, central retinal sensitivity, particularly on the nasal side, is more likely to deteriorate after ERM and ILM removal in cases with glaucoma9,10,11. Starting membrane peeling from the area with complete loss of VF sensitivity, avoiding ICG usage, and sparing ILM are recommended in eyes with glaucoma33. Herein, ILM removal, ICG dyeing, and DONFL did not affect outcomes.
This study had certain limitations, including its small sample size and retrospective nature. Further, PPV was not performed by a single surgeon. During the period, only the Goldmann perimeter began to be utilized from the middle instead of the Humphrey VF Analyzer, especially in severe glaucoma with central VF defect. Therefore, changes in VF after long-term observation could not be evaluated. Moreover, baseline characteristics including ERM stage, glaucoma severity, or visual functions varied between the groups, likely because retinal surgeons tend to be reluctant to perform PPV in patients with severe glaucoma despite ongoing controversy about indications in cases with ERM concomitant with OAG. In the peeling group, triamcinolone acetonide (TA)-assisted ILM peeling was performed in 25 eyes. Unlike ICG or Brilliant Blue G, TA cannot stain ILM directly. Limited visual function tests were conducted, although axial length was not measured. Fluorescein angiography was not performed, and prostaglandin eye drops, which could cause CME, were not discontinued in the follow-up period.
In conclusion, we identified MME and thinner CFT as risk factors for long-term visual deterioration in eyes with ERM concomitant with OAG. PPV with ERM peeling for eyes with OAG did not lead to critical visual deterioration over the long term; however, it improved MME. Long-term observation may help assess the visual outcomes of ERM concomitant with glaucoma because glaucomatous VF deterioration progresses slowly. Larger prospective studies are required to validate our results and examine the long-term effects on VF.
Methods
Participants
This retrospective observational study included consecutive cases with ERM concomitant with OAG who visited Osaka University Hospital between August 2017 and August 2022, with more than 2 years of follow-up since the disease diagnosis (baseline). Eyes undergoing membrane peeling with PPV in the follow-up period were assigned to the peeling group, and those without PPV were grouped into the observation group. The study protocol was approved by the Institutional Review Board of Osaka University Hospital (approval number: 09260) and adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived because this study was retrospective. The exclusion criteria were patients with diabetic retinopathy, retinal vein occlusion, uveitis, corneal disease, retinal detachment, age-related macular degeneration, macular hole, lamellar macular hole, optic neuropathy, multiple sclerosis, and any previous history of PPV. In the peeling group, cases followed up for less than 2 years after PPV were excluded. Eyes that underwent cataract or glaucoma surgery within 3 months were also excluded.
Diagnosis and treatment of OAG
A VF test (Humphrey VF Analyzer II, 30 − 2 Swedish interactive threshold algorithm, Humphrey–Zeiss instrument, Dublin, CA, USA) was performed on all eyes at baseline. Glaucomatous optic neuropathy was defined as partial or diffuse neuro-retinal rim thinning with or without a retinal nerve fiber defect. OAG was diagnosed based on the presence of glaucomatous optic neuropathy and its responsible VF defect in eyes with elevated IOP and without angle-closure4. Therefore, OAG can be diagnosed in patients with low C/D ratio if they have a partial neuro-retinal rim thinning and its responsible VF defect. OAG was diagnosed by glaucoma specialists. Central VF defect was defined as a VF defect within 10º of fixation in the 30 − 2 VF test with at least one point at P< 1% in the pattern deviation plot12,34.
Ophthalmic examinations
The medical and surgical records of all patients were reviewed retrospectively for the following parameters: age, sex, bilaterality, BCVA, MD in the VF test, CFT, EZ disruption, DONFL appearance, C/D ratio, cataract surgery, size of sclerotomy, fluid-air-exchange, agent to visualize ILM and follow-up period. BCVA was measured using the Landolt C acuity chart and converted to the logarithm of the minimum angle of resolution. Retinal and glaucoma conditions were assessed using swept-source OCT (Deep Range Imaging OCT Triton Swept Source OCT, Topcon, Japan 8 eyes), spectral-domain OCT (Cirrus HD-OCT, Carl Zeiss Meditec Inc, Germany 54 eyes), or RS-3000 (Nidek Co., Ltd., Gamagōri, Japan 38 eyes). Vertical and horizontal cross-sectional scans were acquired for each eye. ERM was staged according to the classification proposed by Govetto et al.35MME was defined as a hyporeflective cystic area with clear boundaries in the inner nuclear layer around macula area19,20. Gelfand identified MME using two adjacent B-scans19. However, because this study is retrospective and several OCT devices were utilized, we identified MME in some eyes using only one B-scan as described in a previous report20. The appearance of DONFL was evaluated using postoperative OCT16. All eyes were classified into three groups based on the C/D ratio in fundus photographs as follows: 0.1–0.4 (mild), 0.5–0.7 (moderate), and over 0.8 (advanced)13. EZ disruption was defined as the discontinuation of EZ. ERM stage, MME, EZ disruption, DONFL, and C/D ratio were assessed by two masked graders (M.K. and T.O.), and discrepancies were resolved by S.S. CFT and GCC thickness were measured by a single observer (M.K.) who was blinded to patients’ clinical information using the ImageJ software (National Institutes of Health) to delete segmentation errors.
Procedures of PPV with membrane peeling
In total, 31 eyes of 26 patients underwent PPV with a 25-gauge or 27-gauge vitrectomy system (CONSTELLATION, Alcon Inc., Germany) under retrobulbar anesthesia by surgical retina specialists. Sclerotomy ports were made in the pars plana, and core vitrectomy was performed after the vitreous gel was visualized by injecting TA into the vitreous cavity. Posterior vitreous detachment was created when it was not present. The peripheral vitreous was shaved with scleral indentation. ERM was peeled using micro forceps with TA. The peeling began from the area with the VF defect in severe glaucoma. After ERM peeling, ILM was confirmed and peeled using TA, Brilliant Blue G, or ICG to prevent recurrent ERM. Fluid-air exchange and maintenance of a postoperative facedown position for a couple of days were recommended in only one case where retinal tears were observed during surgery. Combined phacoemulsification and intraocular lens implantation were performed on patients with cataracts.
Statistical analysis
Independent or paired t-tests and Mann–Whitney U tests were used to compare the averages of continuous variables. The chi-squared, Fisher exact, and McNemar tests were used to compare the proportions of categorical variables between groups. Univariate and multivariate logistic regression analyses assessed prognostic risk factors associated with visual acuity worsening. R (R Foundation, version 4.1.2) and Statcel software (Statcel, version 4, OMS Ltd.) were used for all statistical analyses. A two-sided P value < 0.05 was statistically significant.
Data availability
The datasets used and analyzed in this study are available from the corresponding author on reasonable request.
Change history
11 March 2025
The original online version of this Article was revised: In the original version of this article, Table 3 did not display correctly. This error has now been corrected in the PDF version of the Article; the HTML version was correct from the time of publication.
References
Bu, S. C., Kuijer, R., Li, X. R. & Hooymans, J. M. Los, L. I. Idiopathic epiretinal membrane. Retina 34, 2317–2335 (2014).
Ng, C. H. et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology 118, 694–699 (2011).
Okamoto, F., Sugiura, Y., Okamoto, Y., Hiraoka, T. & Oshika, T. Time course of changes in aniseikonia and foveal microstructure after vitrectomy for epiretinal membrane. Ophthalmology 121, 2255–2260 (2014).
Foster, P. J., Buhrmann, R., Quigley, H. A. & Johnson, G. J. The definition and classification of glaucoma in prevalence surveys. Br. J. Ophthalmol. 86, 238–242 (2002).
Medeiros, F. A. et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am. J. Ophthalmol. 139, 44–55 (2005).
Asrani, S., Essaid, L., Alder, B. D. & Santiago-Turla, C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol. 132, 396–402 (2014).
Lee, J. Y., Sung, K. R. & Kim, Y. J. Comparison of the prevalence and clinical characteristics of epiretinal membrane in pseudoexfoliation and primary open-angle glaucoma. J. Glaucoma. 30, 859–865 (2021).
Sakimoto, S. et al. Cross-sectional imaging analysis of epiretinal membrane involvement in unilateral open-angle glaucoma severity. Invest. Ophthalmol. Vis. Sci. 59, 5745–5751 (2018).
Tsuchiya, S., Higashide, T. & Sugiyama, K. Visual field changes after vitrectomy with internal limiting membrane peeling for epiretinal membrane or macular hole in glaucomatous eyes. PLoS One. 12, e0177526 (2017).
Kaneko, H. et al. Effect of internal limiting membrane peeling on visual field sensitivity in eyes with epiretinal membrane accompanied by glaucoma with hemifield defect and myopia. Jpn J. Ophthalmol. 65, 380–387 (2021).
Tsuchiya, S., Higashide, T., Udagawa, S. & Sugiyama, K. Glaucoma-related central visual field deterioration after vitrectomy for epiretinal membrane: Topographic characteristics and risk factors. Eye (Lond). 35, 919–928 (2021).
Ko, Y. C. et al. Factors related to unfavorable visual outcome after idiopathic epiretinal membrane surgery in patients with glaucoma. Retina 42, 712–720 (2022).
Peck, T. et al. Epiretinal membrane surgery in eyes with Glaucoma: Visual outcomes and clinical significance of inner microcystoid changes. Ophthalmol. Retina 6, 693–701 (2022).
Uemura, A., Kanda, S., Sakamoto, Y. & Kita, H. Visual field defects after uneventful vitrectomy for epiretinal membrane with indocyanine green-assisted internal limiting membrane peeling. Am. J. Ophthalmol. 136, 252–257 (2003).
Tadayoni, R., Svorenova, I., Erginay, A., Gaudric, A. & Massin, P. Decreased retinal sensitivity after internal limiting membrane peeling for macular hole surgery. Br. J. Ophthalmol. 96, 1513–1516 (2012).
Tadayoni, R. et al. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology 108, 2279–2283 (2001).
Mitamura, Y. & Ohtsuka, K. Relationship of dissociated optic nerve fiber layer appearance to internal limiting membrane peeling. Ophthalmology 112, 1766–1770 (2005).
Steel, D. H., Dinah, C., White, K. & Avery, P. J. The relationship between a dissociated optic nerve fibre layer appearance after macular hole surgery and Muller cell debris on peeled internal limiting membrane. Acta Ophthalmol. 95, 153–157 (2017).
Gelfand, J. M., Nolan, R., Schwartz, D. M., Graves, J. & Green, A. J. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 135, 1786–1793 (2012).
Burggraaff, M. C., Trieu, J., de Vries-Knoppert, W. A., Balk, L. & Petzold, A. The clinical spectrum of microcystic macular edema. Invest. Ophthalmol. Vis. Sci. 55, 952–961 (2014).
Mahmoudinezhad, G. et al. Risk factors for microcystic macular oedema in glaucoma. Br. J. Ophthalmol. 107, 505–510 (2023).
Govetto, A. et al. Microcystoid macular changes in association with idiopathic epiretinal membranes in eyes with and without glaucoma: Clinical insights. Am. J. Ophthalmol. 181, 156–165 (2017).
Hasegawa, T. et al. Microcystic inner nuclear layer changes and retinal nerve fiber layer defects in eyes with glaucoma. PLoS One 10, e0130175 (2015).
Brazerol, J. et al. Retrograde maculopathy in patients with glaucoma. J. Glaucoma 26, 423–429 (2017).
Lee, D. H., Park, S. E. & Lee, C. S. Microcystic macular edema and cystoid macular edema before and after epiretinal membrane surgery. Retina 41, 1652–1659 (2021).
Yang, X. et al. Clinical features and prognosis in idiopathic epiretinal membranes with different types of intraretinal cystoid spaces. Retina 42, 1874–1882 (2022).
Zur, D., Fischer, N., Tufail, A., Mones, J. & Loewenstein, A. Postsurgical cystoid macular edema. Eur. J. Ophthalmol. 21, S62–68 (2011).
Miguel, A. I. & Legris, A. Prognostic factors of epiretinal membranes: A systematic review. J. Fr. Ophtalmol 40, 61–79 (2017).
Asrani, S. et al. Correlation among retinal thickness, optic disc, and visual field in glaucoma patients and suspects: A pilot study. J. Glaucoma. 12, 119–128 (2003).
Greenfield, D. S., Bagga, H. & Knighton, R. W. Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch. Ophthalmol. 121, 41–46 (2003).
Wen, J. C., Freedman, S. F., El-Dairi, M. A. & Asrani, S. Microcystic macular changes in primary open-angle glaucoma. J. Glaucoma 25, 258–262 (2016).
Diaz-Valverde, A. & Wu, L. To Peel or not to Peel the Internal limiting membrane in idiopathic epiretinal membranes. Retina 38, S5–S11 (2018).
Yoshida, M., Kunikata, H., Kunimatsu-Sanuki, S. & Nakazawa, T. Efficacy of 27-gauge vitrectomy with internal limiting membrane peeling for epiretinal membrane in glaucoma patients. J. Ophthalmol. 7807432 (2019).
Park, S. C. et al. Initial parafoveal versus peripheral scotomas in glaucoma: Risk factors and visual field characteristics. Ophthalmology 118, 1782–17893 (2011).
Govetto, A., Lalane, R. A., Sarraf, D., Figueroa, M. S. & Hubschman, J. P. Insights into epiretinal membranes: Presence of ectopic inner foveal layers and a new optical coherence tomography staging scheme. Am. J. Ophthalmol. 175, 99–113 (2017).
Author information
Authors and Affiliations
Contributions
Overall responsibility: SS. Study supervision: SS and KN. Conception and design: MK, SS and KN. Data collection: MK and SSAnalysis and interpretation: MK, SS, MS, DS, TO, AS, KN, KM, SU, SS, KM, TM and KNManuscript draft and review: MK, SS, TM and KNAll authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kanai, M., Sakimoto, S., Suzue, M. et al. Long-term risk factors for poor visual outcomes in patients with epiretinal membrane and open-angle glaucoma: a retrospective study. Sci Rep 14, 28660 (2024). https://doi.org/10.1038/s41598-024-80020-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80020-1