Abstract
We aimed to differentiate and evaluate the clinical imaging features of pulmonary cryptococcosis with different therapeutic responses. The clinical imaging data of 70 patients with PC (complete response PC 37 cases and incomplete response PC 33 cases) were collected and compared to determine the independent risk factors for different therapeutic responses, and their diagnostic performances were verified by receiver operating characteristic curve analysis. Compared with complete response PC, incomplete response PC was more common with immunosuppression (10.8% vs. 45.5%, P = 0.001), intermediate progress (8.1% vs. 39.4%, P = 0.002), bilateral distribution (21.6% vs. 78.8%, P < 0.001), consolidation-interstitial pattern (10.8% vs. 42.4%, P = 0.003), pleural effusion (0 vs. 27.3%, P = 0.002) and mediastinal lymphadenopathy (0 vs. 24.2%, P = 0.005). Multivariate logistic regression showed that immunosuppression, intermediate progress, and bilateral distribution were independent risk factors, with low to moderate areas under curves (AUC, 0.656–0.786). Their combined performance was good with an AUC of 0.888. The diverse clinical imaging features can reflect the therapeutic response of PC. Immunosuppression, intermediate progress, and bilateral distribution were independent risk factors. Their combination can significantly improve diagnostic effects.
Similar content being viewed by others
Introduction
Pulmonary cryptococcosis (PC) is an opportunistic fungal infection, immune damage and chronic wasting diseases are susceptible factors1. Due to cryptococcal evolution and climate change, the susceptible population of PC has increased, including more immunocompetent individuals2. The clinical imaging manifestation of PC is a dynamic process influenced by immune status, inflammation degree, and therapeutic effect3,4. PC often presents as focal lesions during the non-acute phase or the absorption phase4,5. The acute PC with immune deficiency is often aggressive5,6. For the management of PC, surgery is not suitable for nonmalignant conditions7. Besides, the therapeutic response and evolutionary process of PC cannot be observed after surgical resection. Antifungal therapy is preferred for PC8,9. Nevertheless, immune status, cryptococcal pathogenicity, and therapeutic timing can bring different therapeutic responses. We hypothesize that the relevant reasons may exist in the initial and subsequent clinical imaging information. The evaluation of therapeutic response is helpful for the adjustment of the antifungal regimen. However, antifungal clinical imaging changes have not been fully evaluated. In this study, the clinical imaging data of non-surgical PC were retrospectively analyzed to screen out the independent factors influencing the therapeutic response. Furthermore, their diagnostic values can deepen our understanding of PC.
Materials and methods
Clinical evaluations and therapeutic strategies
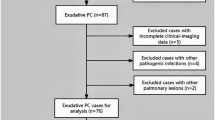
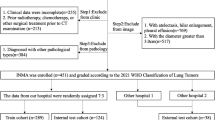
From July 2014 to October 2023, 77 patients with PC without surgical resection were collected. The inclusion criteria: definite pathological diagnosis confirmed by percutaneous biopsy, positive cryptococcal antigen (CrAg, colloidal gold labeled) with possible clinical imaging findings, or positive etiological culture. The exclusion criteria: patients treated with surgery, incomplete clinical and imaging data, or with other pathogenic infections. It was considered to be immunocompromised when a patient met one or more of the following indicators. Clinical indicators: immunosuppressants or glucocorticoid therapy, malignancies with radiochemical therapy, HIV infection, immunosuppressive diseases, or organ transplantation. Humoral immune indicators: serum immunoglobulin IgG < 7.0 g/L, IgA < 0.4 g/L, or IgM < 0.7 g/L. Cellular immune indicators: the percentage of CD3 cells < 60%, the percentage of CD4 cells < 24.5%, the percentage of CD8 cells < 18.5%, or CD4/CD8 ratio < 1.02. The others who had no these predisposing factors were immunocompetent individuals. According to the inclusion and exclusion criteria, 7 cases were ultimately excluded (2 cases treated with surgery, 3 cases with incomplete clinical imaging data, and 2 cases with other pathogenic infections), and 70 cases were retained. Patients with neurological symptoms, positive cerebrospinal fluid (CSF) smears (India ink capsule staining), or positive CSF culture are identified as central nervous system (CNS) dissemination. Results of serum CrAg is also a marker for PC dissemination. All of them received standardized antifungal treatment and their clinical imaging data were retrospectively analyzed.
The standard antifungal therapy was performed according to the immune status, clinical symptoms, or cryptococcal dissemination10,11. Monofluconazole treatment (200–400 mg/d, 3–12 months) was given to asymptomatic and mild to moderate PC. For patients with severe intra- and/or extrapulmonary dissemination, induction therapy (amphotericin B, 0.5-1.0 mg/kg/day and 5-fluorocytosine, 100 mg/kg/day, at least 4 weeks), consolidation therapy (fluconazole, 400 mg/day, 8 weeks), and maintenance therapy (fluconazole, 200 mg/day, 6–12 months) were combined.
Imaging and therapeutic evaluations
Before and after treatment, all 70 PC patients underwent non-enhanced CT scans (16- or 64-section GE scanners, Milwaukee) and were evaluated on the Medcare AnyImage workstation (V4.5). Reconstruction was performed with a thickness of 1 mm to better display the lesions. Imaging indicators included the distribution of lesions (unilateral or bilateral distribution), the morphology of lesions (nodule, consolidation, consolidation-interstitial pattern, and consolidation-nodular pattern), CT signs (lobulation, spiculation, vacuole, cavitation, pleural indentation, and halo sign), pleural abnormalities (pleural-based involvement and pleural effusion) and mediastinal lymphadenopathy. Nodules were round opacities with a maximum diameter of 3 cm or less. A halo sign was defined as the ground-glass opacity around the lesion. Patchy opacity obscuring the underlying structures or accompanied by air-bronchograms was defined as consolidation. The interstitial pattern referred to the fibroreticular opacity or ground-glass opacity, without obscuration of the underlying structures. Malignant signs included lobulation, spiculation, vacuole, cavitation, and pleural indentation. Pleural-based involvement was defined as the wide-based attachment of the lesion to the pleura. A lymph node with a short axis diameter ≥ 10 mm referred to as lymphadenopathy. Imaging data were evaluated by two experienced thoracic radiologists with ten years of diagnosis experience and without knowing the final diagnoses. In case of disagreement, consensus should be reached through consultation.
According to clinical imaging findings, the therapeutic responses of PC were evaluated by two infectiologists (with ten years of working experience) through consultation. Therapeutic response refers to the sensitivity of PC to antifungal drugs and the degree of organic reaction, which is different from the prognostic evaluation. According to the therapeutic response, 70 patients with PC were divided into complete response PC (37 cases) and incomplete response PC (33 cases). Complete response was defined as the clinical imaging improvement within the antifungal course. Incomplete response was defined as the clinical imaging deterioration or intermediate progress within the antifungal course. Clinical deterioration was characterized by repeated exacerbation of clinical symptoms, persistent positive CrAg, or extrapulmonary dissemination. Imaging deterioration was defined as the increased distribution of PC lesions on CT. Intermediate progress was defined as the clinical and/or imaging deterioration after initial improvement during antifungal treatment. Poor prognosis was defined as persistent clinical imaging deterioration or death associated with cryptococcal infection. Good prognosis was defined as the disappearance of clinical symptoms, absorption of lesions, and no recurrence.
Ethical considerations
This study was designed and conducted following the Declaration of Helsinki and was approved by the Ethics Committees of Affiliated Yantai Yuhuangding Hospital of Qingdao University (No. 2023 − 275) and Affiliated Hospital of Yangzhou University (No. 2023-YKL07). Because it was a retrospective study, the Ethics Committees of Affiliated Yantai Yuhuangding Hospital of Qingdao University and Affiliated Hospital of Yangzhou University waived the requirement to obtain informed consent from the study participants.
Statistical analysis
By IBM SPSS version 26.0 (SPSS, Chicago, IL), descriptive statistics were used to characterize the clinical imaging features of both groups. All data were presented as numbers (n), media, or mean ± SD. An Independent t-test (two-tailed) or Mann-Whitney U test was performed for the comparison of continuous variables between the two groups. The Chi-square test or Fisher’s exact test (two-tailed) was performed to assess the difference between dichotomous variables. For the inter-observer variability of CT analyses, the interclass correlation coefficient (ICC) > 0.75 or Kappa ≥ 0.8 was considered a better agreement. The univariate statistical results with significance and feasibility were incorporated into a multivariate logistic regression analysis to determine the independent risk factors for different therapeutic responses. Calculate and compare the areas under curves (AUC) of these clinical imaging features through receiver operating characteristic (ROC) curve analysis and the Delong test. P < 0.05 was defined as statistically significant.
Results
Clinical comparison of both groups
In this study, 34 patients were diagnosed by percutaneous biopsy, 56 patients were diagnosed by positive CrAg, and 10 patients were diagnosed by positive culture. A total of 28 patients underwent percutaneous biopsy and CrAg testing. Four patients with complete response PC had immunosuppression (2 cases of systemic lupus erythematosus with immunosuppressants or glucocorticoid therapy, 1 case of gastric cancer, and 1 case of esophageal cancer). Fifteen patients with incomplete response PC had immunosuppression (9 cases of malignancies with radiochemical therapy, 3 cases of organ transplantation, 2 cases of HIV, and 1 case of membranous nephropathy with immunosuppressants or glucocorticoid therapy). Forty-five patients (64.3%) were treated with fluconazole monotherapy, while 25 patients (35.7%) were treated with an initial antifungal combination.
Both groups had some nonspecific respiratory symptoms, including cough, expectoration, chest distress, chest pain, fever, etc. (P = 0.292). There was no difference in age, sex, and environmental exposure between the two groups (P = 0.338, P = 0.489 and P = 0.406, respectively). In this study, four patients had CNS dissemination, including one patient with complete response PC and three patients with incomplete response PC. Their CrAg titers were 1:320, 1:2560, 1:1024, and 1:4096, respectively. Compared with complete response PC, incomplete response PC was more common with immunosuppression (10.8% vs. 45.5%, P = 0.001) and intermediate progress (8.1% vs. 39.4%, P = 0.002). The intermediate progress of complete response PC (3 cases) occurred within one month after antifungal treatment. The intermediate progress of incomplete response PC (13 cases) occurred in induction therapy (6 cases), consolidation therapy (5 cases), and maintenance therapy (2 cases), respectively. The therapeutic duration for complete response PC was 3–6 months, with a median course of 4 months. The therapeutic duration for incomplete response PC was 6–12 months, with a median course of 9 months. Although the therapeutic response of the two groups was different, their prognoses were good (90.9% vs. 97.3%, P = 0.526). See Table 1 for details.
Imaging comparison of both groups
For these CT analyses, the inter-observer agreements between the two observers were good (ICC = 0.8631, Kappa = 0.8512). Nodule, consolidation, and consolidation-nodular patterns were common CT patterns in both groups (P = 0.213, P = 0.416, and P = 0.372, respectively). There was no difference in malignant CT signs (P = 0.820) and halo signs (P = 0.580) between the two groups. Compared with compete response PC, incomplete response PC was more common with bilateral distribution (21.6% vs. 78.8%, P < 0.001) and consolidation-interstitial pattern (10.8% vs. 42.4%, P = 0.003). Although pleural-based involvement was common in both groups (P = 0.592), incomplete response PC was more often with pleural effusion (0 vs. 27.3%, P = 0.002) and mediastinal lymphadenopathy (0 vs. 24.2%, P = 0.005). See Table 1; Figs. 1 and 2 for details.
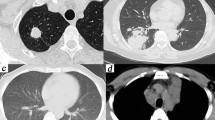
CT findings of complete response PC. Figure 1a-f were from the same patient at different therapeutic stages. Axial CT revealed multiple patchy consolidations and nodules in the left lower lobe with pleural-based involvement and halo sign (a, b). After one month of treatment with fluconazole (400 mg/d), patchy consolidations and nodules were partially absorbed (c, d). After two months of treatment with fluconazole (200 mg/d), only residual fibrosis was visible on CT (e, f).
CT findings of incomplete response PC. Figure 2a-d were from the same patient at different therapeutic stages. Axial CT revealed diffuse ground-glass opacities with air-bronchograms in both lungs (a). One month after induction therapy (amphotericin B, 0.5-1.0 mg/kg/day and 5-fluorocytosine, 100 mg/kg/day), bilateral consolidation-interstitial lesions were partially absorbed (b), but with bilateral pleural effusions and larger mediastinal lymph node (b and c, intermediate progress). Six months after consolidation therapy (fluconazole, 400 mg/day, two months) and maintenance therapy (fluconazole, 200 mg/day, four months), bilateral ground-glass opacities and pleural effusions were completely absorbed on CT (d).
In this study, immunocompromised PC (19 cases, complete response PC 4 cases and incomplete response PC 15 cases) was more common with consolidation-interstitial pattern (18/19, 94.7%). Immunocompetent PC (51 cases, complete response PC 33 cases and incomplete response PC 18 cases) was more common with nodule (9/51, 17.6%), consolidation (18/51, 35.3%), or consolidation-nodular pattern (24/51, 47.1%). Four patients (complete response PC 1 case and incomplete response PC 3 cases) had persistent clinical imaging abnormalities, and three patients with incomplete response PC died. Another nine patients (complete response PC 1 case and incomplete response PC 8 cases) showed clinical improvement without imaging changes, but the lesions were eventually absorbed after continuous treatment. After treatment, PC with a good prognosis showed lesion absorption or residual fibrosis on CT. The PC with poor prognosis (4 cases) showed diffuse consolidation-interstitial lesions and pleural effusion on CT.
Selection and validation of independent risk factors
Multivariate logistic regression showed that immunosuppression, intermediate progress, and bilateral distribution were independent risk factors for therapeutic responses. Although these three clinical imaging features had low to moderate diagnostic values (AUC = 0.656–0.786) in ROC curve analysis, their combined performance was good with an AUC value of 0.888. The Delong test showed that the AUC value of bilateral distribution was slightly better than that of intermediate progress (0.656 vs. 0.786, P = 0.048). There was no diagnostic difference among the other clinical imaging features. However, the diagnostic value of the combined parameter was significantly higher than that of any clinical imaging feature (P ≤ 0.001). See Tables 2 and 3; Fig. 3 for details.
Discussion
Because of occupational exposure, PC was previously thought to have a male predisposition12. In this study, there was no difference in sex, age, and environmental exposure between the two groups. Clinically, more and more PC patients have no clear environmental exposure. Compared to age, gender, and exposure history, immune status is more important for PC pathogenesis13. PC lacks specific symptoms, but vulnerable immune function can exacerbate them and lead to incomplete response and intermediate progress14,15. The previous studies showed that extensive immunocompromised PC caused incomplete remission and poor prognoses16,17. The underlying diseases can also weaken the antifungal efficacy10,11.
PC with different therapeutic responses had some similar imaging findings, but their mechanisms might be different18,19. Nodule and/or consolidation were common CT patterns in both groups, reflecting different cryptococcal invasions or inflammatory evolutions20,21. Complete response PC could localize lesions through granulomatous inflammation, and a powerful inflammatory reaction could form patchy exudation22,23. In some cases, the CT consolidation suggested early and curable lesions without structural damage. Active antifungal intervention could avoid necrosis and cavitation. PC could develop some malignant CT signs and halo signs, because of different pathological evolutions during the treatment. The halo sign and malignant CT signs of complete response PC may be the inflammatory encapsulation and irregular absorption of the lesion24. For incomplete response PC, these signs may indicate local infiltration24,25. The imaging follow-up is necessary to avoid misdiagnosis.
Without effective control, incomplete response PC could spread through the alveoli and lymphatic vessels, resulting in these consolidation-interstitial changes23,26. Furthermore, the interstitial lymphatic involvement resulted in bilateral pulmonary involvement and mediastinal lymphadenopathy27,28. Due to the suitable nutrition and temperature, the subpleural region is a favorable location for Cryptococcus29,30. The similar airway inhalation brought about pleural-based involvement in both groups. Without immune protection, incomplete response PC was more often with pleural effusion by damaging the pleural-airway integrity28,30.
The selection and validation of independent risk factors provided more reliable information.
Bilateral distribution, intermediate progress, and immunosuppression allowed clinicians to consider the possibility of incomplete response PC. Immunosuppression had a negative effect on therapeutic effects, resulting in bilateral distribution and intermediate progress of PC. Previous studies had found that the lesion size of PC was an independent prognostic factor, the larger lesions were more difficult to achieve a good therapeutic response16,17,28. Therefore, bilateral distribution implied a larger lesion size and incomplete response. Perhaps intermediate progress was an external manifestation of poor treatment. Intermediate progress can occur at any stage of the antifungal process, without a specific time point. This is related to the patient’s immune status, inflammatory response, and cryptococcal invasiveness. Although incomplete response PC was more common with intermediate progress, a better prognosis can still be achieved through rational combination therapy. The therapeutic response does not determine the prognosis of PC, but antifungal regimens can be adjusted to achieve a good prognosis. Although none of the three clinical imaging features had a high discriminative effect on PC therapeutic responses, their combination achieved optimal performance. Given the antifungal complexity, a multifactorial evaluation is necessary.
Without unified criteria for immune evaluation, our grouping of immune status was not perfect. The small sample size limited the statistical power of the retrospective analysis. In the future, large-scale longitudinal studies should be conducted with refined immune assessment.
Conclusion
The diverse and dynamic clinical imaging features can reflect the therapeutic response of PC. Immunosuppression, intermediate progress, and bilateral distribution were independent risk factors. Their combination can achieve the best diagnostic performance, which is beneficial for evaluating and monitoring antifungal treatment.
Data availability
Data in this study are available from the corresponding author on reasonable request.
References
Zhang, Y. et al. Clinical analysis of 76 patients pathologically diagnosed with pulmonary cryptococcosis. Eur. Respir J. 40(5), 1191–1200 (2012).
Setianingrum, F., Rautemaa-Richardson, R. & Denning, D. W. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med. Mycol. 57(2), 133–150 (2019).
Xie, L. X. et al. Pulmonary cryptococcosis: comparison of CT fndings in immunocompetent and immunocompromised patients. Acta Radiol. 56(4), 447–453 (2015).
Qu, J., Zhang, X., Lu, Y., Liu, X. & Lv, X. Clinical analysis in immunocompetent and immunocompromised patients with pulmonary cryptococcosis in western China. Sci. Rep. 10(1), 9387 (2020).
Lindell, R. M., Hartman, T. E., Nadrous, H. F. & Ryu, J. H. Pulmonary cryptococcosis: CT findings in immunocompetent patients. Radiology 236(1), 326–331 (2005).
Sui, X. et al. Clinical features of pulmonary cryptococcosis in thin-section CT in immunocompetent and non-AIDS immunocompromised patients. Radiol. Med. 125(1), 31–38 (2020).
Iyer, K. R., Revie, N. M., Fu, C., Robbins, N. & Cowen, L. E. Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat. Rev. Microbiol. 19(7), 454–466 (2021).
MacMahon, H. et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284(1), 228–243 (2017).
Choi, K. H. et al. Treatment of asymptomatic pulmonary cryptococcosis in immunocompetent hosts with oral fluconazole. Scand. J. Infect. Dis. 43(5), 380–385 (2011).
Perfect, J. R. et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 50(3), 291–322 (2010).
Musubire, A. K., Boulware, D. R., Meya, D. B. & Rhein, J. Diagnosis and management of cryptococcal relapse. J. AIDS Clin. Res. Suppl. 3(3), S3–003 (2013).
Hou, X. et al. Pulmonary cryptococcosis characteristics in immunocompetent patients-A 20-year clinical retrospective analysis in China. Mycoses 62, 937–944 (2019).
Deng, H. et al. Clinical features and radiological characteristics of pulmonary cryptococcosis. J. Int. Med. Res. 46, 2687–2695 (2018).
Wang, D. X. et al. Comparison of CT findings and histopathological characteristics of pulmonary cryptococcosis in immunocompetent and immunocompromised patients. Sci. Rep. 12(1), 5712 (2022).
Song, K. D. et al. Pulmonary cryptococcosis: imaging findings in 23 non-AIDS patients. Korean J. Radiol. 11(4), 407–416 (2010).
Yan, Y. et al. Lesion size as a prognostic factor in the antifungal treatment of pulmonary cryptococcosis: a retrospective study with chest CT pictorial review of 2-year follow up. BMC Infect. Dis. 23(1), 153 (2023).
Henao-Martínez, A. F., Chastain, D. B. & Franco-Paredes, C. Treatment of cryptococcosis in non-HIV immunocompromised patients. Curr. Opin. Infect. Dis. 31(4), 278–285 (2018).
Chang, W. C. et al. Pulmonary cryptococcosis: comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest 129(2), 333–340 (2006).
Xiong, C., Lu, J., Chen, T. & Xu, R. Comparison of the clinical manifestations and chest CT findings of pulmonary cryptococcosis in immunocompetent and immunocompromised patients: a systematic review and meta-analysis. BMC Pulm Med. 22(1), 415 (2022).
Wang, D. et al. Comparative study of primary pulmonary cryptococcosis with multiple nodules or masses by CT and pathology. Exp. Ther. Med. 16(6), 4437–4444 (2018).
Guimarães, M. D. et al. Fungal infection mimicking pulmonary malignancy: clinical and radiological characteristics. Lung 191(6), 655–662 (2013).
Yang, R., Yan, Y., Wang, Y., Liu, X. & Su, X. Plain and contrast-enhanced chest computed tomography scan findings of pulmonary cryptococcosis in immunocompetent patients. Exp. Ther. Med. 14(5), 4417–4424 (2017).
Chen, F., Liu, Y. B., Fu, B. J., Lv, F. J. & Chu, Z. G. Clinical and computed tomography (CT) characteristics of pulmonary nodules caused by cryptococcal infection. Infect. Drug Resist. 14, 4227–4235 (2021).
Suwatanapongched, T., Sangsatra, W., Boonsarngsuk, V., Watcharananan, S. P. & Incharoen, P. Clinical and radiologic manifestations of pulmonary cryptococcosis in immunocompetent patients and their outcomes after treatment. Diagn. Interv Radiol. 19(6), 438–446 (2013).
Fang, L. F., Zhang, P. P., Wang, J., Yang, Q. & Qu, T. T. Clinical and microbiological characteristics of cryptococcosis at an university hospital in China from 2013 to 2017. Braz J. Infect. Dis. 24(1), 7–12 (2020).
Hu, Z. et al. Radiological characteristics of pulmonary cryptococcosis in HIV-infected patients. PLoS ONE. 12(3), e0173858 (2017).
Zinck, S. E. et al. Pulmonary cryptococcosis: CT and pathologic fndings. J. Comput. Assist. Tomogr. 26(3), 330–334 (2002).
Wang, R. Y. et al. Cryptococcosis in patients with hematological diseases: a 14-year retrospective clinical analysis in a Chinese tertiary hospital. BMC Infect. Dis. 17(1), 463 (2017).
Zavala, S. & Baddley, J. W. Cryptococcosis. Semin Respir Crit. Care Med. 41(1), 69–79 (2020).
Yamamura, D. & Xu, J. Update on pulmonary cryptococcosis. Mycopathologia 186(5), 717–728 (2021).
Author information
Authors and Affiliations
Contributions
Y.L.Z. conducted clinical research and manuscript preparation. C.R. and Y.L.Z. conducted data collection and interpretation of results. W.L. and C.R. conducted imaging analysis. W.L. was responsiple for the design and supervision of the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Yl., Ran, C. & Li, W. Clinical imaging diagnosis of pulmonary cryptococcosis with different therapeutic responses. Sci Rep 14, 29337 (2024). https://doi.org/10.1038/s41598-024-80875-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80875-4