Abstract
Patients with chronic kidney disease have a high incidence of cardiovascular diseases, and autonomic dysfunction has a determinant role in the relevant declines. Physical exercise influences heart rate variability and cardiac autonomic modulation. Thus, our objective was to systematically review, with a meta-analysis, the correlation between physical exercise interventions and alterations in cardiac autonomic modulation in hemodialysis patients. A customized research strategy was used across four databases. The search yielded 392 studies, with eight randomized clinical trials included (396 participants), indicating that the investigated indices favor the intervention group by increasing autonomic activity. The exercise training probably increases the standard deviation of all NN intervals (20.71 ms CI 95% [9.55, 31.87], p < 0.001, I²=95%) compared to the control group and showing an moderate certainty, was the most commonly used index (seven studies). Mean RR (35.57 ms CI 95% [14.56, 56.57], p = 0.91, I²=0%), the root mean square sum of squares of differences between NN intervals (10.55 ms CI 95% [6.75, 14.34], p = 0.37, I²=4%), and LF/HF (0.28 ms (n.u) [0.11, 0.44], p = 0.18, I²=39%) were also in favor of the training group. However, based on the GRADE analysis we are uncertain whether Mean RR can increase after an exercise intervention, as well RMSSD and LF/HF may increase slightly, we obtained low certainty of this evidence. The exact magnitude of the impact of physical training on the alteration of cardiac autonomic modulation in this patient population has yet to be conclusively defined.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is characterized by a progressive and irreversible decrease in renal function1. According to data from the International Society of Nephrology, hemodialysis is the most prevalent renal replacement therapy, with an average rate of 343 individuals per million2. Patients with CKD have many adverse effects resulting from the disease, such as a reduction in lean mass, mineral bone density, physical exercise intolerance, arterial hypertension, and physical capacity reduction3,4,5.
Another consequence for these patients is the high incidence of cardiovascular disease (CVD), which has emerged as the leading cause of mortality2,6,7. Among the contributing factors to CVD incidence, autonomic dysfunction has a determinant role8. Autonomic dysfunction is characterized by an increase in sympathetic activity and/or a reduction in parasympathetic activity and is strongly correlated with the risk of all causes9,10,11 of mortality. In the past few years, the scientific community has expressed increased interest in implementing a physical exercise routine for patients with CKD to attenuate the deleterious effects caused by kidney failure12,13,14,15,16,17,18,19,20,21. Few studies have evaluated the benefits of physical exercise on cardiac autonomic modulation, given that this system functioning contributes to mortality22, understanding how these interventions impact the heart rate variability (HRV) of these patients becomes important.
One of the adaptations to physical exercise is the modification of HRV through improvements in cardiac autonomic modulation and cardiovascular function23,24,25. A recent review25 demonstrated the effects of physical exercise on cardiovascular outcomes for patients with CKD, however, only studies in which the intervention modality was performed during the intradialytic period were included. Understanding the role of physical exercise beyond the intradialytic period is important for this population because many clinical centers do not offer the possibility of implementing a physical exercise program during hemodialysis, so understanding the effects of exercise beyond this period can make it more widespread26,27. Therefore, this systematic review with meta-analysis aimed to clarify the relationship between physical exercise interventions and changes in cardiac autonomic modulation in hemodialysis patients.
Results
Searches
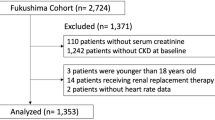
The search resulted in 392 articles. After duplicate removal (n = 32), 360 articles remained for title and abstract evaluation. Seventeen studies met the established criteria for full reading, with nine exclusions (Fig. 1).
Characteristics of the studies
Table 1 summarizes the study’s methods and the HRV analyses. The studies ranged from 3 to 12 months of intervention, three studies exclusively used aerobic exercise28,29,30, four interventions utilized concurrent exercise (aerobic + resistance)31,32,33,34, and one used breath-based lower limb training35. In all the studies, the participants were trained at least three times a week. Most studies used 24-hour ambulatory electrocardiogram recordings obtained from an ECG Holter device on nondialysis days to document HRV data. Only the Pereira28 (Polar RS800CX; Polar Electro™, Kempele, Finland) and Huang35 (8Z11, Enjoy Researcher, Inc., Taiwan) studies used a heart rate monitor to assess HRV.
Risk of study bias
The PEDro scale classified one28 study as methodologically high quality, four were rated as moderate quality29,30,31,32,33,35, and one article as low quality34. Table 2 shows the checklist for this scale.
Certainty of evidence
Only the SDNN index was moderate certainty, while the others were low certainty (LF/HF and RMSSD) and a very low certainty to Mean RR (Supplementary Material 2).
Quality of the training protocol
After analysis with the CERT, none of the articles achieved a high-quality classification. The percentages obtained were the following: Deligiannis et al.34, 21%; Reboredo et al.29, 47%; Huang et al.35, 53%; Mitsiou et al.30, 58%; Pereira et al.28, 58%; Kouidi et al.33, 58%; Kouidi et al.32, 68%; and Michou et al.31, 68%.
Analysis of HRV indices
The HRV data were divided into time domain (SDNN, MeanRR, and RMSSD) (Fig. 2) and frequency domain (LF/HF) data (Fig. 3), presented in milliseconds and milliseconds (n.u), respectively. Analyses that included the article by Kouidi et al.32 had the correlation coefficient calculated from this study and applied to the calculation of the standard deviation of the others.
Time domain
The SDNN index was the most used (seven studies), totaling 310 participants (160 intervention and 150 control). A summary measure of random effects was applied (as well as all the other variables in the time domain), revealing significant differences between groups (20.71 ms CI 95% [9.55, 31.87], p < 0.001, I²=95%), which were favorable for the group that received physical training. Mean RR (35.57 ms CI 95% [14.56, 56.57], p = 0.91, I²=0%), and RMSSD (10.55 ms CI 95% [6.75, 14.34], p = 0.37, I²=4%) also were favorable to intervention but not reached significant difference between the groups (p > 0.05). The absolute difference between means served as the summary measure in three analyses. Although this measure yielded a significant value, the SDNN analysis displayed high heterogeneity, indicating substantial variability (Fig. 2).
Frequency domain
Analyses in the frequency domain were the least common in the studies found. The Huang et al.35 and Reboredo et al.29 studies were eligible for the LF and HF analyses but were not included in the meta-analysis because they did not present data that would allow obtaining the standard deviation as recommended by the Cochrane Handbook and described in our methods. Thus, only LF/HF (0.28 ms (n.u) [0.11, 0.44], p = 0.18, I²=39%) was included in the meta-analysis with a summary measure of absolute differences and the use of random effects. LF/HF showed favorable to the intervention group and moderate heterogeneity (Fig. 3).
Discussion
This systematic review and meta-analysis examined the relationship between physical exercise interventions and alterations in cardiac autonomic modulation among hemodialysis patients. Our analysis included eight clinical trials with a total of 396 patients. One of the main findings is that all the time and frequency domain indices evaluated were favorable for the intervention group, evidenced by an increase in HRV activity. It is important to emphasize that even an index of observed parasympathetic activity (RMSSD) increased significantly in the intervention groups, representing a signal of exercise benefits for cardiovascular health in this population. CVDs are responsible for the majority of deaths in patients undergoing hemodialysis36. Estimations have indicated that 843.6 million people worldwide are affected by CKD, and in 2030, there will be an increase of 49% for individuals in the final disease stage2,37. Therefore, evaluating the autonomic nervous system and treating CVD are valuable, and HRV and physical exercise may complement each other due to their cost-effectiveness38,39.
Patients with CKD are a population that presents autonomic dysfunction, commonly represented by increased sympathetic activity and reduced HRV, as well as suppressed parasympathetic system action even in situations of a marked increase in it, such as at rest28,40,41,42. However, looking at the GRADE analyses, even though it is favorable to the intervention group in the Mean RR analyses, we are uncertain whether intervention can increase this outcome. The RMSSD and LF/HF may increase slightly, but this finding shows low certainty. In contrast, there is moderate certainty that physical exercise interventions lead to a probably increase in the SDNN index for these patients.
Our review elucidates some questions concerning the potential effects of training on the cardiac autonomic modulation of patients on hemodialysis. Among the studies found, Deligiannis et al.34, Kouidi et al.32, and Tourkantonis et al.34 reported an increase in the HRV expressed by the SDNN and a reduction in the vulnerability to arrhythmias in the training group compared to the control group. Kouidi et al.32 reported the same results regarding the SDNN index. Kouidi et al.33,] in addition to the beneficial changes in the SDNN, reported an improvement in parasympathetic activity indices, the RMSSD, and pNN50. Michou et al.31 recently showed that in diabetic kidney disease patients, similar results revealed significant improvements in the SDNN, RMSSD, and LF, as well as improvements in values that represent a better cardiorespiratory system, such as METs and VO2 peak. In this case, however, it is worth noting that, for both studies, the intervention strategy was concurrent training (aerobic + resistant).
Reboredo et al.29 and Huang et al.35 did not find significant changes in the comparison between groups; however, unlike the ones mentioned above, both studies received isolated training intervention, only aerobic training and lower limb exercises with respiratory control. Contrary, Pereira et al.28 reported an increase in HRV in the intervention group for both global (SDNN and SD2) and parasympathetic (RMSSD and SD1) indices. Mitsiou et al.30. reported increases in HRV after the training protocol for SDNN and LF (global indices) and parasympathetic indices (RMSSD and pNN50), as well did the studies by Reboredo et al.29 and Huang et al.35, which involved aerobic training alone for three and six months, respectively. These studies differently obtained a favorable outcome for the training group compared to their control peers, highlighting the gaps found in the current literature in elucidating the role of physical exercise in the HRV of patients on hemodialysis.
The results of Pereira et al.28 and Mitsiou et al.30 are similar to others found in the literature with patients with cardiovascular impairments who received aerobic training between nine and 12 weeks, such as patients with recent coronary events (increased SDNN as well as nocturnal HRV)43, heart failure (increase in SDNN and HF, respectively)44,45, acute myocardial infarction (improvement in LF and HF indices)46 and coronary artery disease (increased HRV)47. The results of long-term interventions involving six months of aerobic exercise were similar to the findings of Deligiannis, Kouidi, and Tourkantonis34 and Kouidi et al.32,33. Mazzuero et al.48 reported an increase in the SDNN index in postinfarcted patients, as did Villafaina and colleagues49; however, patients with fibromyalgia were the subject of the study (this population also presented autonomic impairment). Finally, Pietila et al.50 verified an increase in HRV and the HF index, an index of predominantly vagal activity.
In a recently published review, Verrelli et al.25 aimed to evaluate the cardiovascular outcomes of intradialytic physical exercise interventions. Among the main findings, the role of exercise in promoting pulse wave velocity, diastolic blood pressure, left ventricular ejection fraction and even heart rate variability stands out. These data are in the same direction as our findings and with another reviews40,51,52, suggesting that physical exercise carried out inside or outside the hemodialysis session has a beneficial effect on the cardiovascular health of these individuals. However, the review did not set out to analyze indices of parasympathetic predominance, an important marker of cardiac autonomic modulation, in addition, HRV ends up being a secondary analysis. As mentioned in the introduction, understanding physical exercise beyond the intradialysis moment is important, given that it has several limitations for it to occur during the session, being limited to a few modalities and failing to cover a significant portion of these. patients, as this modality still requires greater implementation in dialysis centers27.
Some of the possibilities for the absence of significance in the other indices of the studies included in this review are related to the low/moderate quality of most studies; intervention; and control groups, which had a small sample size; training strategy; intervention time; overload control; and a low number of articles on the subject included in the analysis. The methods analyses utilizing the CERT in different studies indicate the difficulty of replicating the protocol regarding the choice of overload and training protocol. Thus, the inferences about overload are difficult, even though this is a relevant training parameter to obtaining significant results53. However, this characteristic is not exclusive to the technical literature of this review54.
Additionally, when analyzing the training protocols, it was possible to note that the studies31,32,33,34 that used concurrent training (aerobic + resistance) presented at least one index with an improved response in the training group compared to others that only utilized aerobic exercise. Mitsiou et al.30. and Pereira et al.28. obtained similar results using an isolated training strategy (aerobic), and Reboredo et al.29. did not observe significant improvement in aerobic training adopted in isolation, despite the vast body of literature indicating that this modality can improve cardiorespiratory function, as well as autonomic indices24,55,56,57. Therefore, there is a clear need for more controlled studies using physical exercise in isolation.
Our group recommends conducting new clinical trials due to the limited availability of studies on the topic, which hinders the extrapolation of results, such as subgroup analyses. We observed a lack of studies for all training modalities, whether concurrent, resistant, or aerobic. In addition, the training protocols are often poorly described, and the study methods lack the necessary academic rigor and clarity in their design and presentation. The sample size was sometimes not calculated due to the complexity of this type of intervention in recruiting patients, but adding this parameter to the experiment is necessary for advancing the technical quality of the intrinsic findings of studies and those extrapolated by a meta-analysis. Finally, we suggest the standardization of HRV analysis and the use of the reunited indices, at least concerning the most used to assess the parasympathetic activity (RMSSD and HF), as these indices are closely linked to the occurrence of CVD in this population and are well characterized as being reduced to the detriment of the sympathetic activity.
Our results indicated significant improvements in the autonomic response for the mean RR, SDNN, RMSSD, and LF/HF indices that occurred after the training protocol. However, extrapolation of these findings needs caution, since the level of certainty observed was low and very low for most of them. Nevertheless, these findings suggest a promising direction for physical activity programs for patients with CKD on hemodialysis, due to the cardiac risks in this population. Thus, improvements in the autonomic nervous system may indicate greater longevity for these individuals. Despite these findings, the magnitude of the impact of physical training on changing cardiac autonomic modulation in patients undergoing hemodialysis, as well as the degree of recommendation and certainty of evidence, still need attention.
Among the limitations of this review, we highlight the lack of subgroup analyses by type of physical exercise and moment of intervention (intradialytic and non-dialytic), the small number of included studies making some analyses unfeasible, and the inclusion of studies not being conditioned on their quality. Strengths include the meticulously designed search strategy for each database, methodological rigor in data extraction and analysis using different tools, and the inclusion of only randomized controlled studies with different physical exercise interventions. It is expected that this study can be used as a reference for the application and adoption of physical training programs in clinics and hemodialysis centers as well as to encourage public policies that promote physical exercise for these patients. Furthermore, other authors could understand the current gaps regarding physical training and cardiac autonomic modulation in patients with CKD who undergo renal replacement therapy.
In conclusion, the study reviewed the scientific literature to understand how physical exercise has been applied to improve the autonomic nervous system in patients with CKD undergoing hemodialysis. Results indicated that physical interventions can increase the HRV in these patients for indices in time and frequency domains, suggesting benefits for cardiovascular health.
Methods
Study design
The study is designed to meet the requirements of a systematic review with meta-analysis following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) model58 and the Participants, Interventions, Comparisons, Outcomes, and Study Design (PICOS) strategy59. Following the PICOS model, we defined: (i) Participants: Patients with chronic kidney disease undergoing hemodialysis treatment; (ii) Interventions: Physical exercises; (iii) Comparisons: Control groups without physical exercises; (iv) Outcomes: Heart rate variability; (v) Study Design: Randomized clinical trials. This study was registered in the PROSPERO database (CRD42021255073) to obtain a low risk of bias and reduce the duplication chances of the reviewed topic.
Eligibility criterion
The inclusion criteria for eligible studies were as follows: randomized clinical trials with patients in the end-stage of CKD receiving hemodialysis treatment and a control group; any physical exercise intervention for at least 12 weeks; at least three months of treatment and older than 18 years of age; and HRV as an outcome. The exclusion criteria included commentary articles, summaries, animal model experiments, systematic reviews and/or meta-analyses, studies involving patients not receiving hemodialysis or other renal replacement therapy, and transplant recipients. The searches had no date restriction, prioritizing those published in electronic bibliographic databases, and contact was made with the authors for eventual clarifications about the absence of complete articles or analysis and data contained in your summary.
Search strategy
The search strategy was applied to the following databases: EMBASE, MEDLINE, Cochrane Library (CENTRAL), and PEDro. When necessary, the corresponding authors were contacted to answer questions about the indices used to analyze HRV and further crucial information. The selection of keywords was conducted by searching the MeSH (Medical Subject Headings) platform for the MEDLINE and The Cochrane Library databases, Emtree for EMBASE, and DeCS (Health Sciences Descriptors) for the PEDro database (Table 3). The PICOS59 strategy and a systematic review60 related to the main theme published at the CENTRAL were used to create the descriptor pattern. To the searches, certain terms were added because they were essential for this study, such as heart rate variability (Emtree was the only platform that contained), autonomic modulation, sympathetic hyperactivity, cardiac autonomic modulation, and autonomic dysfunction. The last search update was made on September 20, 2024.
Study selection
Two authors (HSD and PHM) independently conducted the searches on the databases (the search strategy is available in supplementary material 1) following a peer-review model. The authors imported the search results into the software ®Rayyan61 (http://rayyan.qcri.org). After duplicate removal, selections were made based on title and abstract reviews, with full-text assessments conducted when necessary. During the whole process, if the pair disagreed on the inclusion/exclusion of an article, a third author (CSCR) was asked to resolve any disagreements among the pairs.
Both reviewers independently read and included articles that met the eligibility criteria. For this purpose, a standardized form was used, and both reviewers conducted the process independently. Later bias analysis tools, as well as more rigorous methodological quality. It is worth noting that the methodological quality assessment form was solely employed for an initial evaluation of the methodological content within the respective articles, acting as a filter for the types of studies considered.
Outcomes
The investigated outcome, heart rate variability, was analyzed with the following indices: in the time domain, the standard deviation of all NN intervals (SDNN); the root mean square sum of squares of differences between NN intervals (RMSSD), which represents global activity and parasympathetic activity; and the mean RR interval between all regular beats (mean RR), which represents global activity. In the frequency domain, the LF/HF ratio analysis considered it an indicator of the balance between low frequency (LF – global activity) and high frequency (HF – parasympathetic activity).
Summarizing results measures
Effect measures utilized for group comparisons involved analyzing the absolute difference between means (mean difference), as the studies employed the same continuous scale. Following article selection, the application of a fixed or random effect model was also evaluated, depending on the internal disparity of studies.
The analyses were based on the Cochrane Handbook for Systematic Reviews of Interventions, where we computed the differences between post - and pre-mean for each index and group related to the HRV indices for the mean difference. To obtain the standard deviations in analyses with articles that provided sufficient information, we calculated the correlation coefficient for the intervention and control groups and used it to obtain the standard deviation in the other studies analyzed using the same index (Cochrane Handbook Chap. 6.5.2.8). However, in analyses without any study with sufficient information, we adopted the use of the p-value obtained in the comparison of the means between groups of the articles. After this, we obtained the t-value using the Cochrane online calculator. Finally, we calculate the standard error and standard deviation (Cochrane Handbook Chap. 6.5.2.3).
Furthermore, as suggested by the Handbook, we excluded from the analysis studies that did not provide sufficient data to enable the calculations. All the statistical computations for the meta-analysis utilized the Cochrane Collaboration’s Review Manager (RevMan) version 5.4.1 software.
Certainty of evidence
The GRADE assessment (The Grading of Recommendations Assessment, Developing and Evaluation) (https://www.gradepro.org)62 determined the certainty of the evidence for each HRV index. This tool verifies the certainty of evidence using the risk of bias, inconsistency, indirect evidence, imprecision, and publication bias. The PEDro scale (https://pedro.org.au) assessed the risk of bias; however, quality was not a condition for inclusion in this review, and articles with a score ≥ 7 were considered high quality, those with a score of 5 to 6 were moderate, and scores ≤ 4 were low quality.
Therefore, to assess the risk of bias by GRADE, the following criteria were adopted: the PEDro scale score, the weight of the studies with the highest risk of bias, and the proportion of studies included in the analysis. Additionally, the significance of the analysis was supported by studies of lower or higher quality, in which GRADE’s tools were used to assign risk. For inconsistency, the overlap of the 95% confidence intervals was evaluated, as was the heterogeneity and the significance of the heterogeneity. Indirect evidence was assessed using the PICOS strategy, taking into account the research questions and how each study was designed to gather information, with the CERT score serving as a reference. Imprecision was evaluated by considering the minimum information value (number of participants), alongside an analysis of the point estimate, observing the size of the confidence intervals (represented by the analysis diamond) and their position relative to the centerline. The funnel plot and other statistical analysis were not used, because The Cochrane Collaboration recommendations advise against their use in meta-analysis, with 10 or fewer eligible articles for the outcomes. For each of the topics, decisions were made on whether to downgrade each item, grading it as strong, moderate, low, or very low, with the help of the GRADEpro online platform (https://gdt.gradepro.org/app/) created for evaluation of GRADE63, also using the criteria already present in the platform itself in the stratification of the analyses.
Heterogeneity
The I² test was applied to assess the degree of heterogeneity, evaluating the percentage of variation in the estimate of the effect attributed to heterogeneity; interpreting this as 0–40%, heterogeneity might not be important, 30–60% may represent moderate heterogeneity, 50–90% may represent substantial heterogeneity, and 75–100% may indicate considerable heterogeneity63.
Training protocol quality
With the CERT tool, we evaluated the design of the training protocol used in the eligible studies. It consists of a scale of 16 topics totaling 19 items that gather the main information that a physical training protocol should contain (from the material used to the control of the effort intensity)64,65. The tool was applied by two independent evaluators (HSD and VSZ) using a model previously created for Microsoft Excel software (Microsoft Office, Microsoft Corporation, Redmond, Washington, USA) version 2019 based on the checklist of the supplementary material of the official article, which contains the entire description of the tool65. The CERT has a checklist that can reach a result of up to 100% for the present study, and a percentage ≥ 70% was considered high quality of the outlined protocol and less than low/moderate quality66.
Data availability
The summary data present in this study are available from the authors and can be accessed upon prior request to Henrique Santos Disessa the first author via e-mail indexed in this work.
References
Rocco, M. et al. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am. J. Kidney Dis. 66, 884–930 (2015).
Bello, A. K. et al. Global kidney Health Atlas. Int. Soc. Nephrol. 3, 1–168 (2019).
Carrero, J. J. et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin. Nutr. 27, 557–564 (2008).
Johansen, K. L. et al. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 63, 291–297 (2003).
Gamboa, J. L. et al. Skeletal muscle mitochondrial dysfunction is Present in patients with CKD before Initiation of Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 15, 926–936 (2020).
Chang, Y. M. et al. Heart rate variability is an indicator for intradialytic hypotension among chronic hemodialysis patients. Clin. Exp. Nephrol. 20, 650–659 (2016).
Gansevoort, R. T. et al. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382, 339–352 (2013).
Hadaya, J. & Ardell, J. L. Autonomic modulation for Cardiovascular Disease. Front. Physiol. 11, 617459 (2020).
Ranpuria, R., Hall, M., Chan, C. T. & Unruh, M. Heart rate variability (HRV) in kidney failure: measurement and consequences of reduced HRV. Nephrol. Dial Transpl. 23, 444–449 (2008).
Salman, I. M. Cardiovascular autonomic dysfunction in chronic kidney disease: a Comprehensive Review. Curr. Hypertens. Rep. 17, (2015).
Guidline Heart rate variability standards. Eur. Heart J. 17, 354–381 (1996).
Greenwood, S. A. et al. The PrEscription of intraDialytic exercise to improve quAlity of life in patients with chronic kidney disease trial: study design and baseline data for a multicentre randomized controlled trial. Clin. Kidney J. 14, 1345–1355 (2021).
Martin-Alemañy, G. et al. Effect of oral nutritional supplementation with and without Exercise on Nutritional Status and physical function of adult hemodialysis patients: a parallel controlled clinical trial (AVANTE-HEMO study). J. Ren. Nutr. 30, 126–136 (2020).
Martins do Valle, F. et al. Effects of intradialytic resistance training on physical activity in daily life, muscle strength, physical capacity and quality of life in hemodialysis patients: a randomized clinical trial. Disabil. Rehabil. 42, 3638–3644 (2020).
Rosa, C. S. Effect of continuous progressive resistance training during hemodialysis on body composition, physical function and quality of life in end-stage renal disease patients: a randomized controlled trial. Clin. Rehabil. 32, 899–908 (2018).
Bennett, P. N. et al. Effects of an intradialytic resistance training programme on physical function: a prospective stepped-wedge randomized controlled trial. Nephrol. Dial Transpl. 31, 1302–1309 (2016).
Hristea, D. et al. Combining intra-dialytic exercise and nutritional supplementation in malnourished older haemodialysis patients: towards better quality of life and autonomy. Nephrology 21, 785–790 (2016).
Marchesan, M., Krug, R. R., Silva, J. R. L. & Rombaldi, A. J. da C. e, Barbosa, A. R. Physical exercise modifies the functional capacity of elderly patients on hemodialysis. Fisioter. em Mov. 29, 351–359 (2016).
Cheema, B. S., Chan, D., Fahey, P. & Atlantis, E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sport Med. 44, 1125–1138 (2014).
Bennett, P. N. et al. The impact of an exercise physiologist coordinated resistance exercise program on the physical function of people receiving hemodialysis: a stepped wedge randomised control study. BMC Nephrol. 14, 1 (2013).
Segura-Ortí, E. [Exercise in haemodyalisis patients: a literature systematic review]. Nefrologia 30, 236–246 (2010).
Chiang, J. Y. et al. Detrended fluctuation analysis of Heart Rate Dynamics is an important prognostic factor in patients with end-stage renal disease receiving peritoneal Dialysis. PLoS One. 11, 1–10 (2016).
Karemaker, J. M. An introduction into autonomic nervous function. Physiol. Meas. 38, R89–R118 (2017).
Aubert, A. E., Seps, B. & Beckers, F. Heart rate variability in athletes. Sport Med. 33, 889–919 (2003).
Verrelli, D. et al. Effect of Intradialytic Exercise on Cardiovascular outcomes in maintenance hemodialysis: a systematic review and Meta-analysis. Kidney360 5, 390–413 (2024).
Manfredini, F., Mallamaci, F., Catizone, L. & Zoccali, C. The burden of physical inactivity in chronic kidney disease: is there an exit strategy? Nephrol. Dial Transpl. 27, 2143–2145 (2012).
Halle, M., Manfredini, F., Floege, J. & Zoccali, C. Physical exercise in haemodialysis patients: which type of exercise is more convenient? Clin. Kidney J. 17, 1–6 (2024).
Pereira, A. B. N. et al. Physical Exercise affects Quality of Life and Cardiac autonomic modulation in patients with chronic kidney failure submitted to Hemodialysis: a Randomized Clinical Trial. Percept. Mot Skills. 129, 696–713 (2022).
de Reboredo, M. Effects of aerobic training during hemodialysis on heart rate variability and left ventricular function in end-stage renal disease patients. J. Bras. Nefrol orgão Soc. Bras. e Latino-Americana Nefrol. 32, 367–373 (2010).
Mitsiou, M. et al. Effects of a Combined Intradialytic Exercise Training Program and Music on Cardiac Autonomic Nervous System Activity in Hemodialysis Patients. Life 12, (2022).
Michou, V. et al. Effects of Home-Based Exercise Training on Cardiac Autonomic Neuropathy and Metabolic Profile in Diabetic Hemodialysis Patients. Life 13, (2023).
Kouidi, E. J., Grekas, D. M. & Deligiannis, A. P. Effects of Exercise Training on Noninvasive Cardiac measures in patients undergoing long-term hemodialysis: a Randomized Controlled Trial. Am. J. Kidney Dis. 54, 511–521 (2009).
Kouidi, E. et al. Depression, heart rate variability, and exercise training in dialysis patients. Eur. J. Cardiovasc. Prev. Rehabil Off J. Eur. Soc. Cardiol. Work Groups Epidemiol. Prev. Card Rehabil Exerc. Physiol. 17, 160–167 (2010).
Deligiannis, A., Kouidi, E. & Tourkantonis, A. Effects of physical training on heart rate variability in patients on hemodialysis. Am. J. Cardiol. 84, 197–202 (1999).
Huang, H. Y. et al. Breathing-based leg exercises during hemodialysis improve quality of life: a randomized controlled trial. Clin. Rehabil. 35, 1175–1184 (2021).
Jankowski, J., Floege, J., Fliser, D., Böhm, M. & Marx, N. Cardiovascular Disease in chronic kidney Disease Pathophysiological insights and Therapeutic options. Circulation 143, 1157–1172 (2021).
Kovesdy, C. P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int. Suppl. 12, 7–11 (2022).
Michael, S., Graham, K. S. & Oam, G. M. D. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals-a review. Front. Physiol. 8, 1–19 (2017).
Sandercock, G. R. H., Bromley, P. D. & Brodie, D. A. Effects of exercise on heart rate variability: inferences from meta-analysis. Med. Sci. Sports Exerc. 37, 433–439 (2005).
Deligiannis, A., D’Alessandro, C. & Cupisti, A. Exercise training in dialysis patients: impact on cardiovascular and skeletal muscle health. Clin. Kidney J. 14, II25–II33 (2021).
Fadaee, S. B. et al. Oxidative stress is associated with decreased heart rate variability in patients with chronic kidney disease. Redox Rep. 22, 197–204 (2017).
Rubinger, D., Backenroth, R. & Sapoznikov, D. Sympathetic nervous system function and dysfunction in chronic hemodialysis patients. Semin Dial. 26, 333–343 (2013).
Ståhle, A., Nordlander, R. & Bergfeldt, L. Aerobic group training improves exercise capacity and heart rate variability in elderly patients with a recent coronary event: a randomized controlled study. Eur. Heart J. 20, 1638–1646 (1999).
Larsen, A. I. et al. Effect of exercise training in patients with heart failure: a pilot study on autonomic balance assessed by heart rate variability. Eur. J. Cardiovasc. Prev. Rehabil Off J. Eur. Soc. Cardiol. Work Groups Epidemiol. Prev. Card Rehabil Exerc. Physiol. 11, 162–167 (2004).
KIILAVUORI, K., TOIVONEN, L., NÄVERI, H. & LEINONEN, H. Reversal of autonomic derangements by physical training in chronic heart failure assessed by heart rate variability. Eur. Heart J. 16, 490–495 (1995).
Eser, P. et al. Acute and chronic effects of high-intensity interval and moderate-intensity continuous exercise on heart rate and its variability after recent myocardial infarction: a randomized controlled trial. Ann. Phys. Rehabil Med. 65, 101444 (2022).
Lucini, D. et al. Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am. Heart J. 143, 977–983 (2002).
Mazzuero, G., Lanfranchi, P., Colombo, R., Giannuzzi, P. & Giordano, A. Long-term adaptation of 24-h heart rate variability after Myocardiallnfarction*. Chest 304–308. https://doi.org/10.1378/chest.101.5 (1992).
Villafaina, S., Collado-Mateo, D., Domínguez-Muñoz, F. J., Gusi, N. & Fuentes-Garcia, J. P. Effects of exergames on heart rate variability of women with fibromyalgia: a randomized controlled trial. Sci. Rep. 10, 1–8 (2020).
Pietila, M. et al. Exercise training in chronic heart failure: effects on pro-inflammatory markers. Exerc. Train. Hear. Fail. 7, 189–193 (2002).
Barcellos, F. C., Santos, I. S., Umpierre, D., Bohlke, M. & Hallal, P. C. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin. Kidney J. 8, 753–765 (2015).
Afsar, B. et al. The impact of exercise on physical function, cardiovascular outcomes and quality of life in chronic kidney disease patients: a systematic review. Int. Urol. Nephrol. 50, 885–904 (2018).
Schoenfeld, B. J. Squatting kinematics and kinetis and their application to exercise performance. J. Strength. Cond Res. 24, 3497–3506 (2010).
Holden, S. & Barton, C. J. What should i prescribe?’: time to improve reporting of resistance training programmes to ensure accurate translation and implementation. Br. J. Sports Med. 53, 264–265 (2019).
Grässler, B., Thielmann, B., Böckelmann, I. & Hökelmann, A. Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: a systematic review. Eur. Rev. Aging Phys. Act. 18, 1–21 (2021).
Zago, A. S. et al. The impact of aging and estimated training status on blood pressure and antihypertensive medicine consumption. J. Cardiol. Ther. 4, 681–687 (2017).
Iwasaki, K. I., Zhang, R., Zuckerman, J. H. & Levine, B. D. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J. Appl. Physiol. 95, 1575–1583 (2003).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, (2021).
Miller, S. A. & Forrest, J. L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J. Evidenced-Based Dent. Pract. 1, 136–141 (2001).
Bernier-Jean, A. et al. Exercise training for adults undergoing maintenance Dialysis. Cochrane Database Syst. Rev. 12, 135–136 (2022).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5, 1–10 (2016).
Guyatt, G. H., Oxman, A. D., Kunz, R., Vist, G. E. & Falck-Ytter, Y. W. G. GRADE: what is quality of evidence and why is it important to clinicians? Bmj 336, 995–998 (2008).
Higgins, J. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (2023).
Kent, P., O’Sullivan, P. B., Keating, J. & Slade, S. C. Evidence-based exercise prescription is facilitated by the Consensus on Exercise Reporting Template (CERT). Br. J. Sports Med. 52, 147–148 (2018).
Slade, S. C., Dionne, C. E., Underwood, M. & Buchbinder, R. Consensus on Exercise Reporting Template (CERT): explanation and Elaboration Statement. Br. J. Sports Med. 50, 1428–1437 (2016).
Slade, S. C., Finnegan, S., Dionne, C. E., Underwood, M. & Buchbinder, R. The Consensus on Exercise Reporting Template (CERT) applied to exercise interventions in musculoskeletal trials demonstrated good rater agreement and incomplete reporting. J. Clin. Epidemiol. 103, 120–130 (2018).
Funding
This article was funded by the São Paulo State Research Support Foundation (FAPESP – Fundação de Amparo à Pesquisa do Estado de São Paulo) process number 2019/27225-9.
Author information
Authors and Affiliations
Contributions
Authors contributions. Conceptualization: Henrique dos Santos Disessa, Clara Suemi da Costa Rosa, Henrique Luiz Monteiro. Data curation: Henrique dos Santos Disessa, Vitor da Silva Zacharias. Formal Analysis: Henrique dos Santos Disessa, Pedro Henrique Martins Monteiro. Funding acquisition: Henrique dos Santos Disessa, Henrique Luiz Monteiro. Investigation: Henrique dos Santos Disessa, Clara Suemi da Costa Rosa. Methodology: Henrique dos Santos Disessa, Vitor da Silva Zacharias, Pedro Henrique Martins Monteiro, Henrique Luiz Monteiro. Project administration: Henrique dos Santos Disessa, Henrique Luiz Monteiro. Resources: Henrique dos Santos Disessa, Henrique Luiz Monteiro. Software: Henrique dos Santos Disessa, Pedro Henrique Martins Monteiro. Supervision: Henrique dos Santos Disessa, Henrique Luiz Monteiro. Validation: Henrique dos Santos Disessa, Henrique Luiz Monteiro. Visualization: Henrique dos Santos Disessa, Clara Suemi da Costa Rosa, Pedro Henrique Martins Monteiro, Henrique Luiz Monteiro. Writing – original draft: Henrique dos Santos Disessa, Henrique Luiz Monteiro. Writing – review & editing: Henrique dos Santos Disessa, Clara Suemi da Costa Rosa, Vitor da Silva Zacharias, Pedro Henrique Martins Monteiro, Henrique Luiz Monteiro. The original research idea was conceived by researcher Henrique dos Santos Disessa. The searches were conducted by Henrique dos Santos Disessa, Pedro Henrique Martins Monteiro, and Vitor da Silva Zacharias. The critical review was carried out by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no competing interests.
Financial interests
The authors declare that they have no financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
dos Santos Disessa, H., Monteiro, P.H.M., da Silva Zacharias, V. et al. A systematic review and meta-analysis investigating the impact of exercise interventions on heart rate variability in hemodialysis patients. Sci Rep 14, 30818 (2024). https://doi.org/10.1038/s41598-024-81217-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81217-0