Abstract
Despite advancements, the prevalence of HIV-associated neurocognitive impairment remains at approximately 40%, attributed to factors like pre-cART (combination antiretroviral therapy) irreversible brain injury. People with HIV (PWH) treated with cART do not show significant neurocognitive changes over relatively short follow-up periods. However, quantitative neuroimaging may be able to detect ongoing subtle microstructural changes. In this study, we hypothesized that tensor-valued diffusion encoding metrics would provide greater sensitivity than conventional diffusion tensor imaging (DTI) metrics in detecting HIV-associated brain microstructural injury. We further hypothesized that tensor-valued metrics would exhibit stronger associations with blood markers of neuronal and glial injury, such as neurofilament light chain (NFL) and glial fibrillary acidic protein (GFAP), as well as with cognitive performance. Using MRI at 3T, 24 PWH and 31 healthy controls underwent cross-sectional examination. The results revealed significant variations in tensor-valued diffusion encoding metrics across white matter regions, with associations observed between these metrics, cognitive performance, NFL and GFAP. Moreover, a significant interaction between HIV status and imaging metrics in gray and white matter was observed, particularly impacting total cognitive scores. Of interest, DTI metrics were less likely to be associated with HIV status than tensor-valued diffusion metrics. These findings suggest that tensor-valued diffusion encoding metrics offer heightened sensitivity in detecting subtle changes associated with axonal injury in HIV infection. Longitudinal studies are needed to further evaluate responsiveness of tensor-valued diffusion b-tensor encoding metrics in the contest HIV-associate mild chronic neuroinflammation.
Similar content being viewed by others
Introduction
Human immunodeficiency virus (HIV) infiltrates immune system cells and crosses the blood-brain barrier (BBB) shortly after seroconversion and leads to brain injury1. This infiltration triggers a cascade of effects, including axonal disruption, myelin loss, astrogliosis, and to a lesser extent, damage to white matter (WM) tracts2. Approximately 50% of people with HIV (PWH) may experience mild cognitive impairment. These deficits can affect cognitive domains such as executive function, attention, fine motor skills, and information processing speed2,3,4.
Despite the adoption of combination antiretroviral therapy (cART), chronic mild neuroinflammation is believed to be the primary reason for HIV-associated cognitive impairment. The key contributors to neuroinflammation are activated microglia and perivascular macrophages, with some involvement from astrocytes. An additional contribution is the transmigration to the central nervous system (CNS) of activated monocytes, which, after differentiation, increase the pool of perivascular macrophages1,5,6,7,8. As cART becomes more accessible, the understanding of brain abnormalities and cognitive deficits in HIV patients has become increasingly complex. Aging individuals receiving cART may develop comorbid medical conditions that independently lead to brain damage and cognitive changes. Furthermore, certain antiretroviral regimens have been associated with brain damage, complicating treatment strategies9. Hence, there is an urgent need for advanced techniques to deepen our understanding of the pathogenesis of tissue changes in the brain due to HIV infection. As a result, there is a growing interest in sensitive, reliable, readily accessible, and reproducible noninvasive imaging approaches for evaluating the brain injury.
Advanced MRI pulse sequences and post-processing methods provide novel quantitative measures reflective of brain injury10,11,12,13,14. Utilizing micrometer-scale displacement of tissue water, diffusion MRI (dMRI) can noninvasively detect microstructural abnormalities in the brain15,16,17,18. It provides excellent sensitivity to microstructural damage associated with HIV19,20,21,22. However, conventional dMRI approaches (such as diffusion tensor imaging, DTI) are significantly impacted by the dispersion of regional fiber orientations, such as crossing fibers, posing challenges in detecting regional pathology. For instance, the fractional anisotropy (FA) of white matter in DTI is closely linked to densely packed and myelinated axonal structures, as well as the presence of glial cells in disease23,24. However, interpreting the FA is challenging due to the blending of mesoscopic tissue features (e.g., fiber orientation dispersion and crossings) with microscopic features (e.g., axons, cells, and density). These complexities may result in FA changes misinterpreted as pathology25. Given that 90% of white matter voxels involve crossing fibers, the imperfect alignment of axonal fibers makes it nearly impossible to separate tissue microstructural anisotropy from macrostructure using FA26,27,28,29. Higher FA is usually attributed to more intact white matter tracts. However, increased FA may also occur in pathological processes. For example, in Alzheimer’s disease, increased FA may reflect pathological changes in fiber orientation dispersion30.31. However, advanced diffusion techniques like diffusion kurtosis imaging (DKI) offer higher sensitivity than DTI by capturing deviations from Gaussian water diffusivity32.
Tensor-valued diffusion encoding is a new technique which employs diffusion encoding in multiple directions. While encoding in a single direction – as is done for DTI – yields linear tensor encoding (LTE), encoding in all directions with equal sensitivity yields spherical tensor encoding (STE). By contrasting LTE and STE, additional information about the tissue microstructure can be obtained, such as the separation of microscopic anisotropy and orientation dispersion33. While a similar objective has been defined for many modelling methods using LTE, such methods are prone to bias due to modeling degeneracy34. Unlike DTI, where the interpretation of FA relies on both microscopic features and the bulk tract orientation dispersion, tensor-valued diffusion encoding separates these effects through diffusional variance decomposition35,36,37. This approach enables the assessment of axonal integrity by measuring microscopic fractional anisotropy (µFA) as well as isotropic and anisotropic diffusional variance (MKi and MKa) at the sub-voxel level. Thus, tensor-valued diffusion encoding measures may emerge as a sensitive biomarker for evaluating brain microstructure (both gray and white matter) in vivo38,39. To date, tensor-valued diffusion encoding has been used to assess microstructural abnormalities in several diseases36,40,41.
In addition to advanced MRI, cerebrospinal fluid (CSF) and plasma levels of neurofilament light (NFL) chain and glial fibrillary acid protein (GFAP) serve as fluid biomarkers of brain injury. NFL is released into the brain’s extracellular space (ECS) following axonal injury and subsequently detected in CSF and blood42,43. Elevated NFL levels occur in various neurological and neurodegenerative disorders, including HIV infection43,44,45,46. Furthermore, activated glial cells release microparticles expressing GFAP into circulation during brain injury47,48,49,50,51, and have been associated with cognitive impairment52, and viral infections such as HIV infection53,54.
In this study, we hypothesized that tensor-valued diffusion encoding metrics would offer greater sensitivity than conventional DTI metrics in detecting HIV-associated brain microstructural damage. Additionally, we posited that tensor-valued metrics would show stronger correlations with blood biomarkers of neuronal and glial injury, including NFL and GFAP, as well as with cognitive performance.
Methods and materials
Study subjects
Twenty-four PWH (age = 55±10 years, male/female = 17/7) and 31 matched healthy controls (HC) (age = 55±15 years, male/female = 24/7) were enrolled from Rochester NY, and vicinity area. The Institutional Research Subjects Review Board (RSRB) at the University of Rochester thoroughly reviewed and approved the study. All participants provided written informed consent prior to enrollment and underwent clinical, laboratory, neurocognitive, and brain MRI examinations. No participants were excluded from the analyses. All experiments were conducted in accordance with relevant guidelines and regulations. Detailed baseline demographics are presented in Table 1.
Our previous report55, provides detailed descriptions of the inclusion and exclusion criteria as well as all study procedures. To briefly summarize, PWH meeting inclusion criteria had stable cART for a minimum of 3 months before screening and were aged ≥ 18. Exclusions encompassed individuals with symptomatic cardiovascular diseases (angina, myocardial infarction, stroke, or other peripheral atherosclerotic disease) and uncontrolled vascular risk factors such as diabetes mellitus and hypertension. Additionally, those with severe premorbid or comorbid psychiatric disorders (schizophrenia, bipolar disorder, active depression), brain infections other than HIV-1, space-occupying brain lesions, dementia from any cause, and metallic implants were excluded. The control population differed from PWH based on HIV status, level of education and race.
Data acquisition
Blood sample
Whole blood (~ 40 ml) was drawn into sterile, acid-citrate-dextrose (ACD) Vacutainer® blood collection tubes. The plasma was then isolated and used for measuring specified markers. NFL and GFAP levels were measured using Single molecule array (Simoa™)56 kits by Quanterix on a Simoa HD-1 analyzer57,58,59.
Neuropsychological assessments
Assessments of neurocognitive and functional performance were performed in all subjects. Study coordinators trained and supervised by an experienced neuropsychologist, administered all neuropsychological tests. The test battery covered diverse cognitive domains, such as Attention/Working Memory (California Computerized Assessment Package, CalCAP; cognitive reflection test, CRT 4; CRT 14), Speed of Information Processing (Stroop Color Naming, Digit Symbol Modalities Test), Executive Function (Trail Making Test B, Stroop Interference Task), Language (letter and category fluency), Learning (Rey Auditory Verbal Learning Test Trials 1–5; Rey Complex Figure Test Immediate Recall), Memory (Rey Auditory Verbal Learning, RAVLT Trial 7; Rey Complex Figure Test (RCFT) and Recognition Trial, RCFT Delayed Recall), and Motor Skill (Grooved Pegboard). Assessment of premorbid intellectual functioning and English language fluency was limited to the baseline, utilizing the Wide Range Achievement Test (WRAT) 4-Reading subtest.
Before conducting analyses, raw cognitive scores from each test were converted to z-scores using normative data from the test manuals. Cognitive domain scores were then created by averaging the z-scores of the tests within each domain. The cognitive tests are normalized for age and education. A total cognitive score was calculated by summing the z-scores across six cognitive domains: Attention and Working Memory, Processing Speed, Executive Function, Fine Motor Skill, Verbal and Visual Learning, Verbal and Visual Memory, and Language. HAND diagnoses for each participant were determined using the Frascati criteria2.
Magnetic resonance imaging
MRI was performed on a 3T whole-body scanner (MAGNETOM Prisma Fit, Siemens, Erlangen, Germany, software version VE11C) equipped with a 64-channel head coil. The maximum gradient strength is 80 mT/m with a slew rate of 200 mT/m/s.
Anatomical imaging
The T1-weighted (T1w) images were acquired using a 3D magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence with inversion time (TI) = 926 ms, repetition time (TR) = 1840 ms; echo time (TE) = 2.45 ms; flip angle = 8˚; Field of View (FOV) = 256 × 256 × 192 mm3; GRAPPA = 2; number of slices = 192, voxel size = 1.0 × 1.0 × 1.0 mm3, and scan time = 4.16 min 3D T2-weighted FLAIR images were acquired with scan parameters: TI = 1,800 ms, TR = 5,000 ms, TE = 215 ms, FOV = 256 × 256 × 192 mm3; number of slices = 192, voxel size = 1.0 × 1.0 × 1.0 mm3, and scan time = 5.40 min.
Tensor-valued diffusion encoding
We employed a prototype pulse sequence that accommodates free waveform encoding (FWF version 1.13s), available at https://github.com/filip-szczepankiewicz/fwf_seq_resources, based on the diffusion-weighted single-shot spin echo sequence35,60 with encoding waveforms that were compensated for concomitant gradient effects. The imaging protocol involved acquiring 43 images with linear tensor-valued diffusion encoding (LTE) and 37 images with spherical tensor encoding (STE), spread across multiple b-values (b = 0, 100, 700, 1400, and 2000 ms/m2). The imaging parameters included TR = 4100 ms, TE = 91 ms, FOV = 224 × 224 × 30 mm3, partial-Fourier = 6/8, GRAPPA factor = 2, echo spacing (ESP) = 0.6 ms, number of volumes = 80, number of slices = 30, voxel size = 2 × 2 × 4 mm3 and scan time = 5:53 min. The images collected were axial, with phase encoding along the anterior-posterior direction.
Image analysis
Image analyses were performed using a combination of image processing tools, including FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/)61, ANTs (http://stnava.github.io/ANTs/)62, and MATLAB (version 2021b).
All MR images underwent thorough inspection for artifacts, including motion, geometric distortion, and signal dropout. T1w images underwent structural segmentation using the anatomical processing script (fsl_anat) from FMRIB’s Software Library (FSL)63. The processing pipeline involved image reorientation and cropping, radio-frequency bias-field correction, linear and nonlinear registration to MNI 2 mm standard space through FLIRT and FNIRT, brain extraction via BET64, tissue segmentation using FAST, and subcortical structure segmentation employing the FIRST algorithm. White matter lesion segmentation was carried out using volBrain, an automated online MRI brain volumetry system65, based on T1w and FLAIR images. Due to the small size of the lesions, we did not employ lesion filling or exclude any subjects based on their presence.
All diffusion MRI metrics were generated and processed using the MATLAB-based multidimensional diffusion MRI framework66 available at https://github.com/markus-nilsson/md-dmri. Briefly, the diffusion-weighted images from each participant underwent a three-step processing approach: (1) Correction for eddy current-induced distortion and inter-volume subject motion was achieved by registering the images to an extrapolated reference67 using ElastiX (Version 5.0.0)68. The use of extrapolation-based references is crucial for accurate registration of high b-value images67. (2) Smoothing of the images was carried out using a 3D Gaussian kernel with a standard deviation of 0.4 voxels. (3) Voxel-by-voxel normalized anisotropic, isotropic, and total diffusional variance (MKa, MKi, MKt), as well as microscopic anisotropy (µFA), were obtained through linear least squares fitting of the log signal while correcting for heteroscedasticity via the “dtd_covarience” method. Using the same tensor-valued diffusion images, conventional DTI metrics (such as FA and MD) as well as Diffusional kurtosis imaging (DKI) metrics such as mean kurtosis (MK) were computed by employing linear least squares fitting using the same toolbox https://github.com/markus-nilsson/md-dmri69.
ROI analysis
For pre-specified regions of interest (ROIs), we calculated average ROI values for all MRI metrics (DTI – FA, MD; DKI - MK, tensor-valued diffusion encoding - µFA, MKi, MKa, MKt). We used Harvard-Oxford cortical and subcortical, and the Johns Hopkins University WM (JHU-WM) atlases available in FSL in standard MNI152-2 mm space for ROI extraction. Prior to this, all MRI metrics were registered to the high-resolution T1w images of the same individual using a 12-DOF linear registration (FLIRT tool in FSL). Then, individuals’ T1w images were spatially normalized to the MNI-T1-152 standard template using nonlinear registration (using ANTs)62,70. The transformation matrix and the warping field from these two steps were applied to DTI, DKI and tensor-valued diffusion encoding metrics. We then extracted mean values from the MRI metrics for the following: global white matter (GWM), cortical gray matter (CGM), subcortical gray matter (SGM) using the corresponding masks as ROIs, and four white matter tracts encompassing coherent, crossing, and fanning fibers: Genu of Corpus Callosum (GCC), Anterior Corona Radiata (ACR), Forceps Minor (FMin), Superior Fronto-Occipital Fasciculus (SFOF).
Statistical analyses
Statistical analyses were performed in Python (version 3.7.4). An unpaired t-test was used to compare the differences in ROIs between the two cohorts. Spearman correlation analyses were performed to find the associations between imaging metrics and blood markers and cognitive scores after controlling for age. A two-way analysis of variance (ANOVA) was performed to assess the effects of HIV status, MRI metrics, and their interactions on the cognitive status of the subjects. That is,
where “MRI metrics: HIV status” is the interaction of the variables. However, education level and age were not included as covariates in the analysis, as normative data for both were used to calculate total cognitive z-scores from the raw scores of each participant. We also performed similar ANOVA for blood markers to assess the effects of HIV status, MRI metrics and their interactions. A p-value of < 0.05 was considered statistically significant for a single hypothesis testing problem. For inferential problems that involved multiple hypotheses, the Benjamini–Hochberg multiple testing procedure was used to control the false discovery rate (FDR) at the < 0.05 level 71.
Results
Figure 1 represents a T1-weighted anatomical image, voxel-by-voxel tensor-valued diffusion encoding, DKI and DTI maps from a PWH subject.
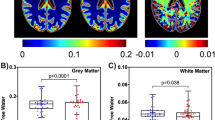
Our analysis revealed significant differences in tensor-valued diffusion metrics in several white matter ROIs, while no significant findings were observed for metrics MK and MD (Table 2). In Fig. 2, we illustrate the comparisons between PWH and HC cohorts across various white matter tracts, encompassing coherent, crossing, and fanning fibers. Notably, we observed a significant decrease in µFA (p = 0.042 for GCC, p = 0.002 for FMin, p = 0.007 for ACR, p = 0.042 for SFOF) and MKa (p = 0.042 for GCC, p = 0.006 for FMin, p = 0.007 for ACR, p = 0.049 for SFOF), along with a significant increase in MKi (p = 0.027 for SCC, p = 0.005 for FMin, p = 0.001 for ACR, p = 0.034 for SFOF) among PWH. In contrast, we observed a significant decrease in FA in the GCC (p = 0.032) and ACR (p = 0.025) in PWH compared to HC. While MD exhibited an increase in PWH, it did not reach statistical significance in any of these ROIs. The trend of changes in DTI metrics is consistent with previous works20,72,73,74,75,76. For example, several previous studies reported that PWH had a decreased FA in several brain regions, including genu and splenium of corpus callosum (GCC, SCC), and SFOF. Although PWH had a decreased µFA and MKa and increased MKi compared to healthy controls in global ROIs i.e., for GWM, CGM and SGM, none of the metrics exhibited significant difference.
Comparison of dMRI metrics. Tensor-valued diffusion encoding metrics show significant differences between PWH and HC cohorts in white matter regions with coherent, crossing, and fanning fibers. GCC: Genu of corpus callosum; ACR: anterior corona radiata; FMin: Forceps Minor; SFOF: superior fronto-occipital fasciculus; Significant p-values are shown as bold; mean diffusivity, MD values are expressed as x10− 3.
Participant characteristics
Detailed information about demographic, clinical, neurocognitive and MRI data of the study participants are presented in Table 1. The Welch’s Two Sample t-test did not reveal any statistically significant age difference between the HC and PWH (p = 0.947). Furthermore, in comparison to PWH, those who were HC exhibited significantly higher education levels and were more likely to be white.
Group comparisons of MRI metrics
Group comparisons of cognitive performance and blood markers
Welch’s two group t-test showed the total cognitive score was lower in the PWH cohort compared to the HC cohort (t = 2.22, p = 0.031). However, while the average concentrations of NFL and GFAP were slightly elevated in the PWH cohort compared to the HC cohort, these differences did not reach statistical significance.
Relationship between cognitive scores and imaging metrics
We investigated the correlation between total cognitive z-scores and tensor-valued diffusion encoding metrics (µFA, MKi, and MKa), DTI metrics (FA and MD) in global white matter (WM), subcortical gray matter (SGM), and cortical gray matter (CGM), among both PWH and HC individuals (see Fig. 3). Significant relationships were observed between total cognitive z-scores and tensor-valued diffusion metrics in the PWH cohort, while no statistical significance was found for HC subjects except for MKa in SGM. Additionally, FA showed no significant associations, except in GWM for PWH whereas MD exhibited significant correlations in both CGM and GWM in PWH. Correlations between total cognitive scores and tensor-valued diffusion encoding metrics in four white matter tracts, which involve coherent, crossing, and fanning fibers (GCC, ACR, FMin, and SFOF), are presented in Supplementary Fig. 1. Similar trends of changes were identified within those ROIs. Additionally, we compared the relationship between total cognitive scores and kurtosis measures MK and MKt (Supplementary Fig. 2). The results clearly indicate that b-tensor-based MKt demonstrates greater sensitivity than DKI-based MK in distinguishing PWH from HC, particularly in the ACR, a region with crossing fibers.
Further, we conducted correlation analyses between cognitive domain scores (i.e., Attention/Working Memory, Speed of Information Processing, Executive Function, Language, Learning, Memory, and Motor Skills) and tensor-valued diffusion metrics, as well as DTI metrics, specifically µFA and FA for global ROIs (see Supplementary Table 1). Our findings suggest that executive function, attention, and motor skills display increased sensitivity to microstructural tissue changes measured by tensor-valued diffusion encoding compared to DTI metrics. The trend of correlations aligns with previous studies involving DTI-derived FA and MD20,72,73,74,75,76.
We also performed two-way ANOVA to measure the effects of HIV status, MRI metrics of five ROIs (such as GWM, CGM and SGM, GCC and ACR) and their interactions on cognitive scores. Table 3 shows the representative results for total cognitive scores. We found a significant interaction between the total cognitive score and both µFA (p = 0.032) and MKa (p = 0.025) in the ACR. Additionally, HIV status showed a significant correlation with tensor-valued encoding metrics in the GCC. Supplementary Tables 2–4 shows two-way ANOVA between tensor-valued encoding metrics and cognitive scores in sub-domains (such as executive function, attention, and motor functions). No significant interactions were found between the individual cognitive domain scores and MRI metrics. Nevertheless, trends within the individual cognitive domains are similar to those of the total cognitive score, albeit less pronounced.
Relationship between blood markers and MRI metrics
Figure 4 and Supplementary Fig. 3 illustrate the associations between average neurofilament light chain (NFL) concentrations, as well as GFAP with tensor-valued diffusion encoding, DKI and DTI metrics. NFL concentrations showed a negative correlation with µFA and MKa while being positively correlated in WM, and significance is mostly found in PWH subjects (p < 0.05). GFAP also shows similar trends. However, DTI and DKI metrics (FA, MD and MK) didn’t show any significant associations with blood markers. Additionally, no significant interactions were found between HIV status, MRI metrics with blood markers (not shown).
Relationship between blood markers (NFL and GFAP) and b-tensor metrics. Scatterplots of blood markers and MRI metrics (µFA, MKi and MKa; FA and MD) in global white matter (WM) for each cohort. Regression lines are drawn with 95% confidence intervals. Spearman correlation coefficients and corresponding p-values are displayed within each plot for each cohort.
Discussion
This is the first study to apply diffusion MRI with tensor-valued diffusion encoding in the context of HIV-associated neuropathology to better understand the underlying brain tissue microstructure and to investigate the association between tensor-valued diffusion metrics and cognitive performance and blood markers of brain injury. Tensor-valued diffusion encoding proves valuable in unraveling orientation dispersion and sub-voxel anisotropy, surpassing the capabilities of conventional diffusion techniques like DTI, as it increases the amount of microstructure information encoded into the diffusion-weighted images34. Our hypothesis posits that axonal injury would associate with elevated plasma levels of NFL and GFAP and lower cognitive performance in PWH. Our findings reveal that (a) tensor-valued diffusion encoding metrics (µFA, MKa, MKi, MKt) demonstrate stronger sensitivity to microstructural abnormalities than DKI MK and DTI MD in PWH comparted to HC; (b) tensor-valued diffusion encoding metrics are significantly associated with cognitive scores in PWH but not with MK, FA and MD; and (c) tensor-valued diffusion encoding metrics in white matter are significantly associated with blood markers such as GFAP and NFL in PWH .
In alignment with observations in other neuroinflammatory and neurodegenerative disorders25,77,78,79, PWH exhibit a reduction in anisotropy-related metrics (FA, µFA, MKa) and an elevation in diffusivity-related metrics (MD, MKi) compared to their healthy counterparts. This indicates a widespread loss of tissue microstructural integrity and possible edema. The mean values for diffusion metrics (both DTI, DKI and tensor-valued diffusion encoding) align with previous studies involving both healthy and diseased subjects36,80,81.
Our findings indicate significant reductions by 10–15% in µFA values in various white matter regions, specifically in coherent (GCC), crossing (SFOF), and fanning (ACR) fibers, while FA showed 9–12% significant changes in GCC and ACR with no significant changes in MD, in PWH compared to healthy controls. This implies that microstructural changes, as measured by µFA, are predominantly due to the loss of local anisotropy rather than disruption of white matter fiber coherence in the HIV cohort. Since µFA is proposed as a measure of axonal integrity rather than myelin24,82, this decrease in µFA suggests widespread axonal damage resulting from HIV infection. Overall, tensor-valued metrics demonstrated more pronounced differences between PWH and healthy controls than DTI metrics across all ROIs. Furthermore, we observed a 17–24% decrease in MKa and up to a 4–10% decrease in MKt, along with a 10–21% increase in MKi, in white matter regions in PWH compared to controls. This is noteworthy, as conventional dMRI without tensor-valued diffusion encoding cannot separate MKi and MKa, as it can only detect MKt, which is the sum of the two. By using tensor-valued diffusion encoding to dissociate the two, larger differences between the groups were found.
The cognitive performance, as measured by the total cognitive Z-score, demonstrated a stronger correlation with tensor-valued diffusion encoding metrics compared to DTI metrics in PWH, underscoring the sensitivity of tensor-valued diffusion encoding metrics. Significantly, the interaction between tensor-valued diffusion metrics and HIV status was observed for the tensor-valued diffusion encoding-based anisotropy metrics (i.e., µFA and MKa in crossing and fanning fiber regions). This suggests that the cognitive effects in the HIV cohort are primarily linked to the loss of local anisotropy, impacting cognitive performance. Moreover, cognitive domain scores, particularly in executive function, attention, and motor functions, exhibited robust associations with anisotropy metrics, specifically µFA, compared to FA. However, trends in individual cognitive domains are similar to the total cognitive score, though weaker. The observed trend in correlations aligns with previous studies involving DTI-derived FA. Decreased FA has also been noted in various white matter regions, correlating with decreased memory and executive function in PWH exhibiting HIV-associated neurocognitive disorders, particularly in studies with larger sample size76,83,84.
In addition, this study unveils a notably stronger association between tensor-valued diffusion encoding metrics and both NFL and GFAP in white matter, compared to DTI and DKI metrics in PWH.
However, it is essential to acknowledge several limitations within this study. Firstly, despite the careful age matching between PWH and healthy controls, there exists an imbalance in the proportion of male and female participants. This discrepancy could introduce gender-related confounding factors. Despite concerted efforts to include female participants, the representation remains at a minimum of 25% in each cohort. Nevertheless, the proportion of males and females in both cohorts are not significantly different. However, the proportion of White s and African Americans was significantly imbalanced. Moreover, due to a lack of Simultaneous Multi-Slice (SMS) in the early implementation of the FWF sequence the collection of tensor-valued diffusion encoding metrics encountered limitations in image resolution (2 × 2 × 4 mm3). These limitations should be considered when interpreting the results and may warrant further investigation in future studies with larger and more diverse cohorts.
Conclusion
In this study, we investigated the effectiveness of tensor-valued diffusion encoding and associated analysis in delineating tissue microstructural degradation in PWH. Our findings indicate that metrics based on tensor-valued diffusion encoding demonstrate greater sensitivity in quantifying subtle changes associated with HIV infection. Moreover, we demonstrated a significant correlation between tensor-valued diffusion encoding metrics, cognitive scores, and plasma levels of NFL and GFAP in PWH. Therefore, the utilization of tensor-valued diffusion encoding offers a more comprehensive and clinically relevant insight into abnormalities in brain tissue microstructure related to HIV infection compared to the conventional DTI approach. Further studies with larger sample sizes and longitudinal designs are warranted.
Data availability
Anonymized data will be made available on reasonable request, pending appropriate institutional review board approvals.
References
Glass, J. D., Fedor, H., Wesselingh, S. L. & McArthur, J. C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: Correlations with dementia. Ann. Neurol. 38(5), 755–762 (1995).
Antinori, A. et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 69(18), 1789–1799 (2007).
Woods, S. P., Moore, D. J., Weber, E. & Grant, I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol. Rev. 19(2), 152–168 (2009).
Li, T.-Q. et al. Extensive heterogeneity in white matter intensity in high-resolution T2*-weighted MRI of the human brain at 7.0 T. Neuroimage. 32(3), 1032–1040 (2006).
Veenstra, M. et al. CCR2 on Peripheral Blood CD14(+)CD16(+) Monocytes Correlates with Neuronal Damage, HIV-Associated Neurocognitive Disorders, and Peripheral HIV DNA: Reseeding of CNS reservoirs?. J. Neuroimmune Pharmacol. 14(1), 120–133 (2019).
Williams, D. W. et al. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol. (R) Neuroimmunol. Neuroinflam. 1(3), 36 (2014).
Fischer-Smith, T., Bell, C., Croul, S., Lewis, M. & Rappaport, J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: Lessons from human and nonhuman primate studies. J. Neurovirol. 14(4), 318–326 (2008).
Singh, M. V. et al. Characterization of platelet-monocyte complexes in HIV-1-infected individuals: Possible role in HIV-associated neuroinflammation. J. Immunol. 192(10), 4674–4684 (2014).
Robertson, K., Liner, J. & Meeker, R. B. Antiretroviral neurotoxicity. J. Neurovirol. 18, 388–399 (2012).
Filippi, M., Preziosa, P. & Rocca, M. A. Microstructural MR imaging techniques in multiple sclerosis. Neuroimag. Clin. 27(2), 313–333 (2017).
Mallik, S., Samson, R. S., Wheeler-Kingshott, C. A. & Miller, D. H. Imaging outcomes for trials of remyelination in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 85(12), 1396–1404 (2014).
Williams, R., Buchheit, C. L., Berman, N. E. & LeVine, S. M. Pathogenic implications of iron accumulation in multiple sclerosis. J. Neurochem. 120(1), 7–25 (2012).
Langkammer, C. et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 267(2), 551–559 (2013).
Schneider, T. et al. Sensitivity of multi-shell NODDI to multiple sclerosis white matter changes: A pilot study. Funct. Neurol. 32(2), 97 (2017).
Chenevert, T. L. et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. JNCI: J. Nat. Cancer Inst. 92(24), 2029–2036 (2000).
Horsfield, M. A. & Jones, D. K. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases–a review. NMR Biomed.: Int. J. Devot. Dev. Appl. Magn. Reson. Vivo. 15(7–8), 570–577 (2002).
Lebel, C., Walker, L., Leemans, A., Phillips, L. & Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 40(3), 1044–1055 (2008).
Figley, C. R. et al. Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Front. Neurosci. 15, 799576 (2022).
Chang K, Premeaux TA, Cobigo Y, Milanini B, Hellmuth J, Rubin LH, et al. Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS. 2019.
Gongvatana, A. et al. White matter tract injury and cognitive impairment in human immunodeficiency virusinfected individuals. J. Neurovirol. 15(2), 187–195 (2009).
Tang, Z. et al. Identifying the white matter impairments among ART-naïve HIV patients: A multivariate pattern analysis of DTI data. Eur. Radiol. 27, 4153–4162 (2017).
Kamat, R. et al. Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J. Clin. Exper. Neuropsychol. 36(8), 854–866 (2014).
Basser, P. J. & Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. 213(2), 560–570 (2011).
Beaulieu, C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed.: Int. J. Devot. Dev. Appl. Magn. Reson. Vivo. 15(7–8), 435–455 (2002).
Andersen, K. W. et al. Disentangling white-matter damage from physiological fibre orientation dispersion in multiple sclerosis. Brain Commun. 2(2), fcaa077 (2020).
Oouchi, H. et al. Diffusion anisotropy measurement of brain white matter is affected by voxel size: Underestimation occurs in areas with crossing fibers. Am. J. Neuroradiol. 28(6), 1102–1106 (2007).
Wang, Y. et al. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. 138(5), 1223–1238 (2015).
Vos, S. B., Jones, D. K., Viergever, M. A. & Leemans, A. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 55(4), 1566–1576 (2011).
Wheeler-Kingshott, C. A. & Cercignani, M. About, “axial” and “radial” diffusivities. Magn. Reson. Med.: Off. J. Int. Soc. Magn. Reson. Med. 61(5), 1255–1260 (2009).
Teipel, S. J. et al. Fractional anisotropy changes in Alzheimer’s disease depend on the underlying fiber tract architecture: a multiparametric DTI study using joint independent component analysis. J. Alzheimer’s Dis. 41(1), 69–83 (2014).
Douaud, G. et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage. 55(3), 880–890 (2011).
Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H. & Kaczynski, K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med.: Off. J. Int. Soc. Magn. Reson. Med. 53(6), 1432–1440 (2005).
Szczepankiewicz, F. et al. Quantification of microscopic diffusion anisotropy disentangles effects of orientation dispersion from microstructure: applications in healthy volunteers and in brain tumors. Neuroimage. 104, 241–252 (2015).
Lampinen, B. et al. Probing brain tissue microstructure with MRI: Principles, challenges, and the role of multidimensional diffusion-relaxation encoding. NeuroImage. 282, 120338 (2023).
Szczepankiewicz, F., Sjölund, J., Ståhlberg, F., Lätt, J. & Nilsson, M. Tensor-valued diffusion encoding for diffusional variance decomposition (DIVIDE): Technical feasibility in clinical MRI systems. PloS one. 14(3), e0214238 (2019).
Nilsson, M. et al. Tensor-valued diffusion MRI in under 3 minutes: An initial survey of microscopic anisotropy and tissue heterogeneity in intracranial tumors. Magn. Reson. Med. 83(2), 608–620 (2020).
Szczepankiewicz, F., Hoge, S. & Westin, C.-F. Linear, planar and spherical tensor-valued diffusion MRI data by free waveform encoding in healthy brain, water, oil and liquid crystals. Data Brief. 25, 104208 (2019).
Lampinen, B. et al. Neurite density imaging versus imaging of microscopic anisotropy in diffusion MRI: A model comparison using spherical tensor encoding. Neuroimage. 147, 517–531 (2017).
Alexander, D. C., Dyrby, T. B., Nilsson, M. & Zhang, H. Imaging brain microstructure with diffusion MRI: practicality and applications. NMR Biomed. 32(4), e3841 (2019).
Nery, F. et al. In vivo demonstration of microscopic anisotropy in the human kidney using multidimensional diffusion MRI. Magn. Reson. Med. 82(6), 2160–2168 (2019).
Lasič S, Szczepankiewicz F, Dall’Armellina E, Das A, Kelly C, Plein S, et al. Motion‐compensated b‐tensor encoding for in vivo cardiac diffusion‐weighted imaging. NMR in Biomedicine. 2019.
Teunissen, C. E. & Khalil, M. Neurofilaments as biomarkers in multiple sclerosis. Mult. Scler. J. 18(5), 552–556 (2012).
Gisslén, M. et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: A cross-sectional study. EBioMedicine. 3, 135–140 (2016).
Gattringer, T. et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology. 89(20), 2108–2114 (2017).
Zhou, W. et al. Plasma neurofilament light chain levels in Alzheimer’s disease. Neuroscience letters. 650, 60–64 (2017).
Pasternak, O. et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J. Neurosci. 32(48), 17365–17372 (2012).
Tian, Y. et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 125(13), 2151–2159 (2015).
Willis, C. M. et al. A refined bead-free method to identify astrocytic exosomes in primary glial cultures and blood plasma. Front Neurosci. 11, 335 (2017).
Schindler, S. M., Little, J. P. & Klegeris, A. Microparticles: A new perspective in central nervous system disorders. Biomed Res Int. 2014, 756327 (2014).
Sartori, M. T. et al. Circulating microparticles of glial origin and tissue factor bearing in high-grade glioma: A potential prothrombotic role. Thromb Haemost. 110(2), 378–385 (2013).
Zhao, Z. et al. Cellular microparticles and pathophysiology of traumatic brain injury. Protein Cell. 8(11), 801–810 (2017).
Wallensten, J. et al. Leakage of astrocyte-derived extracellular vesicles in stress-induced exhaustion disorder: a cross-sectional study. Sci Rep. 11(1), 2009 (2021).
Kodidela S, Gerth K, Sinha N, Kumar A, Kumar P, Kumar S. Circulatory astrocyte and neuronal EVs as potential biomarkers of neurological dysfunction in HIV-infected subjects and alcohol/tobacco users.Diagnostics (Basel). 10 (6), (2020).
Rocha, N. P. et al. Blood biomarkers of neuronal/axonal and glial injury in human immunodeficiency virus-associated neurocognitive disorders. Dement. Geriatr. Cognit. Disorders. 51(6), 467–474 (2022).
Murray, K. D. et al. Pathomechanisms of HIV-associated cerebral small vessel disease: A comprehensive clinical and neuroimaging protocol and analysis pipeline. Front. Neurol. 11, 595463 (2020).
Mora, J. et al. Next generation ligand binding assays-review of emerging technologies’ capabilities to enhance throughput and multiplexing. Aaps J. 16(6), 1175–1184 (2014).
Sahler, J., Woeller, C., Spinelli, S., Blumberg, N. & Phipps, R. A novel method for overexpression of peroxisome proliferator-activated receptor-gamma in megakaryocyte and platelet microparticles achieves transcellular signaling. J. Thromb. Haemost.: JTH. 10(12), 2563–2572 (2012).
Sahler, J., Woeller, C. F. & Phipps, R. P. Microparticles engineered to highly express peroxisome proliferator-activated receptor-gamma decreased inflammatory mediator production and increased adhesion of recipient monocytes. PLoS One. 9(11), e113189 (2014).
Weber, E. A. et al. Novel mechanism of microvesicle regulation by the antiviral protein tetherin during HIV infection. J. Am. Heart Assoc. 9(17), e015998 (2020).
Szczepankiewicz, F., Westin, C.-F. & Nilsson, M. Gradient waveform design for tensor-valued encoding in diffusion MRI. J. Neurosci. Methods. 348, 109007 (2021).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. Fsl. Neuroimage. 62(2), 782–790 (2012).
Avants, B. B., Tustison, N. & Song, G. Advanced normalization tools (ANTS). Insight J. 2(365), 1–35 (2009).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23, S208–S219 (2004).
Smith, S. M. Fast robust automated brain extraction. Human brain mapping. 17(3), 143–155 (2002).
Manjón, J. V. & Coupé, P. volBrain: An online MRI brain volumetry system. Front. Neuroinf. 10, 30 (2016).
Nilsson M, Szczepankiewicz F, Lampinen B, Ahlgren A, De Almeida Martins JP, Lasic S, et al., editors. An open-source framework for analysis of multidimensional diffusion MRI data implemented in MATLAB. Proc Intl Soc Mag Reson Med (2018).
Nilsson, M., Szczepankiewicz, F., van Westen, D. & Hansson, O. Extrapolation-based references improve motion and eddy-current correction of high b-value DWI data: Application in Parkinson’s disease dementia. PloS one. 10(11), e0141825 (2015).
Klein, S., Staring, M., Murphy, K., Viergever, M. A. & Pluim, J. P. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging. 29(1), 196–205 (2009).
Westin, C.-F. et al. Q-space trajectory imaging for multidimensional diffusion MRI of the human brain. Neuroimage. 135, 345–362 (2016).
Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 54(3), 2033–2044 (2011).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc.: Series B (Methodol). 57(1), 289–300 (1995).
Wu, Y. et al. Diffusion alterations in corpus callosum of patients with HIV. Am. J. Neuroradiol. 27(3), 656–660 (2006).
Filippi, C. G., Uluǧ, A. M., Ryan, E., Ferrando, S. J. & van Gorp, W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. Am. J. Neuroradiol. 22(2), 277–283 (2001).
Nir, T. M. et al. Mapping white matter integrity in elderly people with HIV. Human Brain Mapp. 35(3), 975–992 (2014).
Chang, L. et al. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J. Neuroimmune Pharmacol. 3(4), 265–274 (2008).
Oh, S. W., Shin, N.-Y., Choi, J. Y., Lee, S.-K. & Bang, M. R. Altered white matter integrity in human immunodeficiency virus-associated neurocognitive disorder: A tract-based spatial statistics study. Korean J. Radiol. 19(3), 431–442 (2018).
Boito, D. et al. MRI with generalized diffusion encoding reveals damaged white matter in patients previously hospitalized for COVID-19 and with persisting symptoms at follow-up. Brain Commun. 5(6), fcad284 (2023).
Li, X. et al. Microscopic fractional anisotropy detects cognitive training-induced microstructural brain changes. Tomography. 8(1), 33–44 (2022).
Kamiya, K. et al. Brain white-matter degeneration due to aging and Parkinson disease as revealed by double diffusion encoding. Front. Neurosci. 14, 584510 (2020).
Buyukturkoglu, K., Fleyser, L., Byrd, D., Morgello, S. & Inglese, M. Diffusion kurtosis imaging shows similar cerebral axonal damage in patients with HIV infection and multiple sclerosis. J. Neuroimag. 28(3), 320–327 (2018).
Cysique, L. A. et al. White matter measures are near normal in controlled HIV infection except in those with cognitive impairment and longer HIV duration. J. Neurovirol. 23, 539–547 (2017).
Lampinen, B. et al. Searching for the neurite density with diffusion MRI: Challenges for biophysical modeling. Human brain Mapp. 40(8), 2529–2545 (2019).
Sanford, R., Fellows, L. K., Ances, B. M. & Collins, D. L. Association of brain structure changes and cognitive function with combination antiretroviral therapy in HIV-positive individuals. JAMA Neurol. 75(1), 72–79 (2018).
Sasson, E., Doniger, G. M., Pasternak, O., Tarrasch, R. & Assaf, Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front. Neurosci. 7, 32 (2013).
Acknowledgements
This study was supported by grant from the NIH (R01AG054328, R01MH118020, R01AG077156) and the University of Rochester Center for Advanced Brain Imaging and Neurophysiology (UR CABIN) Pilot. We are greatly indebted to the patients and healthy volunteers for participating in this study. In addition, we are grateful to the flow core and Cancer Control & Psychoneuroimmunology Lab (CCPL) who did the Simoa assays.
Author information
Authors and Affiliations
Contributions
MNU: Conceptualization, image processing, data analysis and interpretation, manuscript writing, and funding acquisition; MS: Analysis of the blood markers and manuscript review for intellectual content; AF: Image processing and manuscript review for intellectual content; FS: developed the b-tensor pulse sequence and processing pipeline and manuscript review for intellectual content; MN: developed the b-tensor pulse sequence and processing pipeline and manuscript review for intellectual content; ZDB: Analysis of the blood markers; KRS: Analysis of the blood markers; MET: Manuscript review for intellectual content; JZ: Manuscript review for intellectual content; LW: Statistical analysis; XQ: Statistical analysis and interpretation, and manuscript review for intellectual content; MTW: Cognitive data collection and manuscript review for intellectual content; GS: Project administration, funding acquisition, study concept and interpretation, manuscript review for intellectual content. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
FS and MN are inventors on patents related to b-tensor encoding and declares financial conflicts of interest. None of the other authors have any competing financial or non-financial interests in relation to the work described in this manuscript.
Approval for human experiments
All protocols were reviewed and approved by the Research Subjects Review Board (RSRB) at the University of Rochester Medical Center. All participants signed a written consent form.
Consent to participate
All participants signed a written consent form.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Uddin, M.N., Singh, M.V., Faiyaz, A. et al. Tensor-valued diffusion MRI detects brain microstructural abnormalities in HIV infected individuals with cognitive impairment. Sci Rep 14, 28839 (2024). https://doi.org/10.1038/s41598-024-80372-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80372-8
Keywords
This article is cited by
-
Model-based deep learning with fully connected neural networks for accelerated magnetic resonance parameter mapping
International Journal of Computer Assisted Radiology and Surgery (2025)