Abstract
An efficient modified QuEChERS procedure was established for the simultaneous determination of 37 pesticide residues in fresh and processed edible button mushroom by employing GC/ GC-MS and LC-MS/MS. The effectiveness, reliability and accuracy of the method were assessed through validation parameters such as linearity, LOD, LOQ, precision, accuracy, uncertainty, and matrix effect. The linearity calibration for all the selected pesticides at standard concentrations (0.003, 0.01, 0.025, 0.05, 0.075, and 0.1 µg mL− 1) obtained were ≥ 0.970 in both GC and LC-MS/MS. The LOD and LOQ values were 0.003 and 0.01 µg g− 1, respectively. The recoveries obtained in the spiking level of 0.01–0.1 µg g− 1 were in the acceptable range (71.69 to 117.90%) with RSD less than 20%. Matrix effect and uncertainty for all target compounds were in the acceptable range. Monitoring of edible button mushroom samples collected from market revealed the presence of cypermethrin residues in four out of 25 fresh button mushroom samples and no detectable residues were found in processed mushroom products tested. Dietary risk assessement was calculated to the detected cypermethrin in the fresh button mushroom samples and RQ value obtained was <1, where risk is acceptable.
Similar content being viewed by others
Introduction
Mushrooms are recognised as a healthy balanced diet with an important cradle of bioactive compounds for use like antitumor, antiviral, antihypertensive, anti-inflammatory, immune modulating properties and to prevent hypercholesterolemia and cardiovascular diseases1,2,3. They are regarded as good supplement to cereals, due to their abundance in nutrients. Mushroom contains high amount of protein (2–40%), fat (2–8%), carbohydrates (1–55%), fibre (3–32%), vitamins (1.4 mg-2.2 mg of thiamine, 6.7-9 mg of riboflavin, 21.1–33.3 mg of pantothenic acid, and 1.2–1.4 mg of folic acid) and minerals (aluminium, iron, manganese, magnesium, zinc and selenium)4.
China stands first in the world by producing 39 MT (Million Tonnes) followed by Japan (0.45 MT)5. Total mushroom production in India has reached 0.28 MT per annum6. Bihar is the top-producing state in India, with production of about 28,000 tonnes of mushroom in the year 2021-22. Commonly cultivated species for edible purpose in India are button mushroom, Agaricus bisporus (Lange) Imbach, oyster mushroom (Pleurotus sp.), shiitake mushroom (Lentinus sp.), milky mushroom (Calocybe sp.) and paddy straw mushroom (Volvariella sp.)7. Out of these, button mushroom Agaricus bisporus (Lange) Imbach accounts for about 73 per cent of total mushroom production in India8. Button mushroom is mainly grown in temperate regions of Himachal Pradesh, Maharashtra and hilly regions of Tamil Nadu9.
Mushrooms are grown in indoor conditions with closed moist environment and lack of light which might be a favoring factor for incidence of insect pests and diseases thereby reducing yield and quality. The most important insect pest of mushroom leading to significant yield loss up to 40 per cent is sciarid fly (Lycoriella sp.)10. The other major pests are phorids (Megaselia sp.), springtails (Lepidocyrtus sp.) and mites (Tyrophagus sp.)11.
Pesticides are frequently used, primarily throughout the growth period of crops, to reduce yield loss12. Unconstrained use of these pesticides leads to pesticide residues on farm produce13. At present in India, registered pesticides for mushroom production are not available as per Central Insecticide Board and Registration Committee (CIB and RC). Compost is the substrate used for cultivating button mushroom, which favours mycelial growth and eventually produces fruiting bodies and they may also serve as source of inoculum for pest and disease. Paddy straw and wheat straw are common substrates used in mushroom production on which pesticides belonging to organophosphate (OP), carbamate, synthetic pyrethroid and neonicotinoid group are applied and may leave residues in edible mushrooms.
Few earlier studies are available on method standardization and detection of pesticide residues in edible mushrooms but not in processed products (mushroom in brine and powder).
Conversely, published literature on pesticide residues in edible mushroom or its processed products are not available from the study region or country for the commonly used pesticides. The primary objective of the study is to develop a practical analytical technique for multi-pesticide residue analysis in fresh and processed button mushrooms (in brine and powder), using GC/ GC-MS and LC-MS/MS.
Materials and methods
Chemicals and reagents
Certified Reference Materials (CRM) were purchased from Sigma Aldrich, Bangalore, India. LC-MS grade acetonitrile (C2H3N) (LiChrosolv), n-hexane of HPLC grade, sodium chloride and anhydrous magnesium sulphate of analytical grade were purchased from Merck (Mumbai, India). Formic acid (≥ 99.00% purity) was bought from Fisher chemicals (Czech Republic). Sodium chloride (NaCl) and anhydrous magnesium sulfate (MgSO4) were prepared by heating in a muffle furnace for four hours at 650 °C and kept in airtight desiccators until use. Graphitized Carbon Black (GCB) and Primary Secondary Amine (PSA) (particle size of 58.6 μm) were purchased from Agilent Technologies (Palo Alto, CA, USA). Sodium citrate dibasic sesquihydrate and sodium citrate tribasic dihydrate were procured from Sigma Aldrich, Mumbai, India. Ultrapure water (18 Ω) was obtained from the Millipore Q purification system (Merck Millipore, Burlington, USA).
Primary stock solution was prepared by dissolving respective analyte in 25 mL of LC-MS grade acetonitrile for LC-MS/MS analysis and n-hexane for GC analysis separately to prepare 400 µg mL− 1 primary stock solutions. The flask was labeled and kept at a temperature of -20 ˚C in a deep freezer. Intermediate stock solutions of 40 µg mL− 1 (10 mL) of selected pesticides were prepared by adding one mL primary stock solution with 9 mL of respective solvent acetonitrile. Working standard solutions of 0.003, 0.01, 0.025, 0.05, 0.075 and 0.1 µg mL− 1 were prepared by adding respective solvent acetonitrile with appropriate quantity of the intermediate standard stock solution. The matrix-matched standard solutions at 0.01 to 0.1 µg mL− 1 concentrations were prepared by adding standards to the blank extracts of fresh and processed button mushroom (in brine and powder) separately.
Sample pretreatment and cleanup sorbents
Optimization
Fresh (250 g) and processed (250 g) button mushroom samples were collected from the markets. The samples were taken to the laboratory and homogenized separately in a blender and were examined immediately and/or stored at -20 °C for further analysis.
The samples were processed using a modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) method14. A representative sample of 5 g was taken in a 50 mL centrifuge tube, 20 mL of acetonitrile was added and the mixture was vortexed for one minute. Then, four grams of anhydrous MgSO4, one gram of NaCl, 0.5 g of sodium citrate dibasic sesquihydrate and one gram of sodium citrate tribasic dehydrate were added, again thoroughly mixed by the vortexer and centrifuged for 10 min at 6000 rpm. Six mL of the supernatant aliquot from the centrifuged sample was transferred into a 15 mL centrifuge tube prefilled with 600 mg of anhydrous magnesium sulphate, 100 mg of PSA and 10 mg of GCB vortexed for one minute, and centrifuged at 3000 rpm for 10 min. From the upper layer, 4 mL was taken for LC-MS/MS analysis and other 4 mL was taken for GC analysis. The aliquot was transferred into a turbo vap tube for drying under a gentle stream of nitrogen in a turbo vap evaporator at 35˚ C for 30 min. One ml of final volume was reconstituted using acetonitrile for LC-MS/MS and n-hexane for GC compounds analysis. The residue was then filtered through a 0.22 μm PTFE nylon filter and placed in 1.5-mL glass auto-sampler vial for LC-MS/MS and GC analysis separately.
Instrument operating conditions
Selected pesticides include; Neonicotinoids: Acetamiprid, imidacloprid, thiamethoxam, thiacloprid, clothianidin, dinotefuran; Benzoyl phenyl urea: Novaluran; Organophosphorous: Dimethoate, monocrotophos, quinalphos, profenophos, malathion, chlorpyriphos; Fungicides: Azoxystrobin, trifloxystrobin, tebuconazole.
Samples were analyzed by using both LC-MS/MS and GC-MS for identification and confirmation of selected pesticides. The presence of pesticides was confirmed with the help of two parameters, Retention time and (RT) and Mass Spectrum (MS).
LC-MS/MS parameters
Analysis of target pesticides was carried out on the Water Alliance e2695separation model (Waters corp., Milford, MA, USA) with an integrated solvent and sample management system, coupled with Mass Spectrometer Detector with Electrospray Ionization (ESI+) interface in positive ion mode. Chromatographic separation for LC was done in an analytical column XTerra C 18 (Waters Corp., Milford, MA, USA with a pore size of 5 μm and a dimension of 4.6 × 250 mm) with a flow rate of 0.5 mL min− 1. The temperature of the column oven was maintained at 30 °C. Mobile phases A and B were water and acetonitrile, respectively. Formic acid @ 0.1% was used as an ionising agent. The gradient elution program was as follows: 0 min, 60% A: 40% B; 1 min 50% A: 50% B, 2 min, 30% A: 70% B; 10 min 30% A: 70% B; 11 min, 20% A: 80% B; 15 min 30% A: 70% B; 16 min, 30% A: 70% B; 17 min 20% A: 80% B; 18 min, 10% A: 90% B; 20 min 30% A: 70% B; 21 min, 40% A: 60% B. The analytical run time was 22 min. Masslynx® version 4.1 software was employed for analytical instrumental control, sample data processing and examining.
The MS conditions were as follows: the capillary voltage was 3.90 KV, the cone voltage was 24 V, the cone gas was 80 L hr− 1, and the desolvation gas was 1100 L hr− 1; the source and desolvation temperatures were maintained at 120 ˚C and 500 ˚C respectively. The flow of collision gas was 0.18 mL min− 1. Multiple reaction monitoring transitions were employed, combined with retention time matching, to confirm the analytes. The MS/MS parameters were standardized for tuning purposes by using the Intellistart® software.
GC and GC-MS parameters
Following pesticides were analyzed in GC; Organochlorines: Alpha HCH, beta-HCH, lindane, delta HCH, dicofol, alpha endosulfan, p,p-DDE, endosulfan sulfate, p,p-DDD, p,p-DDT, beta-endosulfan, Synthetic pyrethroids: Bifenthrin, fenpropathrin, lamdacyhalothrin, cyfluthrin, cypermethrin, fenvalerate, fluvalinate, deltamethrin; Fungicides: Propiconazole and hexaconazole. The assessment of multi residue method of selected above pesticides was performed using GC (Shimadzu, series 2010) equipped with Electron Capture Detector (ECD) and a split programmed temperature injector. Samples (1µL) were injected at 280º C in split mode. The chromatographic separation was done using capillary column (Agilent) (DB-5w, 30 m ×0.25 mm i.d. × 0.25 μm film thickness) and flow rate 0.73 mL min− 1. The oven temperature was set initially at 150 ˚C (hold for 2 min), increased to 200 ˚C @ 4 ˚C min− 1 (hold for 7 min), increased to 230 ˚C @ 2 ˚C min− 1 (hold for 0 min), and then increased to 280 ˚C min− 1 @ 3.5 ˚C/min (hold for 11 min). Nitrogen at a flow rate of 30 mL min− 1 was used as a carrier gas. The analytical run time was 61.79 min.
The samples with detection of pesticide residues were confirmed in GC-MS and optimized parameters were as follows: Model Shimadzu GC-MS QP 2010 Plus equipped with column (DB-1, 30 m ×0.25 mm i.d. × 0.25 μm film thickness) and detector (MS in SIM mode m/z 76, 300 ºC). The column flow rate was 0.8 mL min− 1. Samples (1µL) were injected at 250º C in split mode. The oven temperature was set initially at 40 ˚C (hold for 2 min), increased to 80 ˚C @ 12 ˚C min− 1 (hold for 4 min), increased to 200 ˚C @ 2 ˚C min− 1 (hold for 20 min), and then increased to 280 ˚C min− 1 @ 5 ˚C min− 1 (hold for 11 min). Helium was used as a carrier gas. The analytical run time was 61.79 min. GC-MS solution 2.5 software was used for analysis.
Method validation
The validation of an analytical method is a crucial step to ensure that the method is accurate, reliable, and suitable for its intended purpose. The analytical method for the determination of pesticide residues in fresh and processed button mushroom was validated in accordance with the SANTE15 criteria. In this study, method was validated with the parameters of linearity, Limit of Detection (LOD), Limit of Quantification (LOQ), accuracy, precision and uncertainty. The identical samples of fresh, and processed button mushroom free of pesticide residues were used as control in all tests. Linearity of detector response was assessed by injecting a total of six solvent calibration solutions of 0.003, 0.01, 0.025, 0.05, 0.075, and 0.1 µg mL− 1 in LC-MS/MS and GC with six replicate injections per concentration. The LOD and LOQ of spiked fresh, and processed button mushroom were computed by injecting samples at a spiking calibration of 0.01,0.025, 0.05, 0.075 and 0.1 µg mL− 1, following six replications. LOD is the lowest concentration that can be reliably detected, while LOQ is the lowest concentration that can be quantified with acceptable accuracy and precision. In order to calculate, LOD and LOQ, the standard deviation, standard error and x variable were used15. The following equations 16,17 was used to determine the matrix influence of various mushroom on target ion intensity.
Samples were homogenized and spiked at analyte concentrations of 0.01, 0.025, 0.05, 0.075, and 0.1 µg mL− 1 and replicated six times. Computation of the relative standard deviation, matrix effect, recovery percentage, and residue concentration were made by using data obtained from the chromatogram. The following equation was used for the residue quantification.
As - Peak area of the sample.
Astd- Peak area of the standard.
Wstd- Weight of the standard in ng.
Ws - Weight of the sample in g.
Vstd – Volume of standard injected in µL.
Vs - Volume of the sample (final extract in one mL).
A1- Injected volume of standard (µL).
A2 - Injected volume of sample (µL).
The uncertainty of method was calculated by the formula given by Ellison and Williams18,
where R Ucomb is the relative combined uncertainty.
R urec is the relative uncertainty from repeated observations (recovery).
RUvol sample and temp is the relative uncertainty from sample volume and temperature.
R Upurof ref std is the relative uncertainty from purity of the reference standard used.
R Uvolof ref std is the relative uncertainty from volume of reference standard.
R Um ref std is relative uncertainty from mass of reference standard.
Dietary risk assessment
The dietary intake risk of cypermethrin in fresh button mushroom samples was carried out by computing Estimated Daily Intake (EDI) and Risk Quotient (RQ). EDI of cypermethrin residue was calculated by multiplying the highest residue (mg kg− 1) obtained from monitoring data with the average consumption rate of 0.082 g/day19 divided by the mean body weight of an adult men (65 kg) and adult women (55 kg).
Where DC indicates daily consumption (g/ person/day); MIR denotes the maximum concentration (mg kg− 1); bw: indicates average body weight. Long-term risk assessment was performed by assessing the Risk quotient (RQ) = EDI/ ADI20. If RQ value is less than 1, then risk is acceptable, whereas RQ value more than 1 considered as risk is not acceptable.
Results and discussion
Optimization of QuEChERS method
Mushroom matrices are characterized by their high complexity, encompassing a range of components such as high fiber content, chitin, proteins, polysaccharides, and amino acids21. These constituents can introduce both signal enhancement and suppression effects making accurate analysis challenging. Hence, selection of suitable extractant and clean up procedure is critical for precise and accurate detection and quantification of pesticide residues in mushroom.
Acetonitrile was used as an extraction solvent for mushroom matrix in our study. Tian et al.22 showed that recovery of five diamides in mushroom was good when acetonitrile was used rather than ethyl acetate, acetone and n-hexane as extraction solvents due to its appropriate polarity for most pesticide compounds and less co-extracted matrix components. In order to increase the extraction efficiency of the developed method, and also to prevent the degradation of certain sensitive compounds at high or low pH, citrate buffers such as sodium citrate dibasic sesquihydrate and sodium citrate tribasic dehydrate citrate buffer (relatively low buffering capacity)23 were used in our study.
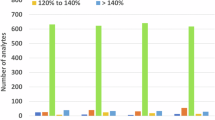
Many studies have employed a combination of the QuEChERS extraction method along with suitable adsorbents to effectively mitigate the impact of the complex sample matrix and improve analytical results. To optimize clean-up, sorbents such as PSA, C18, GCB, and florisil, in three different combinations were used. PSA play a role in selective adsorption of polar compounds, such as organic acids and pigments, from non-polar samples, whereas C18 remove non-polar and medium polar compounds from the polar samples13. GCB was found to be more effective sorbent in removing colour pigments24 and MgSO4 used for absorption of moisture content. The recovery study carried out by using acetonitrile as extraction solvent with 50 mg florisil + 100 mg PSA + 600 mg MgSO4 as clean-up sorbents has resulted in low recovery (40–60%). Similarly, recovery of 10–50 per cent only could be achieved when 10 mg C18 + 100 mg PSA + 600 mg MgSO4 was used as clean-up sorbent with acetonitrile as extracting solvent. Finally, we tried 10 mg GCB + 100 mg PSA + 600 mg MgSO4 with acetonitrile as extracting solvent and could achieve satisfactory recovery within the acceptable range (70–120%) satisfying SANTE15 criteria (Fig. 1) for both fresh and processed button mushroom.
Method validation
Linearity, LOD and LOQ
Linearity study was carried out by injecting six different concentrations of 0.003, 0.01, 0.025, 0.05, 0.075 and 0.1 µg mL− 1 of standard solutions with six replications. The instrument response was determined separately against six different concentrations of the standard mix. The linearity of the calibration curve was established and shown good relationship with the R2 value 0.979 to 0.999 (Table 1). The LOD and LOQ values for all pesticides in both LC-MS/MS and GC were 0.003 µg g− 1 and 0.01 µg g− 1, respectively. The LOQ value standardized is equal to the EU MRL of 0.01 µg g− 1 for mushroom.
The linearity for 187 pesticides in different mushrooms at concentrations from 0.01 to 85 µg kg− 1 showed correlation coefficient R² value ≥ 0.990 for all test analytes16. The LOD and LOQ values for five neonicotinoid insecticides in different mushrooms were 0.03–0.7 µg kg− 1 and 0.1-2 µg kg− 1, respectively25. Le et al.26 developed a highly sensitive and reliable approach to simultaneously identify 180 pesticides in different mushrooms (Enoki, button, oyster and shiitake) using UPLC-MS/MS. The LOQ varied from 2 to 5 µg kg− 1, which is significantly less than the MRL (Maximum Residue Limit) set by the European Union (EU) (10–50 µg kg− 1).
Accuracy and precision
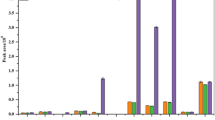
The accuracy and precision of the developed method is expressed as the mean recovery and RSD (Relative Standard Deviation), respectively. RSD indicates the repeatability of the replicates, the intraday precision of the method. Mushrooms were fortified with pesticide standards in five different concentration levels (0.01–0.1 µg g− 1) with five replications at each concentration. According to the criteria of SANTE15, the mean recovery and RSD range should be 70–120% and < 20%, respectively. In our study, the mean recoveries were in the range of 71.69 to 117.90% and RSD values were 0.12–9.92% (Tables 2, 3,4). The average recoveries of 28 organophoshorous compounds in mushroom were in the range of 85.26 to 100.21 per cent with RSD ranging from 1.6 to 6.9 per cent by employing gas purge microsyringe extraction coupled with GC-MS/MS27. Tian et al.28 used acetonitrile as extraction agent and 50 mg PSA + 150 mg MgSO4 as clean up sorbents for simultaneous determination of ten pyrethroid (Bifenthrin, fenpropathrin, cyhalothrin, permethrin, cyfluthrin, cypermethrin, flucythrinate, tau-fuvalinate, fenvalerate and deltamethrin) insecticide residues in six different edible mushrooms (oyster, shiitake, eryngii, crimini, enoki and bunashimeji) using GC-MS/MS. The method showed 72.8 to 103.6% average recoveries for the six different edible mushrooms at three levels (10, 100, and 1000 µg/kg). The LOQ were < 5.57 µg/kg. Le et al.26 developed a highly sensitive and reliable approach to simultaneously identify 180 pesticides in different mushrooms (Enoki, button, oyster and shiitake) using UPLC-MS/MS. Acetonitrile was used as extraction agent and 100 mg of C18 + 100 mg of PSA as clean up sorbents. Recovery rates ranged from 70 to 120% and the RSD of repeatability and reproducibility were less than 20 per cent.
Uncertainty
The uncertainty of measurement was performed as per SANTE15 guidelines, for all selected pesticides. Uncertainty analysis involves identifying and quantifying sources of error or variability in the analytical process. It provides a measure of the confidence in the reported results. The uncertainty of method was calculated by the formula given by Ellison and Williams17 at LOQ level 0.01 µg g− 1 for selected pesticides in both GC and LC-MS/MS. The uncertainty obtained was well below the acceptable range (20%)15. The results were shown in Tables 5 and 6.
Matrix effect
Erney and his coworkers29, given the first interpretation of the matrix effect, and their study states that as the concentration of one base increases, the response of other organic base reduced. Method accuracy and reproducibility, both can be significantly influenced by the matrix effects28. In order to mitigate the matrix effect, the analyte in the fresh button mushroom, button mushroom in brine, button mushroom powder samples were quantified by matrix match standards. In this study, matrix effect for 37 pesticides in three matrices was less than ± 20 per cent which satisfies SANTE15, guidelines (Tables 2, 3 and 4). In contrast to other research26,30,31, our study has shown negligible matrix effect which proved that our developed method was most suited with less interferences of matrices for the analysis of selected 37 pesticides in mushroom matrix.
Application to real samples
Monitoring is carried out by collecting samples to demonstrate the reliability and effectiveness of modified method. Twenty five fresh button mushroom samples and ten processed button mushroom samples were collected from different markets of India (Tamil Nadu, Haryana and Maharashtra). The developed method was used to examine selected pesticide residues. In four samples (16%) of fresh button mushroom residues of cypermethrin in concentration ranging from 0.103 to 0.309 µg g− 1 were detected. The detected cypermethrin residues were higher than 0.01 µg g− 1 MRL set by EU32. The EU data was chosen for our study as MRL for the pesticide cypermethrin were not available as per FSSAI33 and CODEX34. The processed button mushroom samples (brine and powder) showed no detectable residues of pesticides screened.
A study from National Research Centre for mushroom, Solan, India reported detectable residues of α-HCH, β-HCH, γ-HCH, δ-HCH, α-endosulfan, β-endosulfan, o,p-DDT, p,p-DDT, p,p-DDE, chlorpyriphos and chlorpyriphos methyl in fruiting bodies of mushroom which might have resulted from composting materials used like poultry manure, cotton seed cake, and casing material35. Another monitoring study by Tian et al.28 showed the presence of pyridaben, chlorpyriphos and phoxim residues in oyster, shiitake, button mushroom samples collected from Bejing local market of China. In button mushroom, pyridaben was found which ranged from 0.008 to 0.015 mg kg− 1.
Dietary intake risk was estimated in terms of Risk Quotient (RQ) = EDI (Estimated Daily Intake)/ADI (Acceptable Daily Intake) of detected cypermethrin residues in monitoring study. Estimated daily Intake (EDI) was calculated by multiplying maximum concentration of residues (µg g− 1) detected and average consumption rate of 0.082 g /day19 divided by mean body weight of an adult men (65 kg) and adult women (55 kg)36. The RQ values of the cypermethrin detected in the fresh button mushroom samples were <1, and hence the risk is acceptable (Table 7).
Conclusions
In this study, a modified QuEChERS method was adopted to analyze 37 pesticides in fresh and processed button mushroom using both LC-MS/MS and GC. The standardized method fulfilled all the criteria of SANTE15 guidelines. The good linearity of the standard pesticides was achieved with R2 value 0.979 to 0.999. The recoveries obtained for all 37 pesticides were in the range of 70–120 per cent and RSD values were below 20 per cent as per SANTE15, guidelines. Matrix effect analysis showed values less than ± 20 per cent15. The LOD and LOQ values were 0.003 and 0.01 µg g− 1, respectively. The LOQ values obtained were equal or less than MRL of EU. The processed products showed no detectable residues of screened pesticides. Though cypermethrin residues detected in fresh mushroom collected from market were exceeding EU MRL, the dietary risk assessment studies showed RQ value < 1 and the risk is at acceptable level.
Data availability
All data generated or analysed during this study are included in this article.
Change history
18 April 2025
The original online version of this Article was revised: In the original version of this Article, the author's name of B.Tulsi was incorrectly indexed. The original Article has been corrected.
References
Daba, A. S., Kabeil, S., Botros, W., El-Saadani, M. & A. & Production of mushroom (Pleurotus Ostreatus) in Egypt as a source of nutritional and medicinal food. World J. Agric. Sci. 4, 630–638 (2008).
Cheung, P. C. The nutritional and health benefits of mushrooms. Nutr. Bull. 35 (4), 292–299 (2010).
Waktola, G. & Temesgen, T. Application of mushroom as food and medicine. Adv. Biotechnol. Microbiol. 11(3), 10–19080 (2018).
Kakon, A. J., Choudhury, M. B. & Saha, S. Mushroom is an ideal food supplement. J. Dhaka Natl. Med. Coll. Hosp. 18(1), 58–62 (2012).
Singh, M., Kamal, S. & Sharma, V. P. Species and region-wise mushroom production in leading mushroom producing countries-China, Japan, USA, Canada and India. Mushroom Res. 30(2), 99–108 (2021).
Indiastat (2022) selected state wise mushroom production in india. https://www.indiastat.com/table/agriculture/selected-state-wise-production-mushroom-india-2022/1424761
Raman, J. et al. K. Y. Current prospects of mushroom production and industrial growth in India. J. Korean Mushroom Soc. 16(4), 239–249 (2018).
Amin, Z., Wani, F. F., Gulzar, H., Dar, W. A. & Sheikh, P. A. Diseases of White Button mushroom (Agaricus bisporus) - a potential threat to mushroom industry. Int. J. Curr. Microbiol. Appl. Sci. 10(2), 2076–2085 (2021).
Karthick, K. & Hamsalakshmi, H. Current scenario of mushroom industry in India. Int. J. Commer. Manag. 3, 23–26 (2017).
Lewandowski, M., Sznyk, A. & Bednarek, A. Biology and morphometry of Lycoriella Ingenua (Diptera: Sciaridae. Biol. Lett. 41(1), 41–50 (2004).
Rijal, R., Maity, P. & Kumar, A. Pests of mushroom and their ecological management strategies: A review. Biol. Forum - Int. J. 13(1), 375–387 (2021).
FAO. Retrieved Oct 2021, from (2018). http://www.fao.org/faostat/en/#data/TM
Chen, C. et al. Evaluation of pesticide residues in fruits and vegetables from Xiamen, China. Food Control. 22(7), 1114–1120 (2011).
Anastassiades, M., Lehotay, S. J., Stajnbaher, D. & Schenck, F. J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J. AOAC Int. 86(2), 412–431 (2003).
SANTE. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed Sante 11312/2021. (2024). https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf Accessed 10 Oct 2024.
Chang, Q. et al. Determination of 187 pesticide residues in edible fungi by liquid chromatography-tandem mass spectrometry. Anal. Methods. 6(12), 4288–4304 (2014).
Tiryaki, O. Validation of QuEChERS method for the determination of some pesticide residues in two apple varieties. J. Environ. Sci. Health B. 51(10), 722–729 (2016).
Ellison, S. L. & Williams, A. Quantifying uncertainty in analytical measurement. Eurachem 3, 18–30 (2012).
Shirur, M., Shivalingegowda, N. S. & Chandregowda, M. J. Preethi. Mushroom consumer Behaviour: dimensions and measurement model. Mushroom Res. 24(1), 57–63 (2015).
Patel, B. V. et al. Residue decline and risk assessment of fluopyram + tebuconazole (400SC) in/on onion (Allium cepa). Environ. Sci. Pollut Res. 23, 20871–20881 (2016).
Wang, X. M. et al. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 151, 279–285 (2014).
Tian, F. et al. Development and validation of a method for the analysis of five diamide insecticides in edible mushrooms using modified QuEChERS and HPLC-MS/MS. Food chem. 333, 127468 (2020a).
Anastassiades, M., Scherbaum, E., Tasdelen, B. & Stajnbaher, D. Recent developments in QuEChERS methodology for pesticide multiresidue analysis. Pesticide Chemistry: Crop Prot. Public. Health Environ. Saf. 439–458 (2007).
Kinsella, B. et al. Current trends in sample preparation for growth promoter and veterinary drug residue analysis. J. Chromatogr. A. 1216(46), 7977–8015 (2009).
Lu, Z. et al. Simultaneous determination of five neonicotinoid insecticides in edible fungi using ultrahigh performance liquid chromatography-tandem mass spectrometry (UHPLC MS/MS). Food Anal. Methods. 11, 1086–1094 (2018).
Le, L. H. T., Tran-Lam, T., Cam, T., Nguyen, T. Q., Dao, Y. H. & T. N. & Pesticides in edible mushrooms in Vietnam. Food Addit. Contaminants: Part. B. 14(2), 139–148 (2021).
Nan, J. et al. Novel and rapid method for determination of organophosphorus pesticide residues in edible fungus using direct gas purge microsyringe extraction coupled on-line with gas chromatography-mass spectrometry. Talanta 142, 64–71 (2015).
Tian, F. et al. Simultaneous determination of phoxim, chlorpyrifos, and pyridaben residues in edible mushrooms by high-performance liquid chromatography coupled to tandem mass spectrometry. Food Anal. Methods. 9, 2917–2924 (2016).
Erney, D. R., Gillespie, A. M., Gilvydis, D. M. & Poole, C. F. Explanation of the matrix-induced chromatographic response enhancement of organophosphorus pesticides during open tubular column gas chromatography with splitless or hot on-column injection and flame photometric detection. J. Chromatogr. A. 638(1), 57–63 (1993).
Du, P. et al. Rapid residue analysis of pyriproxyfen, avermectins and diflubenzuron in mushrooms by ultra-performance liquid chromatography coupled with tandem mass spectrometry. Anal. Methods. 5(23), 6741–6747 (2013).
Tian, F. et al. Method development and validation of ten pyrethroid insecticides in edible mushrooms by modified QuEChERS and gas chromatography-tandem mass spectrometry. Sci. Rep. 10 (1), 7042 (2020b).
European Union MRL DATABASE. (2022). https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/products/details/197. Accessed 20 August 2022.
FSSAI. Food Safety and Standard Authority of India (2021). https://www.fssai.gov.in/upload/uploadfiles/files/Compendium_Contaminants_Regulations_20_08_2021.pdf. Accessed 05 April 2023.
CAC. Pesticide Index. Codex Alimentarius Commission. (2022). https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/tr/. Accessed 20 June 2023.
Dhar, B. L. et al. Organic button mushroom (Agaricus bisporus) production, quality produce and pesticide residue analysis,. In Mushroom biology and mushroom products. Proceedings of the Sixth International Conference on Mushroom Biology and Mushroom Products, Bonn, Germany, 203–211 (2008).
Dong, M. et al. Dissipation and safety evaluation of novaluron, pyriproxyfen, thiacloprid and tolfenpyrad residues in the citrus field ecosystem. Food Chem. 269, 136–141 (2018).
Acknowledgements
The authors are grateful to Pesticide Toxicology Laboratory, Department of Agricultural Entomology, Centre for Plant Protection Studies, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India for providing facilities.
Funding
The authors declare that no funding, grants were received during the study period.
Author information
Authors and Affiliations
Contributions
TB: Methodology, Investigation, Formal analysis, Data curation, Writing-original draft. BK: Conceptualization, Visualization, Methodology, Supervision, Project administration, Validation , Resources, Writing-review & editing. SA: Supervision. TG: Conceptualization, Resources, Supervision. GP: Resources, Supervision. SC: Formal analysis, Validation, LC-MS/MS analysis. MV: Formal analysis, Validation, GC/ GCMS analysis. KP: Formal analysis, Data curation. AVC: Formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tulasi, B., Kaithamalai, B., Angappan, S. et al. Standardization of an analytical technique for determination of pesticide residues in fresh and processed button mushroom Agaricus bisporus (Lange) Imbach. Sci Rep 14, 30747 (2024). https://doi.org/10.1038/s41598-024-80690-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80690-x