Abstract
Objectives: Investigate the relationship between bone mineral density (BMD) at various body sites and dental caries. Materials and methods: Based on the National Health and Nutrition Examination Survey (NHANES) database from 2011 to 2016, the correlation between BMD at various body sites and the DMFS index among 7044 adults aged 20–59 years was analyzed. Multiple linear regression, restricted cubic splines (RCS), piecewise linear regression, logistic regression, weighted quantile sum regression (WQS) and mediation effects analysis were integrated to explore the relationship between BMD and dental caries. Results: Under the linear assumption, except for arm BMD, the BMDs of all other sites are negatively correlated with the DMFS index of dental caries. RCS analysis indicates a U-shaped relationship between head BMD and the DMFS index (p for nonlinear < 0.0001). WQS analysis indicates that mixed BMD is significantly negatively correlated with the DMFS index for dental caries (estimate, − 0.023; 95% CI, − 0.025 ~ − 0.020), and head BMD has the most significant impact on the DMFS index (weight = 91.4%). Simple mediation analysis of the effect of dental caries on BMD levels mediated by inflammation levels showed negative results, suggesting that dental caries may not influence BMD through inflammation levels. Conclusion: Monitoring BMD should be combined with appropriate oral healthcare and caries management strategies to effectively address these interconnected health issues, and particular attention should be paid to the monitoring of head BMD.
Similar content being viewed by others
Introduction
Dental caries, stands as one of the most common chronic diseases globally1, affecting individuals of all ages2,3. The connection between bone health and oral diseases has been substantiated through numerous studies. For instance, osteoporosis has been linked to decreased alveolar bone height4, while BMD has been shown to influence caries and periodontitis5,6. Furthermore, research has explored the relationship between BMD and caries across diverse populations and obtained many in-depth insights. Kostik MM and colleagues discovered a negative correlation between caries staging and BMD in children7. Among Korean males and postmenopausal females with osteoporosis, the caries DMFT indices are higher compared to those with normal BMD8. Additionally, studies by Fabiani L et al. and Rezazadeh F et al. highlighted younger age and higher BMD as protective factors against caries in European adolescents5, and a negative correlation between the DMFT index and spinal femoral T-score in Iranian women9, respectively. These findings underscore the intricate relationship between BMD and dental health across varied demographics. However, compared to other oral diseases, the linkage between dental caries and BMD has received less attention, with considerable variability in sample sizes, measurement techniques, and demographics across studies, potentially skewing outcomes. Addressing these gaps, our research leverages the NHANES dataset, employing the authoritative DXA technique for bone densitometry over six years (2011–2016) to secure a robust sample size. This study aims to delve into the relationship between BMD at different body sites and dental caries in adults aged 20–59, with the goal of gaining insights from various perspectives.
There is a correlation between BMD and dental caries, however the causal direction between them has not yet been established. Osteoporosis may influence the occurrence of dental caries by reducing the height of the alveolar bone, among other mechanisms4; Dental caries can trigger inflammatory responses, which inhibit metabolic pathways, reduce erythropoiesis, and interfere with systemic or local bone metabolism10,11,12. Therefore, there may be a bidirectional causal relationship between BMD and dental caries. This study aims to explore the mediating variables that influence the relationship between BMD and dental caries through mediation effect analysis, providing new insights into the complex relationship between dental caries and BMD.
Methods
Study design and study population
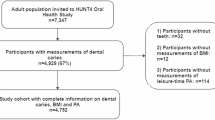
The analysis specifically includes data from adults aged 20–59 years who participated in the 2011–2012, 2013–2014, and 2015–2016 NHANES cycles. From 2011 to 2016, a total of 17,508 participants underwent whole-body DXA scans for BMD measurements. The applied exclusion criteria are as follows: lack of BMD data (n = 3777) and absence of caries DMFS data or other covariates, including socio-demographic data, BMI, smoking status, presence of a family smoker, hypertension, and diabetes (n = 6687). The flowchart is shown in Fig. 1.
Variables
BMD data were collected from all parts of the body, including the head, left arm, left leg, right arm, right leg, left ribs, right ribs, thoracic spine, lumbar spine, pelvis, trunk bone, and total body bone. For ease of analysis, BMD was defined as the arithmetic mean of left versus right for arms, legs, and ribs. Because the T-score and Z-score currently are mostly used to determine the presence of osteoporosis, and there is no standard definition of high BMD for the time being14,15,16, this study utilizes percentiles to segment BMD intervals for logistic regression analysis. BMD data were collected from the NHANES ‘Dual-Energy X-ray Absorptiometry - Whole Body’ data tables for 2011–2016.
The collection of the DMFS index involves assessing untreated carious surfaces (DS), missing teeth due to caries (MS), and previously treated carious surfaces in permanent teeth (FS). This index sums the number of these components, with higher values indicating a greater extent of dental decay, loss, and interventions. The DMFS index, by evaluating the condition of each tooth surface, provides a more sensitive measure of caries severity compared to the DMFT index and offers a detailed insight into oral health status. DMFS data were collected from the NHANES ‘Oral Health - Dentition’ data tables for 2011–2016.
Other collected covariates included sociodemographic data (race, sex, age, education, and income), BMI, smoking status, presence of family smokers, hypertension, and diabetes. Race was categorized into Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Non-Hispanic Asian, and the others (including Multiracial). Education levels were classified as less than 9th grade, 9th-11th grade, high school graduate/GED or equivalent, some college or AA degree, and college graduate or above. Household income relative to the poverty threshold represented income level. Smoking status was categorized as never smoker, current smoker, and former smoker.
A directed acyclic plot of the above variables was constructed in DAGitty v3.1 to filter out the smallest sufficient adjustment set (including age, cigarette use, diabetes, gender, household smokers, hypertension, race) to be used as the adjustment set of covariates for subsequent analyses. The plotted directed acyclic graphs are shown in supplementary Figure S1.
Statistical analysis
The primary sampling units and strata of the complex NHANES survey design were considered in the data analysis. Sampling weights, stratification and clustering provided in the NHANES dataset were incorporated into the analysis to obtain the appropriate estimates. All analyses were performed in RStudio, except the mediated effects analysis.
Study of linear and nonlinear relationships: Using BMD values from various body sites as independent variables and adjusting for all covariates, this study constructs nine multivariate linear regression models with the DMFS index as the dependent variable. It then investigates the nonlinear relationships between BMD at these sites and the DMFS index using RCS analysis. RCS analyses are conducted both with and without adjustments for all covariates, underscoring the findings’ robustness. Additionally, piecewise linear regression is used to calculate the breakpoint positions in the relationship between BMD at various sites and the DMFS index.
Binary Logistic Regression: Binarize the DMFS index into a binary variable (DMFS = 0 and DMFS > 0), and divide the BMD data of each site into quartiles for binary logistic regression analysis.
WQS analysis: The WQS regression is used to assess the combined impact of BMD indicators at various sites on the DMFS index for dental caries, as well as the proportional weights of each site.
Mediation effect analysis: To determine whether four inflammatory markers serve as mediating variables in the relationship between dental caries and BMD, this study conducts an analysis using PROCESS v4.3 in SPSS.
Results
Basic characteristics of participants
Table 1 shows a comparison of each of the characteristics of participants with caries (DMFS > 0) and without caries (DMFS = 0). Normality tests were conducted for data categorized into caries-experienced and non-caries-experienced groups, revealing non-normal distributions across all datasets. Consequently, Mann-Whitney U test and chi-square test were used to calculate the p-value. Individuals with a history of caries were found to generally possess a higher BMI and be older. They were also more likely to be current or former smokers, exposed to secondhand smoke, and suffer from diabetes or hypertension. There are also certain differences in BMD levels at different sites between populations with and without caries. To demonstrate the distribution of each variable, violin plots were also created in this study (see supplementary Figure S2).
Study of linear and nonlinear relationships
In view of previous studies showing a possible negative correlation between BMD and caries5,7,8,9, the present study explored the linear relationship between BMD and DMFS using multiple linear regression analysis in the NHANES population based on the assumption that there is a linear relationship between BMD at each site and DMFS for caries. Nine multiple linear regression models were built by using BMD at each site as the independent variable and caries DMFS index as the dependent variable, and all covariates were included in the adjustments, resulting in the final results presented in the supplementary Table S4. The results showed that except for the arm BMD and caries DMFS linear correlation could not be confirmed, the negative correlation between BMD and caries DMFS for all sites reached a significant level. However, the adjusted R-squared values in the multivariate linear regression model were relatively low (see supplementary Table S3), indicating that the relationship between BMD at various sites or other covariates and caries DMFS may be more complex and nonlinear. Despite the low R-squared values, this does not necessarily undermine the reliability of the significant negative correlations identified in the multivariate linear regression model.
To elucidate the nonlinear relationships between BMD at various sites and the DMFS index, we conducted RCS analysis on the entire sample. The results are shown in Fig. 2. From the figure, it can be seen that the head BMD and DMFS index exhibit a U-shaped relationship (p for nonlinear < 0.0001). Apart from the possibility of a more complex non-linear relationship between arm BMD and the DMFS index, RCS plots for other sites demonstrate an L-shaped relationship where the DMFS index for dental caries initially decreases rapidly and then slowly decreases (or even slightly increases) as BMD increases. Finally, to determine the risk thresholds of BMD at various sites for the impact on dental caries, aiding in clinical decision-making, this study calculated the breakpoints of BMD at each site using piecewise linear regression, with results shown in the supplementary Table S1.
Binary logistic regression
Given the interpretability of the logistic regression model and the ability to adapt more intuitively to the non-linear relationship between the independent and dependent variables compared to the RCS analysis, the dependent variable was defined in this study as a dichotomous variable (with caries: DMFS > 0 and without caries: DMFS = 0), and the BMD at each site was divided according to quartiles and analyzed by logistic regression, using Q1 as a reference.
As shown in the binary logistic regression results in Table 2, individuals in the highest quartile of arm BMD had a 48% higher risk of dental caries compared to those in the lowest quartile. In Model 2, individuals in the highest quartile of head BMD had a 30% increased risk of dental caries compared to those in the lowest quartile, although this did not reach the significance level of p = 0.05, it did reach p = 0.1, suggesting that a relatively high head BMD may increase the risk of dental caries. This finding is consistent with the U-shaped relationship between head BMD and the DMFS index found in the RCS analysis.
WQS analysis
To investigate which BMD location has the greatest impact on the DMFS index, this subsection continued with a WQS regression analysis. The model is adjusted for all covariates, as shown in the Table 3. WQS analysis shows that mixed BMD is significantly negatively correlated with the DMFS index for dental caries (estimate, − 0.023; 95% CI, − 0.025 ~ − 0.020). Head BMD has the most significant impact on the DMFS index (weight = 91.4%), followed by lumbar spine BMD (weight = 6.56%).
Discussion
Considering previous studies that have demonstrated a negative correlation between BMD levels and dental caries5,7,8,9, this study initially hypothesized a linear relationship between BMD and dental caries. Using multiple linear regression, we analyzed the linear relationships between BMD at various sites and the DMFS index among NHANES participants. The results showed a negative correlation between BMD at all sites except the arms and the DMFS index, confirming earlier research. Notably, the relationship between arm BMD and the DMFS index may be more complex and non-linear, leading to non-significant linear results (in RCS analysis, as arm BMD increased, the DMFS index initially decreased, slightly increased, and then decreased again, showing a non-linear trend). Additionally, logistic regression analysis indicated that individuals in the highest quartile for arm BMD had a 1.48 times higher risk of caries compared to those in the lowest quartile, which differs from previous studies and warrants further investigation. Moreover, RCS analysis revealed a U-shaped relationship between head BMD and the DMFS index, where both low and high head BMD increased the risk of caries. This relationship has been rarely reported in past studies. A possible explanation for this U-shaped curve is that low head BMD might signify bone loss in the head, which could lead to osteoporosis and a decrease in alveolar bone height4, subsequently causing gum recession and food impaction that leads to secondary caries; there is a correlation between BMD and chewing function13, and high head BMD might indicate excessive chewing, which could cause tooth wear, reducing the teeth’s resistance to erosion and increasing the risk of caries. The pathogenesis of dental caries is still unclear, and growing evidence suggests that genetic susceptibility plays a critical role in the development of dental caries14,15. The U-shaped relationship between cranial BMD and the DMFS index may also be due to genetic differences at the molecular level.
The WQS regression, a tool used for studying the combined impact of multiple exposure factors on health outcomes, indicated a negative joint effect of BMD at various sites on dental caries (estimate, − 0.023; 95% CI, − 0.025 ~ − 0.020). Moreover, head BMD was identified as the most significant influencing variable (weight = 91.4%), suggesting that compared to other sites, head BMD requires the most attention and management as a risk factor. The occurrence of caries in the teeth of the head might explain why head BMD has the greatest impact on the DMFS index.
There may be a bidirectional causal relationship between BMD and dental caries. Osteoporosis can lead to reduced alveolar bone height, which may cause gum recession and food impaction, eventually leading to dental caries. Severe dental caries can result in difficulty chewing, affecting food intake and overall metabolic levels, which in turn can influence BMD. Analysis shows a negative correlation between BMD and the DMFS index of dental caries; however, the mechanisms by which they affect each other are not fully understood. Therefore, this study conducted an exploratory mediation effect analysis on potential features that could act as mediators between dental caries and BMD.
Dental caries can trigger inflammatory responses in and around the tooth, and the oral microbiota associated with caries can spread through the root canal to the body, subsequently altering systemic inflammation levels and being associated with various systemic diseases16,17,18. Moreover, long-term chronic inflammation may negatively affect bone calcium balance19,20. Considering that dental caries might influence BMD levels through inflammatory responses, this study used four systemic immune markers as mediators: the systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), to explore whether these markers mediate the effect of dental caries on BMD. The results (see Supplementary Table S2) indicate that NLR may have a masking effect on the impact of dental caries on lumbar spine BMD, possibly because NLR is more sensitive and direct in reflecting the systemic inflammatory response caused by caries on lumbar spine BMD, but this result should be interpreted with caution. The other mediation analyses did not reach significant levels, suggesting that these four inflammatory markers may not be effective or primary pathways connecting dental caries and BMD levels. There may be more complex nonlinear causal relationships between dental caries, inflammation levels, and BMD that are difficult to capture through simple mediation effect analysis, and further exploration using methods such as structural equation modeling (SEM) may be needed in the future. Although there may be a bidirectional causal relationship between BMD and dental caries, this study did not explore the potential mediating effects through which BMD might influence dental caries, due to the lack of relevant characteristic data in NHANES that would allow for identifying potential mediators.
This study also has some limitations. Due to the use of a cross-sectional design, it is not possible to assess the causal relationship between BMD and the DMFS index. Additionally, in the multivariate linear regression model, the BMD at various sites (except for arm BMD) was significantly negatively correlated with the DMFS index. A similar decreasing trend was observed in the RCS model, and the nonlinear relationships between BMD at most sites and the DMFS index reached significant levels. However, the adjusted R-squared values in the multivariate linear regression model were relatively low (see supplementary Table S3), indicating that the relationship between BMD at various sites or other covariates and caries DMFS may be more complex and nonlinear. Despite the low R-squared values, this does not necessarily undermine the reliability of the significant negative correlations identified in the multivariate linear regression model. Future research may need to include other potential important variables in the model and more carefully consider the nonlinear relationships among variables in the multivariate linear regression model to enhance the model’s interpretability.
Conclusion
Under the linear assumption, except for arm BMD, the BMDs of all other sites are negatively correlated with the DMFS index of dental caries. RCS analysis indicates a U-shaped relationship between head BMD and the DMFS index. WQS analysis indicates that head BMD has a greater impact on the DMFS index for dental caries compared to BMD at other sites. Simple mediation analysis of the effect of dental caries on BMD levels mediated by inflammation levels showed negative results, suggesting that dental caries may not influence BMD through inflammation levels. In conclusion, monitoring BMD should be combined with appropriate oral healthcare and caries management strategies to effectively address these interconnected health issues, with particular attention to the monitoring of head BMD.
Data availability
All data supporting the findings of this study are openly available in the Zenodo repository at the following persistent link: https://zenodo.org/records/13318909. We confirm that this link has been accurately referenced in the data availability statement within the submission system to ensure transparency and accessibility of the data.
References
Selwitz, R. H., Ismail, A. I. & Pitts, N. B. Dental caries. Lancet 369 (9555), 51–59 (2007).
Al-Nasser, L. & Lamster, I. B. Prevention and management of periodontal diseases and dental caries in the older adults. Periodontology 2000. 84 (1), 69–83 (2020).
Zhang, J., Sardana, D., Wong, M. C., Leung, K. C. & Lo, E. C. Factors associated with dental root caries: a systematic review. JDR Clin. Translational Res. 5 (1), 13–29 (2020).
Payne, J. B., Reinhardt, R. A., Nummikoski, P. V. & Patil, K. D. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos. Int. 10, 34–40 (1999).
Fabiani, L. et al. Dental caries and bone mineral density: a cross sectional study. Eur. J. Paediatr. Dent. 7 (2), 67–72 (2006).
Kim, J. W. et al. The association between bone mineral density and periodontitis in Korean adults (KNHANES 2008–2010). Oral Dis. 20 (6), 609–615 (2014).
Kostik, M. M., Kuzmina, D. A., Novikova, V. P., Larionova, V. I. & Scheplyagina, L. A. Caries in adolescents in relation to their skeletal status. J. Pediatr. Endocrinol. Metab. 28 (3–4), 399–405 (2015).
Lee, Y. H. & Myong, J. P. Relationship between bone Mineral Density and Dental Caries in koreans by Sex and Menopausal State. Int. J. Environ. Res. Public Health. 19 (11), 6917 (2022).
Rezazadeh, F., Emad, S. & Emad, M. Relationship between bone mineral density and oral health status among Iranian women. Int. J. Prev. Med. ;10. (2019).
Sheiham, A. Dental caries affects body weight, growth and quality of life in pre-school children. Br. Dent. J. 201 (10), 625–626 (2006).
Farges, J. C. et al. Dental pulp defence and repair mechanisms in dental caries. Mediat. Inflamm. 2015 (1), 230251 (2015).
Redlich, K. & Smolen, J. S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discovery. 11 (3), 234–250 (2012).
Hasegawa, Y. et al. The relationship between bone density and the oral function in older adults: a cross-sectional observational study. BMC Geriatr. 21, 1–0 (2021).
Li, X., Su, Y., Liu, D. & Yang, J. The association between genetic variants in lactotransferrin and dental caries: a meta-and gene-based analysis. BMC Med. Genet. 21, 1–8 (2020).
Werneck, R. I., Mira, M. T. & Trevilatto, P. C. A critical review: an overview of genetic influence on dental caries. Oral Dis. 16 (7), 613–623 (2010).
Sabharwal, A., Stellrecht, E. & Scannapieco, F. A. Associations between dental caries and systemic diseases: a scoping review. BMC Oral Health. 21, 1–35 (2021).
Scannapieco, F. A. & Cantos, A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontology 2000. 72 (1), 153–175 (2016).
Scannapieco, F. A. The oral microbiome: its role in health and in oral and systemic infections. Clin. Microbiol. Newsl. 35 (20), 163–169 (2013).
Mazzaferro, S. et al. Bone, inflammation and chronic kidney disease. Clin. Chim. Acta. 506, 236–240 (2020).
Wu, D. et al. T-cell mediated inflammation in postmenopausal osteoporosis. Front. Immunol. 12, 687551 (2021).
Funding
Natural Science Foundation of Zhejiang Province (LY22H270002, LY24H270007), China Postdoctoral Science Foundation (2023TQ0295); Postdoctoral research projects merit-based funding in Zhejiang province (First-class funding) (ZJ2023021); the China Scholarship Council (No.202308330210).
Author information
Authors and Affiliations
Contributions
Haonan Zhang:Conceptualization, Methodology, Writing – original draft. Weifeng Jin: Writing - review and editing, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The cohort included in this study received approval of Ethics Review Board of the National Center for Health Statistics to enroll patients, and all participants provided written informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, H., Jin, W. New insights into the correlation between bone mineral density and dental caries in NHANES 2011–2016. Sci Rep 14, 29143 (2024). https://doi.org/10.1038/s41598-024-80109-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80109-7
Keywords
This article is cited by
-
Dental treatment affordability and oral outcomes among U.S. adults: NHANES 2015–2018
BMC Oral Health (2025)