Abstract
Growth-regulating factors (GRFs) are plant-specific transcription factors involved in the regulation of plant growth, development, and abiotic stress processes. However, the function of Brassica juncea (L.) Czern & Coss GRFs remain largely unknown. In this study, 34 BjGRF genes were identified in B. juncea. BjGRF members of the same subfamily were found to share a similar motif composition and gene structure. In total, 663 cis-acting elements were found in the promoter regions of BjGRF genes, which were related to light response, hormone response, environmental stresses, and plant growth/development. Additionally, 48 pairs of segmental duplication genes were identified during gene duplication events, and no tandemly duplicated genes were identified. qRT-PCR analysis showed that the 34 BjGRF genes were primarily expressed in the roots, followed by the leaves. Furthermore, the 10 BjGRF genes were screened in response to drought stress, and the expression patterns of the genes were relatively consistent, with a maximum expression level at 3 or 24 h, and the selected BjGRF03 and BjGRF32 might be involved in drought stress. This preliminary study clarifies the response of the BjGRF gene family to drought stress and provides ideas for further analyses of the biological functions of BjGRF genes.
Similar content being viewed by others
Introduction
Growth-regulating factors (GRFs) are plant-specific transcription factors (TFs) with highly conserved QLQ (Gln, Leu, Gln) and WRC (Trp, Arg, Cys) domains at the N-terminal1. The QLQ domain is responsible for the SNH domain of GRF-interacting factor binding. The WRC domain plays a role in transcriptional regulation by combining with cis-elements of downstream genes, as well as DNA-binding motifs, namely, the zinc finger structure and nuclear localization signal region1,2. The C-end contains TQL (Thr, Gln, Leu), FFD (Phe, Phe, Asp), GGPL (Gly, Gly, Pro, Leu), and other structural domains3,4. Unlike the conserved N-terminal amino acid (aa) residues, the C-terminus comprises of variable aa residues and has a transcriptional activation function5.
GRF TFs are subject to post-transcriptional regulation by miR396 and inhibit the expression of GRFs by degrading their encoded mRNAs or inhibiting their translation through complementary pairing with mRNAs6,7. Therefore, GRF-miR396 is involved in the regulation of plant growth, development, and abiotic stress tolerance through regulatory networks8. The expression of Sp-miR396a-5p in tomato is upregulated under salt and drought stress. After the heterologous expression of Sp-miR396a-5p, the expression of NtGRF1, NtGRF3, NtGRF7 and NtGRF8 in transgenic plants is down-regulated9. The detection of physiological and biochemical indicators showed that the osmotic regulation ability of transgenic plants is increased but the amount of reactive oxygen species is decreased, indicating that the ability to resist drought, salt and low-temperature stress is enhanced9. Additionally, overexpression of the AtGRF7 gene enhances resistance to osmotic and drought stress8. The atgrf7 mutant is more resistant to salt and drought stress than wild-type plants10. Sakuma et al. found that AtGRF7 can improve tolerance to salt and drought stress by inhibiting the expression of the dehydration response element-binding protein DREB2A11. These results indicate that GRF TFs play a regulatory role in plant drought resistance responses.
The seasonal distribution of precipitation in Guizhou, China, is uneven, with more precipitation in spring and summer than in autumn and winter. During autumn and winter, rapeseed seedlings are prone to drought stress, which significantly affects production. Brassica juncea is a characteristic oilseed crop with strong drought resistance and is cultivated in Guizhou. It can be planted in mountainous environments and is a rich resource for drought-resistance genes. Therefore, exploring drought-resistant genes in B. juncea is critical for the improvement of its varieties and germplasm resource innovation. The GRF family plays critical roles in plant growth, development, and drought stress response. At present, GRF genes have been studied in Arabidopsis2, rice (Oryza sativa)12, Brassica napus13, cotton (Gossypium hirsutum)14, wheat (Triticum aestivum)15, foxtail millet (Setaria italica)16 and Brassica rapa17; however, no GRF gene has been reported in B. juncea. In this study, we identified GRF family genes of B. juncea at the whole-genome level. Next, we comprehensively analyzed the physical and chemical characteristics, evolutionary relationships, homology, conserved motifs, gene structure, gene duplications, cis-elements and expression patterns of BjGRF genes under drought stress at the seedling stage (four-leaf stage) to provide a scientific basis for further research on the potential function of BjGRF genes in the drought response and provide candidate genes for the drought-tolerant breeding of B. juncea.
Results
Acquisition of BjGRF family members and prediction of physical and chemical properties
Thirty-four BjGRF genes in the B. juncea genome were identified using two HMMER searches, and all contained QLQ and WRC domains. The CDS sequences of the identified BjGRF genes were provided in Supplementary Table S1. BjGRF01–BjGRF34 were named based on their position on the chromosomes. The physicochemical properties of the family revealed that the length of amino acids varied significantly, ranging from 261 aa (BjGRF19) to 905 aa (BjGRF28). The isoelectric point of BjGRFs ranged from 6.19 (BjGRF02) to 9.35 (BjGRF03), with an average value of 8.33, and 88.24% of BjGRFs were basic protein. The predicted molecular weight of BjGRFs ranged from 29.82 kDa (BjGRF19) to 102.90 kDa (BjGRF28). The protein instability indices of BjGRF proteins were between 51.13 (BjGRF08) and 78.24 (BjGRF19), both of which were greater than 40, indicating that these proteins are unstable. The fatty acid index ranged from 43.65 (BjGRF01) to 78.78 (BjGRF22), the mean value of hydrophilicity (GRAVY) was between –1.07 (BjGRF31) and –0.45 (BjGRF22), and the GRAVY of all hydrophilic BjGRF proteins was negative, which may be attributed to the absence of hydrophobic residues. Subcellular localization prediction showed that 31 encoded BjGRF proteins may be located in the nucleus, BjGRF04 may be localized to the peroxisome, BjGRF25 may be located in the cytoplasm, and BjGRF28 may be located in the chloroplast (Table 1), indicating that BjGRFs play a major regulatory role as a transcription factor in the nucleus.
Phylogenetic tree of GRF proteins
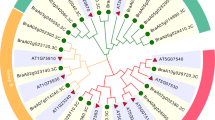
Phylogenetic analysis of GRF families of different species can help explore gene function. Therefore, the full-length amino acids sequences of 35 B. napus, 16 B. rapa, 12 rice, 10 foxtail millet and 9 Arabidopsis GRFs were downloaded to construct a phylogenetic tree based on the 34 identified BjGRF genes (Fig. 1). The three subfamilies contained varying numbers of members; 116 GRF TFs were divided into three distinct subfamilies (Groups A–C), which comprised 59 (50.86%), 34 (29.31%) and 23 (19.83%) GRFs, respectively. Among them, 34 members of the BjGRF family members were dispersed across the three subfamilies: 13 (38.24%) in Group A, 12 (35.29%) in Group B and 9 (26.47%) in Group C. During the polyploidization of B. juncea, there were differences in the number of genes in different subfamilies of BjGRFs, which may have undergone gene amplification and loss. Interestingly, there was no distribution of rice and foxtail millet GRF in Group C, and there were two rice GRFs and 1 foxtail millet GRF in Group B, and most of the rice and foxtail millet GRFs were clustered in the same branch, indicating that BjGRFs are closely related to dicot GRFs. Among them, the research on GRF function in Arabidopsis was the most in-depth, which provided a basis for the study of BjGRFs function.
Homology analysis between BjGRF gene family and the selected species
With the aim of studying the evolutionary relationship of BjGRF family genes among species, we analyzed the interspecific homology of B. juncea, Arabidopsis, and B. rapa GRF TFs. As shown in Fig. 2, GRF genes were homologous among B. juncea, Arabidopsis (24) and B. rapa (27), indicating that the GRF gene family of B. juncea and B. rapa had the closest homologous evolutionary relationship, and these genes originated from ancestral genes, indicating that they may have similar functions. Additionally, BjGRF31 was associated with 2 GRF genes (Bra021521 and Bra027384) in B. rapa, suggesting that BjGRF31 may play a critical role in the evolution of B. rapa. AtGRF4 (AT3G52910) was homologous to BjGRF10, BjGRF16, BjGRF17, BjGRF29 and BjGRF32, suggesting that these five BjGRF genes may have evolved from AtGRF4. Details of the BjGRFs homologous gene pairs with Arabidopsis and B. rapa GRFs are provided in Supplementary Table S2.
Conserved motif and gene structure of GRFs
Conserved motifs help clarify the biological functions of GRF TFs. In this study, we found 15 conserved motifs in the 94 GRFs of B. juncea, B. napus, B. rapa, and Arabidopsis (Figs. 3a), of which motif 1 constituted the WRC domain, motif 2 constituted the QLQ domain, motif 3 constituted the GGPL domain, and motif 5 constituted the FFD domain. WRC and QLQ were present in all GRF gene families (except Bra021521), indicating that the GRF homologous genes among different species of the cruciferous family were highly conserved. Additionally, AtGRF3, AtGRF4 and BjGRF09, BjGRF10, BjGRF11, BjGRF013, BjGRF16, BjGRF17, BjGRF20, BjGRF28, BjGRF29, BjGRF30, BjGRF32 and BjGRF34 contain FFD. Most notably, BjGRF10, BjGRF16, BjGRF29, Bra019640 and Bnacnng50230D contained similar conserved motif types, which may have similar features.
Conserved motif, and gene structure of GRFs. (a) Conserved motif of GRF family in B. juncea, B. napus, B. rapa and Arabidopsis, with different colors of rods representing different motifs. (b) Structural analysis of the GRF family gene in B. juncea, B. napus, B. rapa and Arabidopsis, with yellow bars representing UTRs, green bars representing exons, and black lines representing introns.
The composition and number of gene introns and exons are critical for studying gene function. This study analyzed the gene structure of the B. juncea, B. napus, B. rapa and Arabidopsis GRF family (Fig. 3b) and found significant differences in the number of exons among different GRF genes: BjGRF, 2–12 exons, B. napus GRF, 2–10 exons, B. rapa GRF, 3–10 exons, and Arabidopsis GRF, 3–6 exons. In 64.7% of BjGRF genes, 38.57% (17) of B. napus GRF genes, 62.5% (10) of B. rapa GRF genes, and 55.56% (5) of B. rapa GRF genes, there were four exons. BjGRF28, Bnacnng50230D, Bra019640 and AtGRF8 have the highest number of exons in B. juncea, B. napus, B. rapa, and Arabidopsis, containing 12, 10, 10 and 6 exons, respectively. Furthermore, a higher number of exons was found in BjGRF10, BjGRF16 and BjGRF29 genes, indicating that the alternatively spliced forms were more complex. The gene structure of some BjGRFs was more complex than that of the GRF genes of B. napus, B. rapa and Arabidopsis.
Chromosomal localization and duplication of BjGRF genes
To elucidate the distribution and amplification characteristics of BjGRFs, gene duplication in the BjGRF family was analyzed (Fig. 4). In total, 48 pairs of duplicated genes were detected in BjGRF genes, all of which were segmental duplications and randomly mapped to 14 chromosomes (except A06, A08, B03, and B04). Three BjGRF genes were found on chromosomes A01, A04, B02, B05, B06 and B08, while a single gene was found on each of chromosomes A02, A09 and B07. Five BjGRF genes were found on chromosome A03, but two BjGRF genes each were found on chromosomes A05 and A07. Four BjGRF genes were located on chromosome B01. Additionally, no tandem duplication events were detected, suggesting that segmental duplications were the main driver for the expansion and evolution of BjGRF family members and played a major role in the evolution of BjGRF genes.
Prediction of cis-acting elements of BjGRF genes
We Used the PlantCARE website to predict and analyze the cis-acting elements in the 1.5 kb region upstream of the start codon of BjGRF genes, which was conducive to exploring the potential biological functions and regulatory mechanisms of BjGRF genes (Fig. 5). In total, 663 cis-acting elements were found in BjGRF genes (Fig. 5a), which were divided into four categories (Fig. 5b, Supplementary Table S3): hormone signaling (192 sites) (Fig. 5c), environmental stress responses (144 sites) (Fig. 5d), growth and development (36 sites) (Fig. 5e) and light-response elements (291 sites) (Fig. 5f). Light-response elements were presented in all BjGRF genes, indicating that BjGRF participated in photo-response regulation. Among the environmental stress-related elements, anoxic induction (GC motif and ARE), drought induction (MBS), and low-temperature response elements were found, suggesting that the BjGRF family played a role in the response to stress. Notably, BjGRF16 and BjGRF29 contained three drought-responsive elements. Additionally, five hormone-related elements, namely, abscisic acid (ABA) cis-acting element (ABRE), methyl jasmonate (MeJA) cis-acting element (TGACG-motif and CGTCA-motif), gibberellin (GA) response element (GARE-motif, P-box and TATC-box), auxin-response element (TGA-box and TGA-element) and salicylic acid (SA) response element (SARE and TCA-element), were found in most BjGRF genes. Circadian elements, meristem-related elements (CAT-box), endosperm expression (GCN4_motif and AACA_motif), and cell cycle regulation (MSA-like) were development-related elements found in BjGRF genes. In summary, the BjGRF genes contained many elements related to hormone responses and environmental stress, suggesting that they respond to adverse environmental effects by regulating different hormone pathways and responding to stress.
Prediction analysis of the BjGRF genes promoter. (a) Distribution of BjGRF genes in the 1.5 kb promoter region. (b) Number of light-responsive cis-elements, hormone-responsive cis-elements, stress-responsive cis-elements, and plant growth–related cis-elements in BjGRF genes. (c) Number of different hormone (MeJA, GA, ABA, auxin, and SA)-responsive cis-elements in BjGRF genes. (d) Number of environmental stress (anaerobic, drought, defense, stress, wound, and low temperature)–related cis-elements upstream of BjGRF genes. (e) Number of plant growth–related cis-elements in BjGRF genes. (f) Number of light–response cis-elements in BjGRF genes.
Expression of BjGRF genes in different tissue parts at seedling stage
To investigate the function of BjGRF genes at the seedling stage of B. juncea, we used qRT-PCR to analyze the expression of 34 BjGRF genes in different tissues (Fig. 6, Supplementary Table S4). There were differences in the expression of BjGRF genes in roots, stems and leaves. Nineteen BjGRF genes (BjGRF01, BjGRF03, BjGRF05, BjGRF08, BjGRF09, BjGRF11, BjGRF13, BjGRF14, BjGRF18, BjGRF19, BjGRF20, BjGRF21, BjGRF24, BjGRF25, BjGRF27, BjGRF28, BjGRF29, BjGRF30 and BjGRF33) were most expressed in roots. Notably, the homologous genes BjGRF11 and BjGRF20 were specifically expressed in roots; 11 BjGRF genes (BjGRF02, BjGRF04, BjGRF06, BjGRF07, BjGRF16, BjGRF17, BjGRF23, BjGRF26, BjGRF31, BjGRF32 and BjGRF34) showed the highest expression levels in leaves. Among those 11 BjGRF genes, the expression level of BjGRF16 in leaves was 11.0 times higher than that in roots, and 7.1 times higher than that in stems. Additionally, the expression of the BjGRF34 gene was relatively high in roots, stems, and leaves. BjGRF10, BjGRF12, BjGRF15 and BjGRF22 showed the highest expression levels in the stems. The various expression patterns suggested that BjGRF genes have different functions in B. juncea growth and development, provideing a basis for subsequent gene function studies.
Expression of BjGRF genes under simulated drought stress
Ten BjGRF genes were selected for qRT-PCR based on the phylogenetic relationship of BjGRF genes, their expression in different tissues and their cis-elements associated with drought stress (Fig. 7, Supplementary Table S5). The genes BjGRF03, BjGRF09, BjGRF16, and BjGRF32 had similar expression patterns, presenting an increase–decrease–increase trend, and the expression reached a peak at 3 h of drought stress. Furthermore, the expression levels of BjGRF03, BjGRF09, BjGRF16 and BjGRF32 genes were 17.6, 16.9, 10.0 and 15.3 times higher than those of the control (0 h) at 3 h after drought stress, respectively. Notably, BjGRF03 exhibited the highest expression level, which was 19.5 times greater than that of the gene with the lowest expression (BjGRF06), and BjGRF32 was induced to the greatest degree at 24 h after drought stress. In addition, the expression of BjGRF06, BjGRF23, BjGRF26, BjGRF29 and BjGRF34 peaked at 24 h, reaching 1.3, 1.7, 3.8, 4.0 and 5.0 times the control levels, respectively, and the expression trends of these five BjGRF genes differed. The expression of BjGRF06 gradually decreased after drought treatment (except for 24 h); BjGRF23 expression showed a decreasing–increasing trend, and the expression level was the lowest at 3 h; The expression level of BjGRF26 and BjGRF34 increased slightly at 3 h of stress and then decreased, but the expression level was the highest at 24 h of stress. The expression trends of BjGRF26 and BjGRF34 were similar, suggesting that they had similar or identical functions. The expression level of BjGRF29 was significantly increased at 3 and 24 h after stress, but the expression level at 24 h was 3.7 times that of BjGRF29 at 3 h. In conclusion, BjGRF03 and BjGRF32 genes may have specific biological functions in drought stress.
Discussion
Owing to the continuous changes in global climate, a study has focused on understanding how crops can resist drought stress and improve their resistance mechanisms18. Plants undergo changes in their morphological structure, gene expression, and metabolic processes after drought, which may lead to the termination of photosynthesis and disruption of metabolism, affecting crop yield and quality19,20,21. When plants perceive drought signals, they produce Ca2+, phosphatidylinositol, and other secondary messenger substances while increasing the concentration of intracellular calcium ions and initiating the regulatory network of the protein phosphorylation pathway22,23. Finally, the target protein directly participates in cell protection or regulates the expression of related stress genes through TFs to improve stress resistance in plants24,25. Therefore, TFs play a critical role in drought stress response. According to the sequence and DNA-binding characteristics of TFs that respond to drought stress, TFs can be divided into different families, such as the GRF, ERF, MYB, and WRKY families26.
The GRF gene family is a group of plant-specific TFs that play critical roles in various aspects of plant growth, development, signal transduction, and plant defense responses27. Since the identification of the first GRF gene in O. sativa28, an increasing number of GRF genes have been identified in multiple species, and they have been shown to affect plant growth, development, and stress responses8,29,30,31,32. With the publication of the genome sequence of B. juncea, the identification of the BjGRF gene family has become possible33. In this study, 34 BjGRF genes were identified in the whole genome of B. juncea, and they were named BjGRF01–BjGRF34 based on their positions on the chromosomes, all of which contained highly conserved QLQ and WRC domains. Analysis of the physical and chemical properties showed that the number of amino acids, and molecular weight of BjGRF (except BjGRF28) proteins were not significantly different, indicating that members of the BjGRF family might have similar functions. Gene structure analysis showed that 64.7% of BjGRF genes contained four exons, indicating that the structure of BjGRF genes was relatively evolutionarily conserved; however, there were exons in BjGRF10, BjGRF16, BjGRP28 and BjGRF29 than in the other BjGRF genes. Research has suggested that the addition or deletion of exons or introns may lead to differences in gene structure and function, resulting in the generation of new genes34,35,36. Therefore, we speculated that the intron of BjGRF was lost during evolution, which may have altered gene function. In line with existing studies, we also found that the number of introns was related to gene expression, and when the number of introns in genes was large, genes could quickly respond to various adverse factors.
Gene duplications are the main driver of genome and genetic evolution37. Related studies have shown that gene duplication not only increases the number of GRF genes but is also a means to produce new genes, which supports plant adaptation to various adverse environments38. In this study, a total of 48 pairs of duplicated genes were detected, all of which were segmental duplications, indicating that segmental duplication is the main mechanism for increasing the number of genes in the family. Segmental duplication has been reported to effectively promote the amplification of GRF gene family members in Arabidopsis and strawberries, and no tandem duplication has been found in this gene family in either species27,39. The results of this study are consistent with those of existing studies on Arabidopsis and strawberry families, suggesting that the GRF family can increase the number of genes and produce new genes through segmental duplication in different plants.
The tissue expression characteristics of genes can reflect the biological functions of genes, which can provide a basis for subsequent functional studies40,41. The GRF gene was found to be strongly expressed in the young leaves, flower buds and bud tips of rice, and affected plant growth and development by regulating cell proliferation12. HtGRF4/6/10/12/13 was found to exhibited higher expression in leaves than in roots and stems during seedling growth42. We found that 11 BjGRF genes were expressed at higher levels in seedlings and leaves than in roots and stems, and 19 BjGRF genes were highly expressed in roots, indicating that different BjGRF genes had different expression patterns and may play different roles in drought stress. Furthermore, the prediction of cis-acting elements revealed that 19 BjGRF genes in this family contained 1–3 drought response elements (MBS). Among them, BjGRF16 and BjGRF29 contained three drought-responsive elements, suggesting that they may be involved in drought stress through drought response elements. ABA is a key hormone involved in plant responses to drought stress. The promoter region of the 20 BjGRF genes had an ABA response element, while BjGRF06 and BjGRF34 contained more ABAE elements, both containing 4 elements, indicating that these BjGRF genes may be involved in the response to drought stress through the ABA signaling pathway. Under drought conditions, plants perceive external stimuli, and the transcription and protein levels of ABA synthase are upregulated upon receiving drought signals, leading to an increase in endogenous ABA content43. Additionally, the responses to drought stress and resistance to drought requiring gene regulation can be divided into two types according to the mode of action of drought stress genes: functional genes that have protective effects on plants and directly participate in improving the drought resistance ability of plants and regulatory genes that regulate signal transduction and gene expression. TFs, as regulatory genes, participate in the response to drought stress, whereas GRFs, as plant-specific TFs, play a role in plant growth and abiotic stress by coordinating the stress response and defense signals4,32,44. Regulatory functions have also been reported in response to drought stress. Du et al. found that the expression levels of MtGRF2 and MtGRF8 in alfalfa are increased under drought stress45. In wheat, the expression of the TaGRF21 gene was found to be significantly up-regulated under drought stress 15. In this study, qRT-PCR showed that BjGRF03, BjGRF09, BjGRF16, and BjGRF32 had a similar expression trend after drought treatment, which reached their maximum expression after 3 h of stress; BjGRF06, BjGRF23, BjGRF26, BjGRF29 and BjGRF34 were significantly induced after 24 h of drought stress, and the induction degree reached the maximum. Notably, the expression level of BjGRF03 peaked at 3 h, and its expression level was the highest among the 10 genes at this time point, which was 17.6 times higher than that of the control (0 h), indicating that BjGRF03 gene could be significantly induced; thus, we speculated that it played a critical role in the signaling pathway of drought stress. Furthermore, BjGRF32 was significantly induced at 3 and 24 h under drought stress. Most GRF genes are the target genes of miR396, which can directly inhibit GRF expression through post-transcriptional regulation, and drought can induce the expression of miR39645. AtGRF7, AtGRF8 and AtGRF9 were confirmed to be the target genes of Ath-miR396 by 5’-RACE, and the drought resistance was enhanced after overexpression of Ath-miR396 46. miR396 was induced in wild-type Arabidopsis leaves after drought stress, but was accompanied by significant down-regulation of AtGRF1 and AtGRF346. Overexpression of SiGRF1 inhibited root growth and reduced susceptibility to drought stress, and SimiR396d and SiGRF1 regulate root growth under drought stress via ethylene signaling47. The expression level of AtGRF7 was inhibited under high salt and drought conditions to activate osmotic stress response genes8. In the phylogenetic relationship, AtGRF7, AtGRF8 and AtGRF9 TFs are clustered in the same clade as BjGRF06 and BjGRF23, and the expression levels of BjGRF06 and BjGRF23 gene decrease after drought stress, suggesting that they are involved in drought stress as miR396 target genes. In summary, BjGRF03 and BjGRF32 genes may have a positive regulatory role in drought stress, but BjGRF06 and BjGRF23 play a role in drought stress as miR396 target genes. However, the detail functions of these genes in B. juncea still need to be further determined in the future.
Conclusions
In this study, 34 BjGRF genes were found in B. juncea, which were divided into three subfamilies exhibiting similar conserved motifs and gene structures. Furthermore, 48 pairs of segmental duplications were found in B. juncea via collinearity analysis. The BjGRF promoter region contained cis-acting elements related to light response, hormone response, environmental stress response, and growth/development. We also detected the expression of 34 BjGRF genes in the seedling stage (roots, stems, and leaves) of B. juncea, as well as the expression patterns of 10 BjGRF genes under drought conditions, We found that the expression patterns of BjGRF genes were similar under drought stress and that they might be involved in the regulation of drought stress. BjGRF03 and BjGRF32 genes might have a positive regulatory role in drought stress, whereas BjGRF06 and BjGRF23 played a role in drought stress as miR396 target genes. In general, our study lays a biological foundation for the future discovery of the functions of BjGRF genes in cruciferous plants.
Methods
Materials and treatments
B. juncea seeds used in this experiment were provided by the Oil Research Institute of Guizhou Province, Guizhou Academy of Agricultural Sciences. Whole seeds were selected and planted in soil (substrate:soil = 3:1) until the four-leaf stage, after which the roots, stems, and leaves were collected. Plants were subjected to a drought simulation treatment with 20% PEG 6000, and the leaves were collected at 0, 3, 6, 12, and 24 h. All plant samples were immediately frozen in liquid nitrogen and then stored in a deep freezer at –80 ℃ for the next experiment.
Identification of BjGRF family genes
To identify GRF genes in the whole genome of B. juncea, the hidden Markov model files of WRC (PF08879) and QLQ (PF08880) domains were downloaded from the Pfam database (http://pfam.xfam.org/)48. All BjGRF proteins in the B. juncea protein database were searched using HMMER software (version 3.0; http://hmmer.org/) with the E-value of < e−5. A specific hidden Markov model of B. juncea was constructed and searched again in the B. juncea protein database, with an E-value threshold of < 10−3. The protein sequences of candidate genes were submitted to the Pfam and NCBI-CDD (https://www.ncbi.nlm.nih.gov/cdd/) databases to verify the WRC and QLQ domains and screen reliable BjGRF candidate genes. The physicochemical properties of proteins, including the number of aa, molecular weight (kDa), theoretical isoelectric point, instability index, aliphatic index, and mean value of hydrophilicity (GRAVY), were predicted using ProtParam (https://web.expasy.org/protparam/)49. The subcellular localization was predicted using WoLF PSORT (https://wolfpsort.hgc.jp/).
Phylogenetic and homology analysis of GRF family members
Phylogenetic relationships were analyzed using full-length amino acids sequences of GRF TFs in Arabidopsis, B. rapa, B. napus, rice, foxtail millet and B. juncea. A phylogenetic tree was constructed using the neighbor-joining (NJ) method in MEGA7.0, with 1,000 bootstrap replications50, and visualized using the online software Evolview (https://evolgenius.info/evolview-v2/)51. The genome annotation file (GFF3) and CDS sequences of Arabidopsis and B. rapa were downloaded from the Ensembl Plants database, and the homology of the GRF gene between BjGRF and Arabidopsis and B. rapa was determined using MCScanX software (http://chibba.pgml.uga.edu/mcscan2/)52.
Conserved motif and gene structure of BjGRFs
The CDS and DNA sequences of BjGRF genes were submitted to the Gene Structure Display Server (http://gsds.gao-lab.org/) website to determine the gene structure53. The conserved motif of BjGRF protein was predicted using the online program MEME (https://meme-suite.org/meme/) with the following parameters: maximum number of motifs 15; motif width, between 6 and 100 aa. The conserved motif was visualized using TBtools (version v2.119) software (https://github.com/CJ-Chen/TBtools-Manual)54.
Prediction of cis-acting elements of the BjGRF gene promoter and gene duplications
The 1.5 kb sequence upstream of the BjGRF gene start code was extracted using Perl language and submitted to the PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) to identify the cis-acting elements in BjGRF genes55. Segmental duplications and tandem repeat genes of BjGRF family members were obtained using MCScanX software52, and segmental duplications and tandem repeat genes between BjGRF genes were analyzed using Circos-0.69-8 software (https://circos.ca/software/download/)56.
Expression pattern analysis
A SteadyPure Plant RNA Extraction Kit (Accurate biotechnology, China) was used to extract total RNA from the roots, stems, and leaves of B. juncea seedlings. RNA was also extracted from the leaves after PEG 6000 treatment, and the concentration and quality of RNA were determined using a micro-ultraviolet spectrophotometer. The cDNAs were synthesized using an EvoM-MLV reverse transcription premix kit (Accurate biotechnology, China) and stored at –20 °C. The ChamQ Universal SYBR qPCR Master Mix kit (Vazyme, China) was used for qRT-PCR, with BjUBQ9 as the internal reference gene, according to the manufacturer’s protocol. The procedure of qRT-PCR was as follows: 95 °C for 5 min at the pre-denaturation stage; 40 cycles of 95 °C for 15 s and 60 °C for 30 s at the PCR stage; and 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s at the melt curve stage, with three repeats for each treatment. The 2−ΔΔCt57 method was used to calculate the relative expression of genes. Data collation, analysis, and visualization were performed using Excel 2010, and one-way analysis of variance was performed using SPSS 22.0 software. The LSD method was used for multiple ratios (P < 0.05). Primers and information for qRT-PCR are listed in Supplementary Table S6.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Abid, M. et al. Genome-wide identification and structural characterization of growth-regulating factors (GRFs) in Actinida eriantha and Actinidia chinensis. Plants 11, 1633 (2022).
Kim, J. H., Choi, D. & Kende, H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 36, 94–104 (2003).
Zhang, D.-F. et al. Isolation and characterization of genes encoding GRF transcription factors and GIF transcriptional coactivators in Maize (Zea mays L.). Plant Sci. 175, 809–817 (2008).
Liu, H. et al. OsmiR396d-regulated OsGRFs function in floral organogenesis in rice through binding to their targets OsJMJ706 and OsCR4. Plant Physiol. 165, 160–174 (2014).
Huang, W. et al. Genome-wide analysis of growth-regulating factors (GRFs) in Triticum aestivum. PeerJ 9, e10701 (2021).
Carrington, J. C. & Ambros, V. Role of microRNAs in plant and animal development. Science 301, 336–338 (2003).
Curaba, J., Singh, M. B. & Bhalla, P. L. miRNAs in the crosstalk between phytohormone signalling pathways. J. Exp. Bot. 65, 1425–1438 (2014).
Kim, J. S. et al. Arabidopsis growth-regulating factor7 functions as a transcriptional repressor of abscisic acid- and osmotic stress-responsive genes, including DREB2A. Plant Cell 24, 3393–3405 (2012).
Chen, L., Luan, Y. & Zhai, J. Sp-miR396a-5p acts as a stress-responsive genes regulator by conferring tolerance to abiotic stresses and susceptibility to Phytophthora nicotianae infection in transgenic tobacco. Plant Cell Rep. 34, 2013–2025 (2015).
Kim, J. H. Biological roles and an evolutionary sketch of the GRF-GIF transcriptional complex in plants. BMB Rep. 52, 227–238 (2019).
Sakuma, Y. et al. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 18, 1292–1309 (2006).
Choi, D., Kim, J. H. & Kende, H. Whole genome analysis of the OsGRF gene family encoding plant-specific putative transcription activators in rice (Oryza sativa L.). Plant Cell Physiol. 45, 897–904 (2004).
Ma, J. Q. et al. Genome-wide analysis and expression profiling of the GRF gene family in oilseed rape (Brassica napus L.). Gene 620, 36–45 (2017).
Cao, J. F. et al. Genome-wide characterization of the GRF family and their roles in response to salt stress in Gossypium. BMC Genom. 21, 575 (2020).
Zan, T., Zhang, L., Xie, T. & Li, L. Genome-wide identification and analysis of the growth-regulating factor (GRF) gene family and GRF-interacting factor family in Triticum aestivum L.. Biochem. Genet. 58, 705–724 (2020).
Chen, H. & Ge, W. Identification, molecular characteristics, and evolution of GRF gene family in foxtail millet (Setaria italica L.). Front. Genet. 12, 727674 (2021).
Wang, F. et al. Genome-wide identification and analysis of the growth-regulating factor family in Chinese cabbage (Brassica rapa L. ssp. pekinensis). BMC Genom. 15, 807 (2014).
Tan, X., Li, S., Hu, L. & Zhang, C. Genome-wide analysis of long non-coding RNAs (lncRNAs) in two contrasting rapeseed (Brassica napus L.) genotypes subjected to drought stress and re-watering. BMC Plant Biol. 20, 81 (2020).
Kaur, G. & Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 61, 201–209 (2017).
Barnabás, B., Jäger, K. & Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 31, 11–38 (2008).
Daryanto, S., Wang, L. X. & Jacinthe, P.-A. Global synthesis of drought effects on cereal, legume, tuber and root crops production: a review. Agric. Water Manag. 179, 18–33 (2017).
Xiong, L., Schumaker, K. S. & Zhu, J. K. Cell signaling during cold, drought, and salt stress. Plant Cell 14, S165–S183 (2002).
Huang, G. T. et al. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 39, 969–987 (2012).
Vinocur, B. & Altman, A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr. Opin. Biotechnol. 16, 123–132 (2005).
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D. & Basra, S. M. A. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29, 185–212 (2009).
Baldoni, E., Genga, A. & Cominelli, E. Plant MYB Transcription factors: their role in drought response mechanisms. Int. J. Mol. Sci. 16, 15811–15851 (2015).
Omidbakhshfard, M. A., Proost, S., Fujikura, U. & Mueller-Roeber, B. Growth-regulating factors (GRFs): A small transcription factor family with important functions in plant biology. Mol. Plant. 8, 998–1010 (2015).
van der Knaap, E. K., Kim, J. H. & Kende, H. A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth. Plant Physiol. 122, 695–704 (2000).
Zhang, B. et al. Identification of growth-regulating factor transcription factors in lettuce (Lactuca sativa) genome and functional analysis of LsaGRF5 in leaf size regulation. BMC Plant Biol. 21, 485 (2021).
Lee, S. J. et al. Growth-regulating factor and Grf-interacting factor specify meristematic cells of gynoecia and anthers. Plant Physiol. 176, 717–729 (2018).
Bao, M. et al. miR396a-mediated basic helix-loop-helix transcription factor bHLH74 repression acts as a regulator for root growth in Arabidopsis seedlings. Plant Cell Physiol. 55, 1343–1353 (2014).
Casadevall, R., Rodriguez, R. E., Debernardi, J. M., Palatnik, J. F. & Casati, P. Repression of growth regulating factors by the microRNA396 inhibits cell proliferation by UV-B radiation in Arabidopsis leaves. Plant Cell 25, 3570–3583 (2013).
Kang, L. et al. Genomic insights into the origin, domestication and diversification of Brassica juncea. Nat. Genet. 53, 1392–1402 (2021).
Xu, G., Guo, C., Shan, H. & Kong, H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. U. S. A. 109, 1187–1192 (2012).
Liang, Z., Li, M., Liu, Z. & Wang, J. Genome-wide identification and characterization of the Hsp70 gene family in allopolyploid rapeseed (Brassica napus L.) compared with its diploid progenitors. PeerJ 7, e7511 (2019).
Rogozin, I. B., Sverdlov, A. V., Babenko, V. N. & Koonin, E. V. Analysis of evolution of exon-intron structure of eukaryotic genes. Brief. Bioinform. 6, 118–134 (2005).
Moore, R. C. & Purugganan, M. D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. U. S. A. 100, 15682–15687 (2003).
Chen, F. et al. Genome-wide identification of GRF transcription factors in soybean and expression analysis of GmGRF family under shade stress. BMC Plant Biol. 19, 269 (2019).
Li, Z., Xie, Q., Yan, J., Chen, J. & Chen, Q. Genome-wide identification and characterization of the abiotic-stress-responsive GRF gene family in diploid woodland strawberry (Fragaria vesca). Plants 10, 1916 (2021).
Weiss, J. et al. Diel pattern of circadian clock and storage protein gene expression in leaves and during seed filling in cowpea(Vigna unguiculata). BMC Plant Biol. 18, 33 (2018).
Wong, D.-C., Sweetman, C. & Ford, C. M. Annotation of gene function in citrus using gene expression information and co expression networks. BMC Plant Biol. 14, 186 (2014).
Ding, B., Yue, Y., Chen, X., Long, X. & Zhou, Z. Identification and expression analysis of miR396 and its target genes in Jerusalem artichoke under temperature stress. Gene 893, 147908 (2014).
Kim, T. H., Böhmer, M., Hu, H., Nishimura, N. & Schroeder, J. I. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61, 561–591 (2010).
Liu, J., Rice, J. H., Chen, N., Baum, T. J. & Hewezi, T. Synchronization of developmental processes and defense signaling by growth regulating transcription factors. PLos ONE 9, e98477 (2014).
Du, W. et al. Genome-wide identification and characterization of growth regulatory factor family genes in Medicago. Int. J. Mol. Sci. 23, 6905 (2022).
Liu, D. et al. Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plant. 136, 223–236 (2009).
Zhang, Y., Xiao, T., Yi, F. & Yu, J. SimiR396d targets SiGRF1 to regulate drought tolerance and root growth in foxtail millet. Plant Sci. 326, 111492 (2023).
Mistry, J. et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 49, D412–D419 (2021).
Duvaud, S. et al. Expasy, the Swiss bioinformatics resource portal, as designed by its users. Nucleic Acids Res. 49, W216–W227 (2021).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Zhang, H., Gao, S., Lercher, M. J., Hu, S. & Chen, W. H. EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res. 40, W569–W572 (2012).
Wang, Y. et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49 (2012).
Hu, B. et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31, 1296–1297 (2015).
Chen, C. et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202 (2020).
Rombauts, S., Déhais, P., Van Montagu, M. & Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 27, 295–296 (1999).
Krzywinski, M. et al. Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645 (2009).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408 (2001).
Acknowledgements
We thank for lab members for their assistance in this study. We would like to thank Editage (www.editage.cn) for English language editing the English text of a draft of this manuscript.
Funding
This work was funded by Guizhou Provincial Basic Research Program (Natural Sciences), No.Qiankehejichu-ZK[2024]generally566;No.Qiankehejichu-ZK[2024]generally560, The National Natural Science Foundation of China, 32060146, The Scientific and Technological Key Program of Guizhou province, No.Qiankehezhicheng[2022] Key 031;No.Qiankehezhicheng[2022] Key 026, The Guizhou Provincial Science and Technology Foundation, No.Qiankehejichu[2019]1306.
Author information
Authors and Affiliations
Contributions
Y.Z. wrote the manuscript. F.L., L.W. and B.Y. helped with bioinformatics analysis and created the figures. H.J., R.T. and S.L. helped revamp the manuscript. H.X. and C.Z. guided the experiments and revised the manuscript. All authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The collection of specimens conformed to the requirement of international ethics, which did not incur any damage to the environment and the species itself. The process and purpose of this experimental research were in line with the rules and regulations of our institute. There are no ethical issues or specific permissions are required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Jiang, H., Liang, F. et al. Genome‑wide identifcation and expression analysis of growth-regulating factors under drought in Brassica juncea. Sci Rep 14, 29835 (2024). https://doi.org/10.1038/s41598-024-80941-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80941-x
This article is cited by
-
Role of HvmiR396 and its Target Gene (HvGRF3a) in Barley Inflorescence Development under Drought Stress
Journal of Plant Growth Regulation (2025)