Abstract
In this study, we investigated the influence of the inclusion of Tenebrio molitor (TM) larvae meal in the diet on the diversity and structure of the bacterial community in the caecal content of Barbary partridges. A total of 36 partridges, selected randomly for slaughter from 54 animals, were divided equally into three treatment groups, including the control group (C) with a diet containing corn-soybean meal and two experimental groups, in which 25% (TM25) and 50% (TM50) of the soybean meal protein was replaced by the meal from TM larvae. After slaughtering, the bacterial community of the 30 caecal samples (10 samples per each experimental group) was analysed by high-throughput sequencing using the V4–V5 region of the 16 S rRNA gene. Alpha diversity showed a higher diversity richness in the TM50 group. Beta diversity showed statistical dissimilarities among the three groups. Firmicutes was the dominant phylum regardless of the diet, with the predominant families Ruminococcaceae and Lachnospiraceae. Clostridia and Faecalibacterium were decreased in both TM groups, Lachnospiraceae was suppressed in the TM50 group, but still this class, genus and family were abundantly present in all samples. Several potentially beneficial genera, such as Bacillus, Ruminococcaceae UCG-009, Oscillibacter and UC1-2E3 (Lachnospiraceae) were increased in the TM50 group. The results showed a beneficial effect of the T. molitor larvae meal on the caecal microbiota of Barbary partridges, particularly in the TM50 group, which showed an increase in bacterial diversity.
Similar content being viewed by others
Introduction
Poultry production has become an important sector of the human diet because of the increasing demand for poultry meat and eggs1,2. Game birds such as pheasants, quails and partridges are not as commercially produced as other poultry species, but interest in their captive breeding is increasing due to their nutritional and economic value3,4. Barbary partridge (Alectoris barbara) is an avian species wildly abundant in North-West Africa, Gibraltar, Canary Islands and Sardinia5,6,7. The European population of Barbary partridge is estimated at 7,500–20,000 pairs, of which about half (5,000–10,000 breeding pairs) occur in Sardinia (Italy)8. Partridges provide an excellent source of protein for human consumption and their popularity is increasing, due to interest in the gourmet food market demand of game meat3. Regardless of the composition of the feed used during rearing, partridge meat is high in protein, low in fat3 and characterised by good organoleptic properties9. Despite these positive characteristics, research on partridges as a valuable food source is very limited and almost non-existent for the Barbary partridge species, with the exception of the works of Loponte et al.10 and Secci et al.3, who investigated various aspects of Alectoris barbara growth performance, carcass characteristics, meat proteins and fat composition as a function of the addition of insect meal into the diet.
Insect meals are increasingly becoming accepted, recognised and valued sustainable source of protein for animal feeding being rich in digestible essential amino acids, monounsaturated and polyunsaturated fatty acids, minerals, vitamins and other bioactive compounds11,12,13. Tenebrio molitor (TM), also known as yellow mealworm, is one of the most widely consumed insects in the world14 and is known to have good nutritional value, digestibility of nutrients and essential amino acids, particularly for monogastric livestock species, and functional ability15,16. These properties are good prerequisites for its use in poultry nutrition. Most studies have reported positive effects of including T. molitor meal in broiler diets on body weight gain17,18,19,20,21,22,23,24,25. On the other hand, several works showed no significant effects on growth performance and carcass characteristics26,27,28. However, the results regarding the effects on feed intake (FI) and feed conversion ratio (FCR) are inconsistent, as well summarised in the review articles11,29.
Insect meal also affects the gastrointestinal microbiota and induced changes could be a valuable parameter to assess the impact on animal performance and health status. The gut microbiome plays an essential role in the intestinal development, metabolic homeostasis, decomposition of food substrates, energy and nutrient production, protection against pathogens and development of the host’s immune system30. It has a major impact on productivity and any negative effect on the gut microbiome could compromise poultry health and negatively affect nutrient utilisation31,32,33,34. The effect of TM meal on the microbiome has mainly been studied in broiler chickens19,35,36,37,38. Although the number of studies is still rather limited, they indicate a positive influence of yellow mealworm diet supplementation on selected microbial taxa and the intestinal health of broilers. However, this research is completely lacking in partridges. The gut microbiome of partridges has been studied in the context of comparing different species and breeds39,40,41 or different insect-free diets42,43,44. Loponte et al.10 and Secci et al.3 investigated the effect of TM meal on wild partridges raised in captivity, both papers indicated the positive effect on growth performance, blood profiles, carcass characteristics and meat quality. These conclusions prompted our study, which focused on the effects of T. molitor larvae meal on the microbiota in the caecum of Barbary partridges, since there is an insufficient knowledge of how these novel feed ingredients affect the gut microbiota of game birds. This gap is significant because the gut microbiota plays a crucial role that directly impacts the birds’ growth performance and meat quality45. Barbary partridge, with its unique evolutionary adaptations and increasing economic importance in the game bird market, may respond differently to dietary changes compared to conventional poultry species. Understanding these specific effects is crucial for development of tailored, sustainable feeding practices that could enhance the health, performance, and economic viability of Barbary partridge farming while meeting the growing demand for alternative protein sources in animal feed46.
The aim of this study is to investigate the effect of soybean meal protein partial substitution (25% and 50%) with T. molitor meal as protein source in the diet on the caecal bacterial population, which was assessed by high throughput sequencing (HTS) of 16 S rRNA fragments. To our knowledge, this is the first study evaluating the effect of dietary yellow mealworm inclusion into diet on gut bacterial diversity, community structure and taxonomic composition of Barbary partridges.
Materials and methods
Ethical statement
The animals were treated humanely and all procedures were carried out in accordance with the European legislation Directive 2010/63/EU on the protection of animals used for scientific purposes. The experimental protocol was approved by the Ethical Animal Care and Use Committee of the University of Napoli Federico II (Prot. n. 2017/ 0017676). The study design and experimental procedures and animal handling were performed in accordance with the published ARRIVE recommendations published by Percie du Sert et al.47.
Animals and diet
The experiment was carried out on a private partridge farm located in Sardinia (Italy), with prior consent from the farm owner. A total of 54 seven-day-old Barbary partridges with an average weight of 25.16 g ± 2.98 g were divided into three dietary treatment groups (6 replicates, 3 partridges per replicate). The animals were housed in cages (100 cm long × 40 cm deep × 40 cm high) and reared until 64 days of age. Initially, the cages were placed under infrared lamps at a distance of 25 cm from the bottom of the cages to ensure a temperature of 32–35 °C at the bird level. The temperature was then gradually lowered until it reached 23–24 °C at 28 days of age. The trial took place under natural lighting (12–13 h of light/day). The birds were fed 3 isoproteic and isoenergetic diets. The control group (C) received a diet based on corn-soybean meal, while in the experimental groups TM25 and TM50, 25% and 50% of the soybean meal protein was replaced by full-fat Tenebrio molitor larvae meal (Gaobeidian Shannon Biology Co., Ltd., Shannong, P. R. China), respectively. More details on the experimental design and diet are described in the previous study10. In brief, feed and fresh water were administered ad libitum. The chemical nutritional characteristics of the protein sources (Table 1) were used to formulate the diets, taking into consideration the needs of the birds (NRC, 1994). The apparent metabolisable energy values for the insect meals used in this study were obtained from the broiler studies of De Marco et al.48. Feed samples were taken eight times at regular intervals during the study to analyse the content of the feed. Their chemical composition was evaluated according to the methods of the AOAC (2004): DM (method 934.01), ether extract (method 920.39), ash (method 942.05), CP (method 954.01) and ADF (method 973.18; AOAC, 2004). In addition, ADF and residual nitrogen in ADF (ADFN; method 954.01; AOAC, 2004) were assessed for the Tenebrio molitor diets and used to estimate the amount of chitin according to Marono et al.49. According to the content of the diet ingredients provided by the producer, the content of essential amino acids was estimated. The metabolisable energy content of the diets was determined based on their chemical composition using the NRC (1994) Eq. 50. The protein contents in TM meals were calculated according to Janssen et al.51. The ingredients and chemical-nutritional characteristics of the diets were calculated and listed in the Table 2.
ADIP protein linked to ADF, ADF acid detergent fibre.
Samples collection
A total of 12 birds per treatment group (2 per replicate) were randomly selected and slaughtered in a specialised slaughterhouse at 64 days of age. The entire gastrointestinal tubes were removed and the caeca were excised in a sterile manner. Their luminal contents were carefully squeezed into sterile microcentrifuge tubes (2.0 ml, Eppendorf®), immediately refrigerated (0° C for about 1 h), transported to the laboratory and stored at -80° C. Caeaca with small intestinal contents were excluded from the analysis and 10 samples for each group were used for microbiota analysis. The samples were lyophilised using the Heto powerdry LL3000 freeze dryer (Thermo Fisher Scientific, Wilmington, DE, USA) and transported to the Institute of Animal Physiology and Genetics of the Czech Academy of Sciences (Prague, Czech Republic) for further analysis. The characteristics of the animals are listed in Table S1 (Supplementary Material).
DNA extraction
Genomic DNA was extracted from the dry caecal samples using the PowerSoil DNA Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocols. The concentration and purity of nucleic acids (ratio 260/280) were checked using a NanoDrop 2000c UV-Vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The eluted DNA was stored at − 20 °C until required for analysis.
Amplification and purification of 16 S rRNA gene
DNA from each sample was diluted 10-fold in nuclease-free water, and 1 µl (~ 20 ng/µl) was used as a template for the PCR reaction. The bacterial variable V4-V5 region of the 16 S rRNA gene was amplified from the extracted DNA with the primer pair BactB-F (GGA TTA GAT ACC CTG GTA GT) and BactB-R (CAC GAC ACG AGC TGACG)52 using the EliZyme™ HS FAST MIX Red Master Mix (Elisabeth Pharmacon, Brno, Czech Republic). The PCR conditions were: Denaturation step for 5 min at 95 ◦C, followed by 25 cycles of 30 s at 95 ◦C, 30 s at 57 ◦C and 30 s at 72 ◦C, ending with a final elongation step at 72 ◦C for 5 min. The quality and length of PCR amplicons (≈ 300 bp) were assessed by 1.5% agarose gel electrophoresis (30 min at 100 V). Amplicons were purified using the Monarch® PCR and DNA Cleanup Kit (New England BioLabs, Ipswich, MA, USA) and their concentration was determined using the NanoDrop 2000c UV-Vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
Library preparation and high-throughput sequencing
The high-throughput sequencing procedure was performed as described previously53. Briefly, library preparation was performed using the NEBNext Fast DNA Library Prep Set for Ion Torrent (New England BioLabs) with Ion Xpress Barcode Adapters 1–96 Kit (ThermoFisher Scientific), and quality was checked using the Agilent 2100 Bioanalyzer instrument (Agilent Technologies, Santa Clara, CA, USA). Libraries were pooled in equimolar ratios based on the concentration measured with the KAPA Library Quantification Kit (KAPA Biosystems) using the QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). Template amplification was performed by emulsion PCR using the Ion PGM™ HiQ™ View OT2 400 kit (Thermo Fisher Scientific, Waltham, MA, USA) in the Ion OneTouch™ 2 instrument. HTS was carried out on an Ion Torrent PGM platform with an Ion 316 Chip Kit v2 BC (ThermoFisher Scientific) using an Ion PGM Hi-Q View Sequencing Kit (ThermoFisher Scientific) according to the manufacturer’s instructions.
Bioinformatic and statistical analysis
The bacterial 16 S rRNA gene sequences were retrieved in FASTQ format and analyzed by QIIME2 pipeline version 2020.254. Quality filtering, denoising and elimination of chimeras were performed using the DADA2 plugin in QIIME2 to extract sequence variants (ASVs)55. Clustering and taxonomy classification was carried our using a VSEARCH based on the SILVA database (version 132) with a 97% threshold56. Rarefaction was conducted to normalise the data based on read depth in all samples, the dataset was subsampled to a minimum of 5500 reads per sample. The rarefaction curves reached a plateau, indicating that the sequencing depth was sufficient and all species were adequately covered in the samples (Figure S1, Supplementary Material). Bacterial diversity was assessed using alpha diversity indices (Chao1 index, Shannon entropy, Faith’s phylogenetic distance and Pielou index), and comparison between groups was performed using the Kruskal-Wallis H test. For beta diversity, principal coordinate analysis (PCoA) based on the Jaccard’s non-phylogenetic distance matrix was generated, and results were plotted using EMPeror57. Permutational multivariate analysis of variance (PERMANOVA) with 999 permutations was conducted to determine the statistical differences between groups, PERMDISP test was also performed to assess the homogeneity of dispersions between animal groups. Linear discriminant analysis (LDA) with an effect size (LEfSe) algorithm58 in the Galaxy web module (http://huttenhower.sph.harvard.edu/galaxy/) was used to identify the significantly differentially abundant taxa, with alpha values of 0.05 and a threshold value of 2.0 on the logarithmic LDA scores for discriminative features (p < 0.05 and LDA score = 2.0). Sequence information was deposited in the Sequence Read Archive under accession number: PRJNA948341.
Results
Characterisation of caecal bacterial community
The bacterial community in the caecal samples of partridges fed different diets was qualitatively and quantitatively analysed for species richness, evenness and phylogenetic diversity. The alpha diversity analysis showed higher diversity richness in the samples of partridges fed diet TM50 with a 50% replacement of soybean meal protein by T. Molitor larvae meal. The results depicted in Fig. 1 and summarised in Tables S2 and S3 (Supplementary Material) show significant differences in Chao1 index, Shannon entropy and Faith’s phylogenetic distance between the control group C and the experimental group TM50. However, Pielou Evenness index showed no significant differences between the basal and TM-based diets, indicating similar evenness. Beta diversity, which evaluates the similarities and differences in bacterial composition among groups, was determined using Jaccard’s nonphylogenetic distance matrix. A Principal Coordinate Analysis (PCoA) plot (Fig. 2) shows the spatial separation of the samples. Statistical analysis revealed significant differences among all the three groups. PERMDISP showed no statistical differences among the groups, with the exception of the control group compared to the TM50 group, which showed inter-group variability (PERMDISP p = 0.046), as indicated in Table 3.
Comparison of diversity indices for caecal bacterial communities of three groups of partridges fed different diets. (A) Bacterial richness estimated with the Chao1 index, (B) bacterial richness and evenness estimated with Shannon entropy, (C) bacterial diversity estimated with Faith’s phylogenetic distance.
Principal Coordinate Analysis (PCoA) showing the Jaccard’s distance matrix between caecal bacterial community compositions of three groups of partridges fed control diet (C) and diets with 25% (TM25) and 50% (TM50) protein substitution levels of Tenebrio molitor meal. Each dot represents one sample. The percentage of variation explained by the plotted principal coordinates is indicated on the axes.
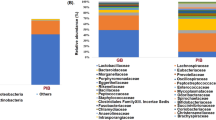
Taxonomical composition
In total, the caecal bacterial community consisted of 6 phyla including 137 bacterial phylotypes. Firmicutes represented the dominant phylum in all three groups of animals (C: 90.6 ± 2.6%; TM25: 87.8 ± 4.6%; TM50: 85.7 ± 3.5%), followed by a minor population of Proteobacteria, Tenericutes, Actinobacteria, Bacteroidetes and Verrucomicrobia. Regardless of the diet, the major order of Firmicutes was Clostridiales, mainly represented at the family level by Ruminococcaceae and Lachnospiraceae, as shown in the Fig. 3(A). At the genus level, the most abundant genera were Faecalibacterium, an unclassified genus within the family Lachnospiraceae, Blautia, Ruminococcus torques group, an unclassified genus within the family Ruminococcaceae and an unclassified genus within the family Lachnospiraceae, which together accounted for more than 41.9% of the sequences in all three groups of partridges. Less abundant genera with a meaningful relative abundance (> 1%) were Negativibacillus, CHKCI001, Subdoligranulum, Escherichia-Shigella, Streptococcus, Lachnospiraceae NK4A136 group, Bacillus, Lactobacillus, Ruminococcaceae UCG-014 and Christensenellaceae R-7 group. The relative abundance (means ± SD) of the bacterial taxa at the different taxonomic levels is listed in the Table S4. Bacterial taxa with a relative abundance of less than 0.5% are summarized as “Others” in the Fig. 3 and listed in the Table S5 (Supplementary Material).
Determination of taxonomic biomarkers
Linear discriminant analysis with effect size (LEfSe) was performed to determine the bacterial taxa with significantly different abundance levels between the control group and the two groups of partridges fed T. molitor larvae meal. The analysis comparing the control group C with the TM25 group revealed 12 bacterial taxa with different abundance levels (LDA score > 2.0). Six taxa had a significantly higher relative abundance in control group C (red bars) and 6 taxa had a significantly higher relative abundance in the TM25 group (green bars). In the control group, the increased class Clostridia (LDA score > 6.0) comprised five significantly more abundant genera, including Ruminococcus gauvreauii group, Eisenbergiella, Lachnospiraceae NK4A136 group, Eubacterium coprostanoligenes group and Faecalibacterium. In the TM25 group, the class Erysipelotrichia and the order Erysipelotrichales were significantly enriched. The family Eggerthellaceae as well as the genera Oscillibacter, Lachnospiraceae FCS020 group and Family XIII AD3011 group also showed a significantly higher relative abundance in the TM25 group (Fig. 4A, B). The comparison of control group C with the TM50 group resulted in a higher number of taxa with significantly different relative abundances. A total of 22 differentially abundant bacteria (LDA score > 2.0) were found. Seven taxa had a significantly higher relative abundance in control group C (red bars), and 15 were significantly more abundant in the TM50 group (green bars). In the control group C, the abundance of phylum Firmicutes (LDA score > 6.0), class Clostridia (LDA score > 6.0), family Lachnospiraceae and four genera, including Fusicatenibacter (family Lachnospiraceae), Eubacterium coprostanoligenes group (family Ruminococcaceae), CHKCI002 (family Lachnospiraceae) and Faecalibacterium (family Ruminococcaceae), were significantly increased. In the TM50 group, six bacterial markers correlated with TM inclusion were of the same taxa as in the TM25 group and a significant increase was observed in a further 9 taxa. These taxa included 3 phylotypes of Bacilli (Bacillales, Bacillaceae and Bacillus) and 6 clostridiales bacteria (Erysipelatoclostridium, Clostridium innocuum group, Ruminococcaceae UCG-009, UC5-1-2E3, ASF356 and DTU089), as shown in Fig. 4C, D.
Linear discriminant analysis (LDA) scores at different taxonomic levels (phylum, class, order, family and genus) of three groups of partridges fed different diets. (A) and (C) show the histogram plots of LDA scores for differentially abundant taxa between the groups. The length of the bar represents the log10-transformed LDA score, indicated by vertical dotted lines. Positive LDA scores (green bars) and negative LDA scores (red bars) represent bacterial taxa that are overabundant in the corresponding group. (B) and (D) represent the cladograms showing the phylogenetic relationship among different groups of organisms with significantly different abundances.
Discussion
In this study, we investigated the effect of Tenebrio molitor larvae meal as a dietary supplement on the bacterial community in the caecum of Barbary partridges. In poultry production, the larvae of T. molitor (yellow mealworm) are now considered a good source of nutrients with a high quantity and quality of proteins and amino acid profile. The crude protein content is around 52.4% (ranging from 47.0 to 60.2%), which on average exceeds the values of soybean meal (49.4%)16. T. molitor larvae meal thus represents an alternative and sustainable source of protein for the animal feed industry. In addition to a good nutritional value, this insect meal also has a good flavour59, digestibility60 and a functional ability due to the chitin contained in the exoskeleton61, which has antimicrobial properties20,62 and positive effects on the immune system21,63. The use of insect meal as feed also has an ecological aspect, as insects require less space for production, have minimal greenhouse gas emissions and water footprint64,65 and insects can be reared on a variety of biological waste66, which contributes to the circular economy and sustainability. The use of insect meal in poultry feeding has an increasing trend as innovative and promising solution to meet the growing demand of meat protein sources for humans. This increasing interest in animal feeding should be accompanied by the acquisition of knowledge on the influence on animal performance and health. Therefore, in our study, we focused on the effect of implementation yellow mealworm into the diet on the caecal microbiome of Barbary partridges.
Caeca play an important role in the nutritional status of poultry due to the fermentation capacity of the resident microbiota and the longest residence time (12–20 h) of digesta in the gastrointestinal tract67. Caeca are inhabited by a rich bacterial population consisting of about 1000 different species forming 1010 − 1011 CFU per gram of digesta34,68. The activity of the bacteria consists mainly of hydrolysis of structural polysaccharides, but also detoxification of harmful substances and protection against colonisation by pathogens69. Microbial fermentation leads to the production of short-chain fatty acids (SCFAs), mainly acetic, butyric and propionic acids. These volatile fatty acids are absorbed, catabolised and provide a source of energy for the host, which is why they are closely related to poultry productivity.
Poultry caeca are generally colonised by two major phyla, Firmicutes and Bacteroidetes and two minor phyla, Actinobacteria and Proteobacteria34,70,71. Their relative abundance is highly variable, and the diversity and composition of the microbiome in the caecum is highly dependent on the age, species and breed of the birds72 and is strongly influenced by diet73.
In our study, the bacterial community composition in the caecal samples of partridges was dominated by Firmicutes, regardless of the type of diet, which is in agreement with several studies conducted in broiler chickens38,74,75, Japanese quails53 or Partridge Shank chicken39,43. However, the extensive absence of Bacteroidetes is exceptional feature, as this phylum is commonly represented in poultry caeca34 and may even dominate36,76. We observed that the inclusion of TM in the diet of Barbary partridges led to a decrease of Firmicutes phylum in both the experimental groups, but significantly in the TM50 group (C: 90.6% vs. TM50: 85.7%). This result is consistent with the study of Biasato et al.36, who used 10 and 15% of TM in broiler´s diet, but in contrast to a previous work using 7.5% TM in the diet of free-range chickens77. Our results also contradict the conclusions of the study by Józefiak et al.37Józefiak et al. [37], who described a decrease of phylum Proteobacteria and an increase of class Clostridia in groups of broilers due to the implementation of a very low amount of TM meal (0.2 and 0.3%) in the broiler diet. In our study, Proteobacteria were nonsignificantly increased in both TM groups, especially in TM50, while Clostridia were significantly reduced in both TM groups, with a greater decrease in TM50.
At the family level, Ruminococcaceae and Lachnospiraceae dominated in all three groups of Barbary partridges, which is in agreement with many previous studies conducted on broilers34,38,74. These two families are essential for the degradation of polysaccharides78,79,80, but they differ in their digestive capabilities. Ruminococcaceae are able to break down cellulose and complex polysaccharides, while Lachnospiraceae are more important in the degradation of less complex polysaccharides78. Ruminococcaceae are the major butyrate producers and play an important role in maintaining the health of the gastrointestinal tract34. Lachnospiraceae are also butyrate producers81 and they have been consistently associated with high chicken productivity33 due to their anti-inflammatory potential78. The Lachnospiraceae family was nonsignificantly increased in the TM25 group, but it decreased significantly with TM50 diet. The TM meal led to a significant increase in Eggerthellaceae in both experimental groups, which could be considered a positive effect, as members of this family produce urolithin82, which plays an important role in muscle health and performance83. The increase of Bacillaceae in the TM50 group cannot be properly assessed, as more than 19,000 species, subspecies and strains have been validly described within this family (UniProt Taxonomy), including both beneficial and pathogenic bacteria. The significant increase of Erysipelotrichia taxa in both TM groups is also difficult to evaluate, due to the lack of knowledge about their role in the poultry microbiota. A positive assessment may be encouraged by a higher abundance of Erysipelotrichaceae in broiler chickens with good FCR performance84. On the other hand, in human research, some members of Erysipelotrichaceae have been found to be highly colitogenic, due to coating with immunoglobulin A (IgA)85.
The influence of T. molitor larvae meal was also observed at the genus level. A significant decrease of Faecalibacterium was detected in both TM groups, especially in TM25. This butyrate-producing genus with anti-inflammatory properties plays an important role in microbiota modulation, gut barrier protection and immune system regulation86, so its decline can be considered an unfavorable shift. However, the abundance of Faecalibacterium in the caeca after TM supplementation is still relatively high and represents the most numerous bacterium. Other decreased taxa are much less abundant genera, but may have important metabolic functions, therefore their decline should not be underestimated. Eubacterium coprostanoligenes group is able to reduce cholesterol to coprostanol87, Lachnospiraceae NK4A136 group is one of the butyrate-producing bacteria known to maintain the integrity of the intestinal barrier in mice88, Eisenbergiella was isolated from the stool of an obese woman after bariatric surgery89 and Ruminococcus gauvreauii was found to be causally linked to neurotransmitters such as glutamine and glutamate90. Fusicatenibacter, which decreased significantly in the TM50 group, produces SCFA (mainly formate and acetate) and is reported to suppress intestinal inflammation based on human studies91,92. On the other hand, according to Medawar et al.93, this genus is associated with unhealthy diets and obesity, thus its role in the digestive tract is still unclear. Oscillibacter, increased in both TM groups, has been enriched in free-range chickens fed TM meal77 and also in high performing broilers94, fat-line chickens95 and fat-induced obese mice96. In our study, the relative abundance of Oscillibacter was generally low, but the incidence was twice as high in the TM25 group as in the TM50 group. Interestingly, in the Barbary partridge´ study by Loponte et al.10, a significantly higher body weight (BW) was found for the TM25 group at 50, 57 and 64 days of age compared to the control, while the BW of the TM50 group was only higher at 57 days of age. However, the association with Oscillibacter is purely hypothetical and further research would be needed. The increased abundance of Bacillus in the TM50 group can be considered positive as this genus is increasingly used in poultry farming as a probiotic microorganism that improves digestion and feed efficiency and reduces disease and mortality97,98,99. Other taxa significantly enriched by the inclusion of TM meal into the diet are low abundant, often uncultured and only for two of them relevant information with respect to poultry microbiota can be found. Clostridia bacterium UC5-1-2E3 was reported as one of the most representative groups of the caecal microbiota of broilers raised without antibiotics (NAE broilers)100 and the abundance of Ruminococcaceae UCG-009 was high in the caecal content of high-market-weight chickens101. However, their physiological role is unknown.
The implementation of TM meal in the diet led to an increase in bacterial diversity in the caeca in both TM groups of Barbary partridges, although a significant increase was only recorded in the TM50 group (α-diversity). Significant differences between the control group and both TM groups were also found in the composition of the bacterial community (β-diversity). This is in agreement with Biasato et al.77, who reported a higher Shannon entropy in free-range chickens fed diet with 7.5% TM meal inclusion and a clear separation of the microbiota of the control and TM group as a function of the diet. However, no increased α-diversity was detected in broiler chicks fed diets containing 5, 10 and 15% TM meals36, whih may indicate a species-dependent response. The studies by Józefiak et al.,38 and Colombino et al.,102 found no differences in alpha and beta diversity between the groups of broilers, but in the former work low TM doses (0.2 and 0.3%) were used, while in the latter work live TM larvae (5% were fed. The increased diversity is a positive outcomeof TM supplementation, as a richer microbiota maintains the stability of the gastrointestinal tract and provides more resilience and resistance to potential pathogens. A loss of diversity can lead to dysbiosis, which causes various health problems, especially in intensive livestock farming103. In poultry studies, bacterial diversity in the gut appears to be the key factor for pathogen exclusion104.
Conclusion
In conclusion, this is the first study evaluating the effect of relatively high doses of Tenebrio molitor larvae meal on the caecal microbiome of Barbary partridges. Despite the significant decrease of Clostridia and butyrate-producing Faecalibacterium in both TM groups and Lachnospiraceae in the TM50 group, these class, genus and family were still abundantly represented. The significantly increased diversity in the TM50 group suggests a beneficial effect of insect meal, as higher bacterial divergence is often associated with a more resilient gut microbiome and greater stability and flexibility in defence against pathogens. Replacing soybean meal protein with 25% and 50% insect meal resulted in varying significant changes in bacterial abundance, making it difficult to recommend an appropriate dose for replacement. The use of TM meal should therefore be further investigated to better understand the effects of different insect meal doses on poultry microbiota, performance, production and welfare.
Data availability
The data presented in this study are openly available in the Sequence Read Archive under the accession number PRJNA948341.
References
Hamidu, J. A., Osie-Adjei, A. & Oduro-Owusu, A. D. Poultry waste management-manure. Encyclopedia Meat Sci. 56, 71. https://doi.org/10.1016/b978-0-323-85125-1.00136-8 (2024).
FAO. Gateway to poultry production and products. Food and Agriculture Organization of the United Nations (2022). https://www.fao.org/poultry-production-products/products-processing/poultry-in-human-nutrition
Secci, G., Moniello, G., Gasco, L., Bovera, F. & Parisi, G. Barbary partridge meat quality as affected by Hermetia illucens and Tenebrio molitor larva meals in feeds. Food Res. Int. 112, 291–298 (2018).
Iqbal, F. et al. A bayesian approach for describing the growth of Chukar partridges. Eur. Poult. Sci. 83, 1–10 (2019).
Cramp, S. & Simmons, K. E. L. Handbook of the Birds of Europe, the Middle East and North Africa, II (Oxford University Press, 1980).
Aourir, M., El Abbassi, A. & Znari, M. Growth patterns in Barbary partridges Alectoris barbara originated from low-and high elevations in West central Morocco. Avocetta 38, 45–51 (2014).
Madge, S. & Gowan, M. P. Book on Pheasants, Partridge and Grouse Including Button Quails, sand Grouse and Allies (Christopher Helm, 2002).
Chiatante, G., Giordano, M., Vidus Rosin, A., Sacchi, O. & Meriggi, A. Spatial distribution of the Barbary Partridge (Alectoris barbara) in Sardinia explained by land use and climate. Eur. J. Wildl. Res. 67, (2021).
Wen, Y. et al. Analysis of the physical meat quality in partridge (Alectoris chukar) and its relationship with intramuscular fat. Poult. Sci. 99, 1225–1231 (2020).
Loponte, R. et al. Growth performance, blood profiles and carcass traits of barbary partridge (Alectoris barbara) fed two different insect larvae meals (Tenebrio molitor and Hermetia illucens). Res. Vet. Sci. 115, 183–188 (2017).
Elahi, U. et al. Insect meal as a feed ingredient for poultry. Anim. Biosci. 35, 332–346 (2022).
Belhadj Slimen, I., Yerou, H., Ben Larbi, M., M’Hamdi, N. & Najar, T. Insects as an alternative protein source for poultry nutrition: a review. Front. Vet. Sci. 10, 1200031 (2023).
Kierończyk, B. et al. Available for millions of years but discovered through the last decade: insects as a source of nutrients and energy in animal diets. Anim. Nutr. 11, 60–79 (2022).
Riaz, K. et al. Growth optimization and rearing of mealworm (Tenebrio molitor L.) as a sustainable food source. Foods 12, 1–13 (2023).
Józefiak, D. et al. Insects - A Natural Nutrient Source for Poultry - A Review. Annals Anim. Sci. 16, 297–313 (2016).
Hong, J., Han, T. & Kim, Y. Y. Mealworm (Tenebrio molitor Larvae) as an alternative protein source for Monogastric Animal: a review. Anim. (Basel). 10, 1–20 (2020).
Khan, S., Khan, R. U., Alam, W. & Sultan, A. Evaluating the nutritive profile of three insect meals and their effects to replace soya bean in broiler diet. J. Anim. Physiol. Anim. Nutr. (Berl). 102, e662–e668 (2018).
Biasato, I. et al. Effects of yellow mealworm larvae (Tenebrio molitor) inclusion in diets for female broiler chickens: implications for animal health and gut histology. Anim. Feed Sci. Technol. 234, 253–263 (2017).
Biasato, I. et al. Yellow mealworm larvae (Tenebrio molitor) inclusion in diets for male broiler chickens: effects on growth performance, gut morphology, and histological findings. Poult. Sci. 97, 540–548 (2018).
Józefiak, A. & Engberg, R. M. Insect proteins as a potential source of antimicrobial peptides in livestock production. A review. J. Anim. Feed Sci. 26, 87–99 (2017).
Benzertiha, A. et al. Tenebrio molitor and Zophobas Morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poult. Sci. 99, 196–206 (2020).
Elahi, U. et al. Evaluation of yellow mealworm meal as a protein feedstuff in the diet of broiler chicks. Animals 10, 1–15 (2020).
Sedgh-Gooya, S. et al. Yellow mealworm, Tenebrio molitor (col: Tenebrionidae), larvae powder as dietary protein sources for broiler chickens: effects on growth performance, carcass traits, selected intestinal microbiota and blood parameters. J. Anim. Physiol. Anim. Nutr. (Berl). 105, 119–128 (2021).
Vasilopoulos, S. et al. Growth performance, welfare traits and meat characteristics of broilers fed diets partly replaced with whole Tenebrio molitor larvae. Anim. Nutr. 13, 90–100 (2023).
de Vilela, S. Black soldier fly larvae in broiler diets improve broiler performance and modulate the immune system. Anim. Nutr. 7, 695–706 (2021).
Pietras, M., Orczewska-Dudek, S., Szczurek, W. & Pieszka, M. Effect of dietary lupine seeds (Lupinus luteus L.) and different insect larvae meals as protein sources in broiler chicken diet on growth performance, carcass, and meat quality. Livest. Sci. 250, 104537 (2021).
Biasato, I. et al. Effects of dietary Tenebrio molitor meal inclusion in free-range chickens. J. Anim. Physiol. Anim. Nutr. (Berl). 100, 1104–1112 (2016).
Bovera, F. et al. Use of Tenebrio molitor larvae meal as protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits. J. Anim. Sci. 94, 639–647 (2016).
Dalmoro, Y. K., Franceschi, C. H. & Stefanello, C. A. Systematic review and Metanalysis on the Use of Hermetia illucens and Tenebrio molitor in diets for Poultry. Vet. Sci. 10, 1–23 (2023).
Wu, H. J. & Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 3, 4 (2012).
Aruwa, C. E., Pillay, C., Nyaga, M. M. & Sabiu, S. Poultry gut health – microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. 12, 1–15 (2021).
Stanley, D., Hughes, R. J. & Moore, R. J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 98, 4301–4310 (2014).
Carrasco, J. M. D., Casanova, N. A., Miyakawa, M. E. F. & Microbiota Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 7, (2019).
Rychlik, I. Composition and function of chicken gut microbiota. Animals 10, 103 (2020).
Benzertiha, A. et al. Tenebrio molitor and Zophobas Morio full-fat meals in broiler chicken diets: effects on nutrients Digestibility, Digestive enzyme activities, and Cecal Microbiome. Animals 5, 248–253 (2019).
Biasato, I. et al. Gut microbiota and Mucin Composition in Female Broiler Chickens Fed diets including yellow mealworm (Tenebrio molitor, L). Anim. 2019. 9, 213 (2019).
Józefiak, A. et al. Full-fat insect meals as feed additive – the effect on broiler chicken growth performance and gastrointestinal tract microbiota. J. Anim. Feed Sci. 27, 131–139 (2018).
Józefiak, A. et al. Improvement of cecal commensal microbiome following the insect additive into chicken diet. Animals 10, 577 (2020).
Sun, J. et al. Comparative analysis of the gut microbial composition and meat flavor of two chicken breeds in different rearing patterns. Biomed Res Int (2018). (2018).
Sztandarski, P. et al. Gut microbiota activity in chickens from two genetic lines and with outdoor-preferring, moderate-preferring, and indoor-preferring ranging profiles. Poult. Sci. 101, 102039 (2022).
Xu, Y. et al. Metagenomic analysis reveals the microbiome and antibiotic resistance genes in indigenous Chinese yellow-feathered chickens. Front. Microbiol. 13, 930289 (2022).
Feng, X. et al. Effects of monobutyrin supplementation on egg production, biochemical indexes, and gut microbiota of broiler breeders. Poult. Sci. 100, 100907 (2021).
Ye, Y. Y. et al. Effects of probiotic supplements on growth performance and intestinal microbiota of partridge shank broiler chicks. PeerJ 9, e12538 (2021).
Zhang, L. et al. Dietary Lasia spinosa Thw. Improves growth performance in Broilers. Front. Nutr. 8, 775223 (2022).
Chen, B. et al. Gut microbiota and meat quality. Front. Microbiol. 13, 951726 (2022).
Borrelli, L. et al. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 7, 16269 (2017).
du Sert, N. P. et al. The arrive guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
De Marco, M. et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 209, 211–218 (2015).
Marono, S. et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 96, 1783–1790 (2017).
NRC, -NATIONAL RESEARCH COUNCIL. Nutrient requirements of poultry. Preprint at. (1994).
Janssen, R. H., Vincken, J. P., Van Den Broek, L. A. M., Fogliano, V. & Lakemond, C. M. M. Nitrogen-to-protein Conversion factors for three Edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 65, 2275–2278 (2017).
Fliegerova, K. et al. Effect of DNA extraction and sample preservation method on rumen bacterial population. Anaerobe 29, 80–84 (2014).
Atallah, E. et al. The effect of different levels of Hermetia illucens oil inclusion on caecal microbiota of Japanese quails (Coturnix japonica, Gould, 1837). J. Insects Food Feed. 1, 1–19 (2023).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology vol. 37 852–857 Preprint at (2019). https://doi.org/10.1038/s41587-019-0209-9
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 13, 581–583 (2016).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ (2016). (2016).
Vázquez-Baeza, Y., Pirrung, M., Gonzalez, A. & Knight, R. EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience 2, 16 (2013).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, (2011).
Kierończyk, B. et al. Do insects smell attractive to dogs? A comparison of dog reactions to insects and commercial feed aromas - a preliminary study. Annals Anim. Sci. 18, 795–800 (2018).
Hammer, L. et al. Mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus) show high total protein in vitro digestibility and can provide good-to-excellent protein quality as determined by in vitro DIAAS. Front. Nutr. 10, (2023).
Song, Y. S. et al. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomol. Res. 48, 227–233 (2018).
Guarnieri, A. et al. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 12, 1–12 (2022).
Malematja, E., Manyelo, T. G., Sebola, N. A. & Mabelebele, M. The role of insects in promoting the health and gut status of poultry. Comp. Clin. Path. 32, 501–513 (2023).
Grau, T., Vilcinskas, A. & Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. fur Naturforschung - Sect. C J. Biosci. 72, 337–349 (2017).
Miglietta, P. P., De Leo, F., Ruberti, M. & Massari, S. Mealworms for food: a Water Footprint Perspective. Water 2015. 7, Pages 6190–6203 (7), 6190–6203 (2015).
Harsányi, E. et al. Evaluation of Organic Wastes as substrates for Rearing Zophobas Morio, Tenebrio molitor, and Acheta domesticus Larvae as Alternative feed supplements. Insects 2020. 11, Page 604 (11), 604 (2020).
Oakley, B. B. et al. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 360, 100–112 (2014).
Montso, P. K., Mnisi, C. M. & Ayangbenro, A. S. Caecal microbial communities, functional diversity, and metabolic pathways in Ross 308 broiler chickens fed with diets containing different levels of Marama (Tylosema esculentum) bean meal. Front. Microbiol. 13, 1–15 (2022).
Svihus, B., Choct, M. & Classen, H. L. Function and nutritional roles of the avian caeca: a review. Worlds Poult. Sci. J. 69, 249–264 (2013).
Sergeant, M. J. et al. Extensive microbial and functional diversity within the Chicken Cecal Microbiome. PLoS One. 9, e91941 (2014).
Ducatelle, R., Goossens, E., Eeckhaut, V. & Van Immerseel, F. Poultry gut health and beyond. Anim. Nutr. 13, 240–248 (2023).
Ocejo, M., Oporto, B. & Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 9, 1–14 (2019).
Kogut, M. H. Role of diet-microbiota interactions in precision nutrition of the chicken: facts, gaps, and new concepts. Poult. Sci. 101, 101673 (2022).
Andreani, N. A., Donaldson, C. J. & Goddard, M. A reasonable correlation between cloacal and cecal microbiomes in broiler chickens. Poult. Sci. 99, 6062–6070 (2020).
Costa, M. C. et al. Different antibiotic growth promoters induce specific changes in the cecal microbiota membership of broiler chicken. PLoS One. 12, e0171642 (2017).
Xu, Q. et al. Comparative characterization of bacterial communities in geese fed all-grass or high-grain diets. PLoS One. 12, e0185590 (2017).
Biasato, I. et al. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet. Res. 14, 1–15 (2018).
Biddle, A., Stewart, L., Blanchard, J. & Leschine, S. Untangling the genetic basis of Fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut communities. Divers. 2013. 5, Pages 627–640 (5), 627–640 (2013).
Devillard, E., McIntosh, F. M., Duncan, S. H. & Wallace, R. J. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. J. Bacteriol. 189, 2566–2570 (2007).
Wong, J. et al. Expansion of urease- and Uricase-Containing, Indole- and p-Cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 39, 230–237 (2014).
Medvecky, M. et al. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genom. 19, 1–15 (2018).
Zhao, L. Y. et al. Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications. Signal. Transduct. Target. Ther. 8, (2023).
Zhao, H. et al. Pharmacological Effects of Urolithin A and Its Role in Muscle Health and Performance: Current Knowledge and Prospects. Nutrients 15, 4441 (2023).
Stanley, D., Hughes, R. J., Geier, M. S. & Moore, R. J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 7, 1–13 (2016).
Palm, N. W. et al. Immunoglobulin a coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Martín, R. et al. Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol. Rev. 47, (2023).
Kenny, D. J. et al. Cholesterol metabolism by uncultured human gut Bacteria influences host cholesterol level. Cell. Host Microbe. 28, 245–257e6 (2020).
Ma, L. et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes. 12, 1–19 (2020).
Togo, A. H. et al. Eisenbergiella massiliensis’, a new species isolated from human stool collected after bariatric surgery. New. Microbes New. Infect. 13, 15–16 (2016).
Yang, J., Yang, Z., Wu, Y., Zhao, T. & Wu, Y. Identifying and ranking causal association between gut microbiota and neuroticism. Prog Neuropsychopharmacol. Biol. Psychiatry. 129, 110886 (2024).
Takeshita, K. et al. A single species of Clostridium Subcluster XIVa decreased in Ulcerative Colitis patients. Inflamm. Bowel Dis. 22, 2802–2810 (2016).
Qiu, X. et al. Characterization of fungal and bacterial dysbiosis in young adult Chinese patients with Crohn’s disease. Th. Adv. Gastroenterol. 13, (2020).
Medawar, E. et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Translational Psychiatry 2021 11:1 11, 1–11 (2021).
Singh, K. M. et al. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 39, 10595–10602 (2012).
Chen, Y. et al. Chicken cecal microbiota reduces abdominal fat deposition by regulating fat metabolism. NPJ Biofilms Microbiomes. 9, 1–16 (2023).
Lam, Y. Y. et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 7, 1–10 (2012).
Ramlucken, U. et al. Advantages of Bacillus-based probiotics in poultry production. Livest. Sci. 241, 104215 (2020).
Luise, D. et al. Bacillus spp. Probiotic strains as a potential Tool for limiting the use of antibiotics, and improving the growth and health of pigs and chickens. Front. Microbiol. 13, 1–19 (2022).
Ogbuewu, I. P., Mabelebele, M., Sebola, N. A. & Mbajiorgu, C. Bacillus Probiotics as Alternatives to In-feed antibiotics and its influence on growth, serum Chemistry, antioxidant status, intestinal histomorphology, and lesion scores in disease-challenged broiler chickens. Front. Vet. Sci. 9, 876725 (2022).
Novoa Rama, E. et al. Characterizing the gut microbiome of broilers raised under conventional and no antibiotics ever practices. Poult. Sci. 102, 102832 (2023).
Wang, L. et al. Metabolic and inflammatory linkage of the chicken cecal microbiome to growth performance. Front. Microbiol. 14, 1–12 (2023).
Colombino, E. et al. Effect of Insect Live Larvae as Environmental Enrichment on Poultry Gut Health: Gut Mucin Composition, Microbiota and Local Immune Response Evaluation. Animals 11, (2021).
Ducatelle, R. et al. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet. Res. 49, 43 (2018).
Pedroso, A. A., Lee, M. D. & Maurer, J. J. Strength lies in diversity: how community diversity limits Salmonella abundance in the Chicken intestine. Front. Microbiol. 12, 1584 (2021).
Author information
Authors and Affiliations
Contributions
Conceptualization, K.O.F., G. P., F.B., and G.M.; methodology, K.O.F., J.M., T.M.M. and E.A.; formal analysis and investigation, T.M.M., E.A.; resources, F.B. and G.M.; data curation, E.A. and T.M.M.; writing—original draft preparation, T.M.M.; writing—review and editing, K.O.F., G. P, F.B. and G.M. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahayri, T.M., Atallah, E., Fliegerová, K.O. et al. Inclusion of Tenebrio molitor larvae meal in the diet of barbary partridge (Alectoris barbara) improves caecal bacterial diversity and composition. Sci Rep 14, 29600 (2024). https://doi.org/10.1038/s41598-024-80341-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80341-1