Abstract
Although intermittent hypoxia training (IHT) and methazolamide (MTZ) alone can prevent high-altitude cerebral edema (HACE) to varying degrees, their efficacy and dispersion remain limited. However, only a handful of trials have explored the effectiveness of the IHT and MTZ combination in preventing HACE. Rats were first exposed to hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% fraction of inspired oxygen (FiO2)) with simultaneous exhaustive exercise (EE) for different durations to determine the ideal condition for establishing a rat model of HACE. Rats receiving various courses of IHT were subjected to this condition, and changes in behaviour, brain water content (BWC), pathology and brain protein expression were evaluated. Meanwhile, rats received different doses of MTZ before and during hypoxia exposure with simultaneous EE. Finally, rats receiving the IHT and MTZ combination were then exposed to hypoxia with simultaneous EE. Systemic inflammation and mild cerebral edema developed in rats after 6 h of hypobaric hypoxia with simultaneous EE. Rats showed severe impairment of spatial and memory functions after 2 days of hypobaric hypoxia with simultaneous EE, and the pathology of their brain showed significant dilated perivascular spaces, cell swelling, vacuolar degeneration and reduced neuron count. BWC, serum inflammatory factors and expression of vascular endothelial growth factor (VEGF) and aquaporin 4 (AQP4) proteins in the hippocampus increased significantly. Both IHT and MTZ differentially counteracted hypobaric hypoxia-induced spatial and memory function impairments and increased BWC, pathological changes and expression of AQP4 and VEGF proteins in the hippocampus. Among these, the long-course IHT (BID, 14 d) combined with MTZ (200 mg/kg/d) showed the most significant improvement, restoring the rats’ indices to normal levels. Continuous hypobaric hypoxia with simultaneous EE for 2 days resulted in significant HACE in rats, which may be used to establish a rat model of HACE. Both IHT and MTZ alleviated HACE in rats to varying degrees, among which long-course IHT (BID, 14 d) combined with MTZ (200 mg/kg/d) effectively prevented HACE in rats.
Similar content being viewed by others
Introduction
High-altitude regions and mountains cover one-fifth of the Earth’s surface, and with the advancement of science and technology, millions of people travel to high-altitude regions for economic development, military, transportation and tourism purposes1,2. Acute mountain sickness (AMS) is a series of symptoms that occur when an individual enters a high-altitude region too quickly and is not suitable to high-altitude conditions. High-altitude cerebral edema (HACE) is a serious illness that occurs in the late stage of AMS progression and is currently the main cause of death from AMS3,4. High altitude is characterised by hypobaric hypoxia, decreased temperature, decreased humidity and enhanced ultraviolet rays5. Among these factors, hypobaric hypoxia is considered to be the major contributor to AMS, which is common in populations that are not adequately acclimatised6. The incidence of HACE varies slightly from region to region, and the estimated incidence of HACE is approximately 0.5–1% among high-altitude travellers, such as tourists, trekkers and mountaineers, with a mortality rate as high as 75–100%7,8. At present, the pathogenesis of HACE remains unclear. Moreover, HACE treatment is limited and its therapeutic effect is still unsatisfactory, accompanied by a high mortality rate. Therefore, an in-depth study of HACE pathogenesis and therapeutic strategies is particularly important. According to the literature, there is currently no gold standard for establishing an experimental animal model of HACE. Thus, establishing a uniform and stable animal model of HACE is the basis for studying the pathogenesis, prevention and treatment of HACE.
Due to its morbidity and mortality, HACE prevention has become the most important aspect9,10. Current prevention strategies include graduated ascents, high-altitude acclimatisation, pharmaceutical prevention, and descent at first signs of symptoms10,11. Intermittent hypoxia training (IHT) is a promising approach that has been used to induce high-altitude acclimatisation and subsequently reduce the risk of developing AMS12. However, the optimal regimen for IHT to prevent AMS has not been determined13. There is emerging evidence to suggest that IHT regimens characterised by mild to moderate hypoxia (9–16% fraction of inspired oxygen (FiO2)), short duration (3–10 min) and 3–15 cycles per day usually produce beneficial results without pathological outcomes14. Nonetheless, only a few relevant studies have investigated the role and optimal course of IHT in the prevention of HACE.
In addition to IHT, pharmacological prophylaxis is also an important HACE prevention strategy. Carbonic anhydrase (CA) inhibiting drugs, like acetazolamide (ACZ) and methazolamide (MTZ), are commonly prescribed for AMS and HACE15. Although ACZ is currently the internationally recognised drug of choice for the prevention of AMS and HACE16, its use is still relatively limited due to adverse effects such as tingling of the hands and feet, lethargy, polydipsia, thirst, tinnitus and gastrointestinal discomfort after administration in some patients17,18,19. MTZ has a similar chemical structure and similar pharmacological effects and mechanism of action to that of ACZ; however, its inhibitory effect on CA inhibition is stronger than that of ACZ, and adverse effects are significantly fewer than those of ACZ, which may be one of the drugs of choice for HACE prevention20,21. While some comparative studies have demonstrated that lower doses of MTZ compared with ACZ produce similar levels of CA inhibition (e.g., oral dosages of 200–250 mg daily for MTZ vs. 500–750 mg daily for ACZ)15,22, others have shown that MTZ administration at 150 mg is as effective as ACZ in preventing AMS with less paresthesia23,24.
Although both IHT and MTZ are effective in preventing HACE to varying degrees, the outcomes remain unsatisfactory or the dissemination is still limited. Additionally, although the IHT and MTZ combination may provide a more desirable preventive effect, clinical studies to substantiate this hypothesis are relatively few. The present study first established an experimental rat model of HACE. Rats were exposed to hypoxia in a hypobaric chamber (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous exhaustive exercise (EE) for different durations to determine the ideal condition for the successful establishment of an experimental rat model of HACE. Subsequently, rats receiving different courses of IHT were subjected to this condition and the changes in behavioural, histological, cellular and molecular levels of the rat were monitored to investigate the effects of short- and long-course IHT in preventing HACE. Meanwhile, different doses of MTZ were administered to the rats before and during hypoxia exposure to study the effect of MTZ in preventing HACE in rats. Finally, rats receiving the IHT and MTZ combination were subjected to hypoxia exposure to study the efficacy of this combination in preventing HACE in rats. Our findings may provide a more precise and effective method of preventing HACE in humans who need to enter the plateau environment for various reasons.
Methods
Animals
Male adult 6-week-old Sprague–Dawley rat (weighing 210 ± 10 g) were purchased from the Laboratory Animal Center of Vital River Experimental Animal Company (Beijing, China). The rats were housed in the animal house of the Beijing Shijitan Hospital at a constant condition (temperature 20 ± 2 °C, humidity 40%–60%, light/dark cycle 12 h) with unlimited access to standard diet and water for 1 week to acclimatize them to the environment. The animal protocol was approved by the Institutional Review Board of Beijing Shijitan Hospital, Capital Medical University, and all procedures were conducted following the relevant guidelines. (Approval number: sjtkyll-lx-2023(010)). All rats were euthanised by decapitation after intraperitoneal anaesthesia with tribromoethanol (15 ml/kg).
HH exposure and MTZ administration
Establishment of rat HACE model
A total of 50 rats were randomly divided into 5 groups as follows: (1) normoxic control group (NC, n = 10). The rats were allowed free access to food and water under normobaric and normoxic conditions; (2)-(5) Hypoxia groups: hypobaric hypoxia 6-h group (HH 6h, n = 10), hypobaric hypoxia 1 day group (HH 1d, n = 10), hypobaric hypoxia 2 days group (HH 2d, n = 10) and hypobaric hypoxia 5 days group (HH 5d, n = 10). The rats were placed in a hypobaric chamber, fed freely, and exposed to continuous hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE (treadmill parameters: incline, 10°; velocity: 12 m/min; continuous movement: 4 h; rest: 15–20 min.) for 6 h, 1 day, 2 days, and 5 days, respectively. The parameters of hypobaric hypoxia exposure and EE in this study were derived from the methods described by Song et al.25 and Guo et al26. (Fig. 1A).
IHT and HH exposure
Forty rats were randomly divided into 4 groups as follows: (1) normoxic control group (NC, n = 10). The rats were allowed free access to food and water under normobaric and normoxic conditions; (2) Hypobaric hypoxia group (HH, n = 10). The rats were placed in a hypobaric chamber, allowed free access to food and exposed to continuous hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days. (3) Short-course IHT group (S-IHT, n = 10), rats were trained with the short-course moderate intermittent hypoxia preconditioning protocol, which is, a 10-min 13% FiO2 alternated by 5 min 21% FiO2 for four sessions. Preconditioning was conducted twice a day for 5 consecutive days. After IHT, the rats were exposed to continuous hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days; (4) Long-course IHT group (L-IHT, n = 10), the rats were trained with the long-course moderate intermittent hypoxia preconditioning protocol, which is, a 10-min 13% FiO2 alternated by 5 min 21% FiO2 for four sessions. Preconditioning was performed twice a day for 14 consecutive days. After IHT, rats were exposed to continuous hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days (Fig. 1B).
MTZ administration and HH exposure
Forty rats were randomly divided into 4 groups as follows: (1) normoxic control group (NC, n = 10). The rats were allowed free access to food and water under normobaric and normoxic conditions, and 0.01 ml/g saline was administered daily to each rat via gavage; (2) Hypobaric hypoxia + saline group (HH + NS, n = 10). The rats were exposed to continuous hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days, and 0.01 ml/g saline was administered daily to each rat via gavage; (3) Hypobaric hypoxia + MTZ 1 group (HH + MTZ1, n = 10). The rats were exposed to continuous hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days, and MTZ was administered at a dose of 150 mg/kg daily via gavage, i.e., 0.01 ml/g saline containing 1.5% MTZ was administered daily via gavage21,23,24. (4) Hypobaric hypoxia + MTZ 2 group (HH + MTZ2, n = 10). The rats were continuously administered hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days, and MTZ was administered at a dose of 200 mg/kg daily via gavage, i.e., 0.01 ml/g saline containing 2% MTZ was administered daily via gavage, respectively15,22 (Fig. 1B).
IHT&MTZ and HH exposure
Forty rats were randomly divided into 4 groups as follows: (1) normoxic control group (NC, n = 10). The rats were allowed free access to food and water under normobaric and normoxic conditions; (2) Long-course IHT group (L-IHT, n = 10), with the same disposition as before; (3) Hypobaric hypoxia + MTZ 2 group (HH + MTZ2, n = 10), with the same disposition as before. (4) Long-course IHT combined with MTZ group (IHT&MTZ, n = 10). The rats were exposed to long-course IHT. After long-course exposure to IHT, the rats were continuously exposed to hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days, and were administered with MTZ at a dose of 200 mg/kg daily via gavage (Fig. 1C).
Morris water maze test (MWM)
The Morris water maze consists of a circular pool of water with a video camera linked to a computer that automates video recording and analysis. The tracking is halted once the designated training period of 120 s concludes or the animal reaches the platform, at which point the swim trajectory is recorded. For this experiment, the pool was filled with fresh water, to which black dye was added, rendering it opaque. The platform was positioned approximately 1.5 cm below the water’s surface, and the water temperature was maintained between 18℃ ~ 20℃. After the experiment, the rats were trained to enter the water once a day from different quadrants, and if the rats did not find the platform within 120 s, they were artificially placed on the platform and allowed to stay for 10 s. Five rats per group underwent training for five days. Following the training, the rats were dried and returned to their cages. After 5 d of training, the rats were placed in the water from the opposite side of the original platform quadrant. The dwell time in the target quadrant, the area where the platform was initially located, along with the frequency of the animal’s entries into that quadrant, were recorded as indicators of spatial memory. The number of crossings over the platform location and the dwell time in the training quadrant were analyzed using computational methods.
Measurement of BWC
After MWM, rats (n = 5 in each group) were euthanised by decapitation after intraperitoneal anaesthesia with tribromoethanol (15 ml/kg), and the brain was immediately harvested and divided into two hemispheres. The right hemisphere was aliquoted and stored at −80℃ until further analysis. The left hemisphere was weighed and dried in a thermostatic oven for 72 h at 110℃ until it was dried to a constant weight. The dried brain was re-weighed and the percentage BWC was calculated as [(wet weight−dry weight)/wet weight] × 100% as an index to assess the severity of HACE in rats26,27,28,29.
HE staining
The remaining rats (n = 5 in each group) were euthanised in the meantime. The whole brain was fixed in 4% paraformaldehyde at 4 °C for 48 h, dehydrated in ethanol and embedded in paraffin. Brain tissue was cut into 10 sections of 5 µm thickness using serial cut method and 5 sections were selected for HE staining. Slides were stained with hematoxylin liquid for 15 min after xylene dewaxing and graded ethanol debenzolization. After washing with tap water, they were separated using ethanol and hydrochloric acid, and then stained with eosin for 2 min. Histopathological changes were examined under an Eclipse E200 microscope (NIKON, Tokyo, Japan).
Detection of inflammatory and anti-inflammatory cytokines in serum
Blood samples were collected from rats (n = 5 per group) via the abdominal aorta and subsequently centrifuged at 2000 rpm for 10 min to obtain serum. Levels of inflammatory cytokines, including interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumour necrosis factor-alpha (TNF-α), and the anti-inflammatory cytokine interleukin-10 (IL-10) were measured immediately using commercial ELISA kits, (FineTest, China; catalog numbers ER0042, ER1094, ER1393, and ER0033), and results were expressed as pg/mL, following the manufacturer’s guidelines.
Western blotting
The frozen hippocampus extracted from the right hemisphere (n = 3 in each group) was washed and homogenized in radioimmunoprecipitation assay (RIPA) buffer on ice. The supernatant was collected after centrifugation (14,000 rpm, 4 ℃, 10 min). Total proteins were quantified using the BCA protein assay Kit (BIOSS, China, C05-02,001), and denatured by boiling with the sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) Sample Loading Buffer (Beyotime, China, P0015L) at 100 °C for 5 min. Subsequently, 30 μg of total tissue protein was loaded on SDS-PAGE electrophoresis. Next, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Merck KGaA, Darmstadt, Germany, IPVH00010) and blocked for 2 h in 5% skimmed milk in TBST buffer. The membranes were immunoblotted with anti-aquaporin 4 (AQP4) (1:1000, Proteintech, USA), anti-vascular endothelial growth factor (VEGF) (1:2000, Proteintech, USA) and anti β-actin (1:4000, Proteintech, USA) primary antibodies overnight at 4 ℃. The membrane was washed three times (10 min each time) to remove TBST, and incubated with goat-anti-rabbit IgG HRP-conjugated secondary antibody for 2 h at room temperature. The membranes were washed with TBST three times (10 min each time) and protein bands were visualized using the enhanced chemiluminescence detection system (HaiGene, M2301) and quantified using the ImageJ software. The expression of target proteins was normalized to that of the reference protein β-actin.
RT-qPCR
The total RNA (n = 5 in each group) was extracted from frozen hippocampus from right hemisphere (aliquoted and stored at −80 ℃) using the TRIzol reagent (Invitrogen). Subsequently, 500 ng of RNA was reverse-transcribed into the complementary DNA using the Primer Script RT reagent (Takara Bio, Inc., Otsu, Japan). The mRNA levels were calculated using SYBR-Green qRT-PCR (Takara Bio, Inc.). The expression of AQP4 and VEGF was normalized to that of actin as the internal reference, calculated using the 2-ΔΔCt. The primers used are as follows:
Rat AQP4: (F)TGGTCCTCATCTCCCTCTGCTT, (R)TGAACCGTGGTGACTCCCAATCC
Rat VEGF: (F)ACGGGCCTCTGAAACCATGAA, (R)TTTCTGCTCCCCTTCTGTCGT
Statistical analyses
Statistical analyses were performed using SPSS Statistics 26.0 software. Continuous variables were tested for normality. Data that showed non-normally distribution pattern were normalized using the Box-Cox transformation and expressed as the mean ± standard deviation. An analysis of variance was conducted using a one-way ANOVA. All the data and figures were prepared using the GraphPad Prism 10.1.2 and Photoshop CS5.0. Differences between the groups were considered statistically significant at a p value < 0.05. Asterisks are used to indicate statistical significance as follows: *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively.
Results
Establishment of the experimental rat model of HACE
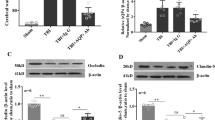
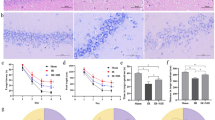
In the MWM, the platform quadrant dwell time and platform crossing number of rats in each hypoxia group (HH 6h, HH 1d, HH 2d and HH 5d) decreased significantly compared with the NC group (all p < 0.05), with the HH 2d group exhibiting the most significant decrease. The dwell time differed significantly between the HH 2d group and the other hypoxia groups (HH 2d vs HH 6h/1d/5d, p < 0.0001, < 0.0001 and 0.0289, respectively). The crossing number differed significantly between the HH 2d group and the HH 6h and HH 1d groups (HH 2d vs HH 6h/1d, p = 0.0002 and 0.0108, respectively) (Fig. 2A). The BWC was significantly increased in all hypoxia groups compared with the NC group (all p < 0.05), with the HH 2d group having the highest mean BWC, which was statistically different from the other hypoxia groups (all p < 0.05) (Fig. 2B). HE staining results revealed normal histological features in the hippocampus and cortex regions of the NC group. Only a few pyknotic neurons and dilated perivascular spaces were observed in the HH 6h group. However, pyramidal cells in the cortex and hippocampus regions of rats in the HH 1d group presented massive derangement, karyopyknosis, hyperchromatic nuclei and irregular shape. Compared with the other hypoxia groups, the HH 2d and HH 5d groups showed the most severe lesions characterised by dilated perivascular spaces, cell swelling, vacuolar degeneration, and reduced neuron count in the cortex and hippocampus regions (Fig. 2C). Compared with the NC group, inflammatory factors such as IL-1β, IL-6 and TNF-α were significantly increased and the anti-inflammatory cytokine IL-10 was significantly decreased in the serum of each hypoxia group (all p < 0.05). IL-1β and IL-6 were most significantly increased in the serum of the HH 2d and HH 5d groups, with statistically significant differences compared with other hypoxia groups (all p < 0.05). IL-10 was most significantly decreased in the HH 2d group, with statistically significant differences compared with other hypoxia groups (all p < 0.05) (Fig. 2D). Compared with the NC group, protein and messenger RNA (mRNA) expression levels of AQP4 and VEGF were significantly increased in the hippocampus of rats in each hypoxia groups (all p < 0.05), with the HH 2d and HH 5d groups showing the most significant increase, and the differences were statistically significant compared with other hypoxia groups (all p < 0.05) (Figs. 2E,F).
Establishment of the experimental rat model of HACE. (A) MWM, the platform quadrant dwell time and platform crossing number of rats in each group; (B) BWC was significantly increased in all hypoxia groups compared to the NC group, among which the mean BWC was highest in the HH 2d group; (C) The histopathological features in the cortex and hippocampus regions were examined by HE staining. The cortex and hippocampus regions of the NC group showed normal histological features (a and f). Only dilated perivascular spaces and a little pyknotic neurons were observed in the HH 6h group (b and g). However, pyramidal cells in the cortex and hippocampus regions of rats in the HH 1d group presented massive derangement, karyopyknosis, hyperchromatic nuclei and irregular shape (c and h). The HH 2d group (d and i) and HH 5d group (e and j) showed the most severe lesions than the other hypoxia groups with the feature of cell swelling, vacuolar degeneration, dilated perivascular spaces and reduced number of neurons in the cortex and hippocampus regions. (D) Inflammatory and anti-inflammatory cytokines in serum; (E) The expression of AQP4 and VEGF proteins. The expression of target proteins was normalized to that of the reference protein β-actin; (F) The relative level of VEGF and AQP4 mRNA expression. The expression of AQP4 and VEGF mRNA was normalized to that of actin as the internal reference, calculated using the 2-ΔΔCt; Asterisks are used to indicate statistical significance as follows: *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively.
IHT intervention
In the MWM, dwell time and crossing number increased significantly in the L-IHT group compared with the HH group (both p < 0.0001). Meanwhile, dwell time increased significantly in the S-IHT group compared with the HH group (p = 0.0168). L-IHT showed more increase but did not recover to the level of the NC group, and the difference was statistically significant (both p < 0.05) (Fig. 3A). Compared with the HH group, both S-IHT and L-IHT groups showed a significant improvement in the BWC (p = 0.0195 and p < 0.0001, respectively), with L-IHT showing comparable results to NC, and the difference was statistically significant (p = 0.0448) (Fig. 3B). Dilated perivascular spaces, cell swelling, and vacuolar degeneration in the cortex and hippocampus regions were significantly relieved in the S-IHT compare with HH groups. The L-IHT group had dilated perivascular spaces and a few pyknotic neurons, which were comparable to the normal histological features (Fig. 3C). IL-1β, IL-6 and TNF-α were significantly decreased and IL-10 was significantly increased in the serum of each IHT group compared with the HH group (all p < 0.05), with the most significant effects observed in the L-IHT group, and the difference was statistically significant compared with the NC group (all p < 0.05) (Fig. 3D). Protein and mRNA expression levels of AQP4 and VEGF reduced significantly in the hippocampus of S-IHT and L-IHT groups compared with the HH group (all p < 0.05), with the L-IHT group showing the most significant decrease. However, protein and mRNA expression levels of AQP4 and VEGF were higher in the S-IHT and L-IHT groups than in the NC group, with statistically significant differences (all p < 0.05) (Figs. 3E,F).
IHT intervention. (A) MWM, the platform quadrant dwell time and platform crossing number of rats in each group; (B) Compared to the HH group, both S-IHT and L-IHT groups showed a significant improvement in BWC, with L-IHT group closer to the NC group, but there was a statistically significant difference between the two groups; (C) In HE staining, cell swelling, vacuolar degeneration, and dilated perivascular spaces in the cortex and hippocampus regions of the S-IHT group (c and g) were significantly relieved, compare with the HH group (b and f). Only dilated perivascular spaces and a little pyknotic neurons were observed in the L-IHT groups (d and h), which are very close to the normal histological features (a and e). (D) Inflammatory and anti-inflammatory cytokines in serum; (E) The expression of AQP4 and VEGF proteins. The expression of target proteins was normalized to that of the reference protein β-actin; (F) The relative level of VEGF and AQP4 mRNA expression. The expression of AQP4 and VEGF mRNA was normalized to that of actin as the internal reference, calculated using the 2-ΔΔCt; Asterisks are used to indicate statistical significance as follows: *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively.
MTZ intervention
In the MWM, platform quadrant dwell time and platform crossing number increased significantly in the HH + MTZ1 and HH + MTZ2 groups compared with the HH + NS group (all p < 0.05), with the HH + MTZ2 group exhibiting the most significant increase, and the platform crossing number recovered to the level of the NC group with no statistical difference (p > 0.999); dwell time statistically significantly differed between the two groups (p = 0.0166) (Fig. 4A). Compared with the HH + NS group, the HH + MTZ1 and HH + MTZ2 groups showed a significant improvement in BWC (p = 0.004 and p < 0.0001, respectively), with the HH + MTZ2 group showing comparable results to NC group, and the difference was statistically significant (p = 0.041) (Fig. 4B). Dilated perivascular spaces, cell swelling, vacuolar degeneration, and reduced neuron count in the cortex and hippocampus regions were significantly relieved in the HH + MTZ1 group compared with the HH group. The HH + MTZ2 group had dilated perivascular spaces and a few pyknotic neurons, which were comparable to the normal histological features (Fig. 4C). IL-1β, IL-6 and TNF-α levels were significantly decreased and the IL-10 level was significantly increased in the serum of each MTZ group compared with the HH + NS group (all p < 0.05), with the most significant effects observed in the HH + MTZ2 group, with no significant difference compared to the NC group (all p > 0.05) (Fig. 4D). Protein and mRNA expression levels of AQP4 and VEGF reduced significantly in the hippocampus of the HH + MTZ1 and HH + MTZ2 groups compared with the HH + NS group (all p < 0.05), with the HH + MTZ2 group showing the most significant decrease. However, protein and mRNA expression levels of AQP4 and VEGF were higher in the HH + MTZ1 and HH + MTZ2 groups than in the NC group, with statistically significant differences (all p < 0.05) (Figs. 4E,F).
MTZ intervention. (A) MWM, the platform quadrant dwell time and platform crossing number of rats in each group; (B) Compared to the HH + NS group, both HH + MTZ1 and HH + MTZ2 showed a significant improvement in BWC, with the HH + MTZ2 group closer to the NC group, but there was statistical significance between the two groups; (C) In HE staining, cell swelling, vacuolar degeneration, and dilated perivascular spaces in the cortex and hippocampus regions of the HH + MTZ1 group (c and g) were significantly relieved, compare with the HH group (b and f). Only dilated perivascular spaces and a little pyknotic neurons were observed in the HH + MTZ2 group (d and h), which are very close to the normal histological features (a and e); (D) Inflammatory and anti-inflammatory cytokines in serum; (E) The expression of AQP4 and VEGF proteins. The expression of target proteins was normalized to that of the reference protein β-actin; (F) The relative level of VEGF and AQP4 mRNA expression. The expression of AQP4 and VEGF mRNA was normalized to that of actin as the internal reference, calculated using the 2-ΔΔCt; Asterisks are used to indicate statistical significance as follows: *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively.
Long-course IHT combined with MTZ intervention
In the MWM, the platform quadrant dwell time and platform crossing number were comparable between the IHT&MTZ and NC groups, with no significant difference between the two groups (p = 0.9935 and 0.8526, respectively) (Fig. 5A). The BWC decreased significantly in the IHT&MTZ group compared with the L-IHT and HH + MTZ2 groups (p = 0.0022 and 0.0372, respectively) and slightly differed from that in the NC group but the difference was not statistically significant (p = 0.9166) (Fig. 5B). No dilated perivascular spaces, cell swelling, vacuolar degeneration, or reduced neuron count were observed in the cortex and hippocampus regions of the IHT&MTZ group compared with the L-IHT and HH + MTZ2 groups, and no significant difference was found between the IHT&MTZ and NC groups (Fig. 5C). IL-1β, IL-6 and TNF-α serum levels were not significantly increased (p = 0.9156, 0.9889 and 0.9637, respectively) and the IL-10 serum level was not significantly decreased (p > 0.9999) in the IHT&MTZ group compared with the NC groups (Fig. 5D). Protein and mRNA expression levels of AQP4 and VEGF reduced significantly in the hippocampus of the IHT&MTZ group compared with the L-IHT and HH + MTZ2 groups, and the differences were statistically significant (all p < 0.05). Moreover, the IHT&MTZ group showed similar results to the NC group, with no statistical differences between the two groups (all p > 0.05), except for protein expression of AQP4 (p = 0.0202) (Figs. 5E,F).
Long-course IHT combined with MTZ intervention. (A) MWM, the platform quadrant dwell time and platform crossing number of rats in each group; (B) Compared to the L-IHT and HH + MTZ2 groups, the IHT&MTZ group showed a significant further improvement in BWC, with no statistical significance between the IHT&MTZ and NC groups; (C) In HE staining, there was no cell swelling, vacuolar degeneration and dilated perivascular spaces in the cortex and hippocampus regions of the IHT&MTZ1 group (d and h), which is basically consistent with the NC group (a and e); (D) Inflammatory and anti-inflammatory cytokines in serum; (E) The expression of AQP4 and VEGF proteins. The expression of target proteins was normalized to that of the reference protein β-actin; (F) The relative level of VEGF and AQP4 mRNA expression. The expression of AQP4 and VEGF mRNA was normalized to that of actin as the internal reference, calculated using the 2-ΔΔCt; Asterisks are used to indicate statistical significance as follows: *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively.
Discussion
The present study found that continuous hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days resulted in significant cerebral edema in rats, which may be used to establish the experimental rat model of HACE. According to the literature, there is currently no gold standard for establishing an animal model of HACE in rats, including 6000 m of hypobaric hypoxia for 72 h29, 6000 m of hypobaric hypoxia combined with hypothermia for 72 h30, 5000 m of hypobaric hypoxia (54.02 kPa, 10.8% FiO2) for 48 h25and 4000 m of hypobaric hypoxia combined with simultaneous EE for 48 h26. Therefore, the establishment of a unified stable animal model of HACE is the basis for conducting studies related to the pathogenic mechanism, prevention and treatment of HACE. To ensure successful modelling, we adopted hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) combined with simultaneous EE as the condition for inducing HACE while setting different exposure times to further clarify the time of HACE induction. Brain edema follows an increase in blood brain barrier (BBB) permeability and astrocyte swelling25. BWC and HE staining are the most direct indicators of cerebral edema in rats. VEGF is an important mediator of angiogenesis and vascular permeability that can increase BBB permeability under hypoxic conditions. Schoch et al. found that hypoxia significantly increased the expression of VEGF in the brain tissue of mice and the degree of increase correlated with the severity of the hypoxic stimulus31. Aquaporins are a family of water channels, of which AQP4 is the most abundant in the brain of primates and rodents, where it is mainly expressed in the perivascular end feet of astrocytes and ependymal cells lining the cerebral ventricles32,33. AQP4 is correlated with the pathological changes in hypoxia-induced cerebral edema34, as a common denominator between edema and neuroinflammation35. Notably, pathological effects of astrocyte swelling and increase in BBB permeability depend on AQP4 upregulation in astrocytes27. AQP4 is also an inflammatory signal potentiator that may be mediated by increased activation of nuclear factor kappa B (NF-κB)36. The present study found that hypobaric hypoxia with simultaneous EE for 6 h induced inflammation and brain edema in rats, consistent with previous findings25. Rats exhibited the highest BWC, the most pronounced impairment of memory and spatial function, the worst histopathological changes and the highest protein and mRNA expression levels of AQP4 and VEGF in the hippocampus after 2 days of hypobaric hypoxia with simultaneous EE. These data suggest that hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days may be the most optimal condition for modelling HACE in rats. In addition, we found that BWC was decreased in HH 5d compared with HH 2d rats, which may be related to hyperventilation37.
IHT alleviated HACE when rats re-entered the hypobaric hypoxia environment, where the effect of long-course IHT (BID, 14 d) was more pronounced than that of short-course IHT (BID, 5 d). In the MWM, the memory and spatial function of rats in the HH group were significantly impaired; these effects were improved by both short- and long-course IHT in hypobaric hypoxia conditions. Compared with the HH group, the S-IHT and L-IHT groups showed differential decreases in BWC and protein and mRNA expression levels of AQP4 and VEGF in the hippocampus, suggesting that IHT can alleviate the HACE that occurs in rats upon re-entry into a hypobaric hypoxia environment. Compared with the S-IHT group, the L-IHT group showed a greater decrease in BWC and a greater improvement in impaired memory and spatial function, as well as lower protein and mRNA expression levels of AQP4 and VEGF in the hippocampus, although neither reached normal levels. These results suggest that IHT can effectively relieve hypobaric hypoxia-induced symptoms, improve tolerance to hypoxia, and reduce systemic inflammatory response and brain damage14. These data also suggest that although long-course IHT (BID, 14 d) was more effective in relieving HACE than short-course IHT, it still does not completely relieve HACE. This may be because the dose we set for IHT is still not optimal. The neuroprotective effects of modest IHT include the reduction of inflammation and oxidative stress and the promotion of angiogenesis, neurogenesis, enhancement of the innate immune system and endothelial NO formation under hypoxic conditions12,38. For example, modest IHT not only rescues spatial learning and memory deficits by inducing hippocampal neurogenesis, synaptogenesis and brain-derived neurotrophic factor expression, but also induces respiratory neuroplasticity such as sustained increases in phrenic and hypoglossal nerve activity and ventilation in several species13. Accumulating evidence suggests that modest hypoxia and low cycle numbers often lead to beneficial effects without detectable pathology, whereas protocols utilizing severe hypoxia and more episodes per day elicit pathology13. Therefore, reducing the risk of IHT-induced pathology and increasing the beneficial effects of IHT by adapting the protocol or other approaches is key to the effectiveness of IHT in preventing HACE.
The prophylactic use of MTZ effectively alleviated HACE that occurred in rats in a hypobaric hypoxia environment, and a 200 mg/kg/d dose of MTZ may be a more optimal dose. The present study found that MTZ significantly enhanced hypoxia tolerance in rats. The experimental results showed that the BWC, protein and mRNA expression levels of AQP4 and VEGF in the hippocampus were significantly lower in the HH + MTZ1 and HH + MTZ2 groups than in the HH group, and rat indices in the 200 mg/kg group were similar to those of the NC group. MTZ significantly improved the ability of rats to tolerate hypoxia and prevented the occurrence of HACE, which may be related to the increase in tissue oxygen capacity or decrease in tissue oxygen consumption. As a CA inhibitor, MTZ exerts its preventive effects against AMS and HACE mainly by acting on the target organs and cells, including the kidney, systemic vascular endothelial cells and the brain39. The main action of MTZ is the inhibition of renal tubular CA and the subsequent loss of bicarbonate and generation of mild metabolic acidosis, which can increase respiration to significantly increase arterial partial pressure of oxygen (pO2) and blood oxygen levels40. Three sites in the brain may be involved in the protective effect of MTZ: the choroid plexus, cerebral vascular smooth muscle and chemoreceptors39. The inhibition of CA by MTZ was related to the increase in carbonic acid produced by the combination of carbon dioxide and water, which increased cerebral blood flow and reduced the production of cerebrospinal fluid, effectively alleviating the symptoms of headache, dizziness, etc.41 In addition, MTZ may act through other mechanisms unrelated to CA inhibition, including a reduction in aquaporin-mediated transmembrane water transport, antioxidant effects, vasodilation and anti-inflammatory effects39. Previous studies found that MTZ significantly reduced BBB permeability and BWC, increased respiratory rate and blood oxygen saturation, activated the activity of the antioxidant stress protomer Nrf2 in the brain, resisted hypoxia-induced oxidative stress injury and reduced hypoxia-mediated cerebrovascular leakage in the rat HACE model42. These data suggest that although 200 mg/kg/d of MTZ is more effective in alleviating HACE than 150 mg/kg/d of MTZ, it still cannot achieve complete relief.
Long-course IHT (BID, 14 d) combined with MTZ (200 mg/kg/d) was effective in preventing HACE. While different courses of IHT and different doses of MTZ each improved the degree of HACE in rats, none achieved complete prevention of HACE. This is the first study, to our knowledge, that investigate the role of IHT and MTZ combination in preventing HACE, to identify a more effective and definitive method to prevent HACE. Compared with the L-IHT and HH + MTZ2 groups, rats in the L-IHT&MTZ group showed a further decrease in BWC, an improvement in impaired memory and spatial function and a further decrease in protein and mRNA expression levels of AQP4 and VEGF in the hippocampus. The histological features in the cortex and hippocampus regions of the L-IHT&MTZ group were not significantly different from those of the NC group. These data suggest that IHT combined with MTZ is more effective in preventing HACE than long-course IHT or MTZ alone. IHT has been recognised by the sports medicine as a effective method for enhancing aerobic exercise performance, improving blood oxygen transport capacity, and inducing altitude acclimatisation13,43. However, after prolonged intermittent hypoxia, adverse factors such as reduced hematocrit-viscosity ratio (an oxygen delivery index) may gradually develop in humans or rats, triggering the side effects of IHT. Interestingly, the hematocrit-viscosity ratio can be dose-dependently increased after MTZ treatment, suggesting that MTZ improves oxygen delivery capacity in rats with excessive erythrocytosis after prolonged intermittent hypoxia20. Furthermore, chronic hypoxia increased plasma total cholesterol and low-density lipoprotein cholesterol levels, which were decreased by MTZ treatment, suggesting that MTZ has a beneficial effect on the metabolism of lipids under conditions of hypoxia20,44. Haptoglobin, an endogenous protective factor, which was decreased in rats after long-term hypoxia and significantly increased after MTZ treatment, may be another important mechanism in preventing HACE20. Taken together, these findings suggest that L-IHT combined with MTZ can achieve more satisfactory results in preventing HACE, probably because MTZ can prevent HACE while reducing the possibility of IHT-caused side effects, i.e., IHT can play a better role in high-altitude acclimatisation under the protection of MTZ without producing corresponding pathological changes. IHT and MTZ combination may be a simple, safe and effective treatment with significant potential for preventing HACE. Although several randomised clinical trials of IHT and MTZ have been conducted, there is still no optimal dose of IHT and MTZ for reference, which warrants further research to promote its effects while reducing potential risks.
Study limitations
Only animal and in vivo experiments were used to investigate the effect of the IHT and MTZ combination in preventing HACE; therefore, further in vitro and human experiments should be carried out to validate our findings.
Conclusions
In summary, the current study demonstrated that continuous hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% FiO2) with simultaneous EE for 2 days resulted in significant cerebral edema in rats, which may be used to establish the experimental rat model of HACE. Preemptive IHT or MTZ alone can alleviate HACE to varying degrees but neither is as effective as its combination. Long-course IHT (BID, 14 d) combined with MTZ (200 mg/kg/d) may be the most ideal method and dosage for preventing HACE.
Data availability
Data is provided within the supplementary information files.
Abbreviations
- IHT:
-
Intermittent hypoxia training
- MTZ:
-
Methazolamide
- HACE:
-
High-altitude cerebral edema
- FiO2 :
-
Fraction of inspired oxygen
- BWC:
-
Brain water content
- AQP4:
-
Aquaporin 4
- VEGF:
-
Vascular endothelial growth factor
- AMS:
-
Acute mountain sickness
- MWM:
-
Morris water maze test
- CA:
-
Carbonic anhydrase
- ACZ:
-
Acetazolamide
- EE:
-
Exhaustive exercise
- BBB:
-
Blood brain barrier
References
Richalet, J.-P., Larmignat, P., Poitrine, E., Letournel, M. & Canouï-Poitrine, F. Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am J Respir Crit Care Med 185, 192–198. https://doi.org/10.1164/rccm.201108-1396OC (2012).
Gallagher, S. A. & Hackett, P. H. High-altitude illness. Emerg Med Clin North Am 22 (2004).
Wilson, M. H., Newman, S. & Imray, C. H. The cerebral effects of ascent to high altitudes. Lancet Neurol 8, 175–191. https://doi.org/10.1016/S1474-4422(09)70014-6 (2009).
Bärtsch, P. & Swenson, E. R. Clinical practice: Acute high-altitude illnesses. N Engl J Med 368, 2294–2302. https://doi.org/10.1056/NEJMcp1214870 (2013).
Netzer, N., Strohl, K., Faulhaber, M., Gatterer, H. & Burtscher, M. Hypoxia-related altitude illnesses. J Travel Med 20, 247–255. https://doi.org/10.1111/jtm.12017 (2013).
MacInnis, M. J., Koehle, M. S. & Rupert, J. L. Evidence for a genetic basis for altitude illness: 2010 update. High Alt Med Biol 11, 349–368. https://doi.org/10.1089/ham.2010.1030 (2010).
Derby, R. & deWeber, K. The athlete and high altitude. Curr Sports Med Rep 9, 79–85, https://doi.org/10.1249/JSR.0b013e3181d404ac (2010).
Hackett, P. H. & Roach, R. C. High altitude cerebral edema. High Alt Med Biol 5, 136–146 (2004).
Aksel, G., Çorbacıoğlu, ŞK. & Özen, C. High-altitude illness: Management approach. Turk J Emerg Med 19, 121–126. https://doi.org/10.1016/j.tjem.2019.09.002 (2019).
Zelmanovich, R. et al. High Altitude Cerebral Edema: Improving Treatment Options. Biologics 2, 81–91 (2022).
Marussi, V. H. R. et al. Teaching NeuroImages: Typical neuroimaging features in high-altitude cerebral edema. Neurology 89, e176–e177. https://doi.org/10.1212/WNL.0000000000004544 (2017).
Zhang, G. et al. Intermittent hypoxia training effectively protects against cognitive decline caused by acute hypoxia exposure. Pflugers Arch 476, 197–210. https://doi.org/10.1007/s00424-023-02885-x (2024).
Navarrete-Opazo, A. & Mitchell, G. S. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307, R1181–R1197. https://doi.org/10.1152/ajpregu.00208.2014 (2014).
Wang, Y. et al. Intermittent hypoxia preconditioning can attenuate acute hypoxic injury after a sustained normobaric hypoxic exposure: A randomized clinical trial. CNS Neurosci Ther 30, e14662. https://doi.org/10.1111/cns.14662 (2024).
Doherty, C. J. et al. The Impact of Acetazolamide and Methazolamide on Exercise Performance in Normoxia and Hypoxia. High Alt Med Biol 24, https://doi.org/10.1089/ham.2022.0134 (2023).
Tapia, L. & Irarrázaval, S. Acetazolamide for the treatment of acute mountain sickness. Medwave 19, e7737. https://doi.org/10.5867/medwave.2019.11.7736 (2019).
Simancas-Racines, D. et al. Interventions for treating acute high altitude illness. Cochrane Database Syst Rev 6, CD009567, https://doi.org/10.1002/14651858.CD009567.pub2 (2018).
Sridharan, K. & Sivaramakrishnan, G. Pharmacological interventions for preventing acute mountain sickness: a network meta-analysis and trial sequential analysis of randomized clinical trials. Ann Med 50, 147–155. https://doi.org/10.1080/07853890.2017.1407034 (2018).
Williamson, J., Oakeshott, P. & Dallimore, J. Altitude sickness and acetazolamide. BMJ 361, k2153. https://doi.org/10.1136/bmj.k2153 (2018).
Zhang, Z. et al. Therapeutic Efficacy of Methazolamide Against Intermittent Hypoxia-Induced Excessive Erythrocytosis in Rats. High Alt Med Biol 19, 69–80. https://doi.org/10.1089/ham.2017.0044 (2018).
Wright, A., Brearey, S. & Imray, C. High hopes at high altitudes: pharmacotherapy for acute mountain sickness and high-altitude cerebral and pulmonary oedema. Expert Opin Pharmacother 9, 119–127 (2008).
Xu, G. Efficiency of methazolamide for hypoxia tolerance in mice and its prevention for acute mountain sickness. Journal of Third Military Medical University (Chinese) 40(41), 12–16. https://doi.org/10.16016/j.1000-5404.201707166 (2018).
Wright, A. D., Bradwell, A. R. & Fletcher, R. F. Methazolamide and acetazolamide in acute mountain sickness. Aviat Space Environ Med 54, 619–621 (1983).
Joyce, K. E., Lucas, S. J. E., Imray, C. H. E., Balanos, G. M. & Wright, A. D. Advances in the available non-biological pharmacotherapy prevention and treatment of acute mountain sickness and high altitude cerebral and pulmonary oedema. Expert Opin Pharmacother 19, 1891–1902. https://doi.org/10.1080/14656566.2018.1528228 (2018).
Song, T.-T. et al. Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J Neuroinflammation 13, 63. https://doi.org/10.1186/s12974-016-0528-4 (2016).
Guo, P., Luo, H., Fan, Y., Luo, Y. & Zhou, Q. Establishment and evaluation of an experimental animal model of high altitude cerebral edema. Neurosci Lett 547, 82–86. https://doi.org/10.1016/j.neulet.2013.05.008 (2013).
Chen, S.-J. et al. Overactivation of corticotropin-releasing factor receptor type 1 and aquaporin-4 by hypoxia induces cerebral edema. Proc Natl Acad Sci U S A 111, 13199–13204. https://doi.org/10.1073/pnas.1404493111 (2014).
Jing, L., Wu, N., He, L., Shao, J. & Ma, H. Establishment of an experimental rat model of high altitude cerebral edema by hypobaric hypoxia combined with temperature fluctuation. Brain Res Bull 165, 253–262. https://doi.org/10.1016/j.brainresbull.2020.10.017 (2020).
Peng, Y., Yin, H., Li, S. & Yang, H. Transcriptome of pituitary function changes in rat model of high altitude cerebral edema. Genomics 114, 110519. https://doi.org/10.1016/j.ygeno.2022.110519 (2022).
Ma, B. et al. Effect of butylphthalide on prevention and treatment of high altitude cerebral edema in rats. Heliyon 10, e27833. https://doi.org/10.1016/j.heliyon.2024.e27833 (2024).
Schoch, H. J., Fischer, S. & Marti, H. H. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 125, 2549–2557 (2002).
Wang, C. et al. Mechanism of aquaporin 4 (AQP 4) up-regulation in rat cerebral edema under hypobaric hypoxia and the preventative effect of puerarin. Life Sciences 193, 270–281. https://doi.org/10.1016/j.lfs.2017.10.021 (2018).
Ameli, P. A. et al. Role of vasopressin and its antagonism in stroke related edema. Journal of Neuroscience Research 92, 1091–1099. https://doi.org/10.1002/jnr.23407 (2014).
Wang, C. et al. Protective effects of puerarin on acute lung and cerebrum injury induced by hypobaric hypoxia via the regulation of aquaporin (AQP) via NF-κB signaling pathway. Int Immunopharmacol 40, 300–309. https://doi.org/10.1016/j.intimp.2016.09.010 (2016).
Fukuda, A. M. & Badaut, J. Aquaporin 4: a player in cerebral edema and neuroinflammation. J Neuroinflammation 9, 279. https://doi.org/10.1186/1742-2094-9-279 (2012).
Mishra, K. P., Ganju, L. & Singh, S. B. Hypoxia modulates innate immune factors: A review. Int Immunopharmacol 28, 425–428. https://doi.org/10.1016/j.intimp.2015.07.008 (2015).
Huang, X. et al. A method for establishing the high-altitude cerebral edema (HACE) model by acute hypobaric hypoxia in adult mice. J Neurosci Methods 245, 178–181. https://doi.org/10.1016/j.jneumeth.2015.02.004 (2015).
Serebrovskaya, T. V., Nikolsky, I. S., Nikolska, V. V., Mallet, R. T. & Ishchuk, V. A. Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men. High Alt Med Biol 12, 243–252. https://doi.org/10.1089/ham.2010.1086 (2011).
Swenson, E. R. Pharmacology of acute mountain sickness: old drugs and newer thinking. J Appl Physiol 1985(120), 204–215. https://doi.org/10.1152/japplphysiol.00443.2015 (2016).
Iturriaga, R., Mokashi, A. & Lahiri, S. Dynamics of carotid body responses in vitro in the presence of CO2-HCO3-: role of carbonic anhydrase. J Appl Physiol 1985(75), 1587–1594 (1993).
Swenson, E. R. Carbonic anhydrase inhibitors and high altitude illnesses. Subcell Biochem 75, 361–386. https://doi.org/10.1007/978-94-007-7359-2_18 (2014).
Lisk, C. et al. Nrf2 activation: a potential strategy for the prevention of acute mountain sickness. Free Radic Biol Med 63, 264–273. https://doi.org/10.1016/j.freeradbiomed.2013.05.024 (2013).
Fulco, C. S., Rock, P. B. & Cymerman, A. Improving athletic performance: is altitude residence or altitude training helpful?. Aviat Space Environ Med 71, 162–171 (2000).
Siques, P. et al. Plasma and liver lipid profiles in rats exposed to chronic hypobaric hypoxia: changes in metabolic pathways. High Alt Med Biol 15, 388–395. https://doi.org/10.1089/ham.2013.1134 (2014).
Acknowledgements
This work were supported by the National Natural Science Foundation of China (NSFC), No. 82171858 (to ZQH), and Science and Technology Research and Development Program of China National Railway Group Co., Ltd, No. K2019Z005 (to ZQH). The funders had no role in study design; data collection, analysis, and interpretation; writing of the paper; or decision to submit the paper for publication.
Funding
National Natural Science Foundation of China (NSFC), 82171858, Science and Technology Research and Development Program of China National Railway Group Co., Ltd, K2019Z005.
Author information
Authors and Affiliations
Contributions
WCP, HYM, and ZQH designed the study; WCP and HYM wrote the main manuscript text; WCP, HYM, RZ, and BJ carried out the experiment; RZ and SX collected the data and statistical analysis; WCP, HYM, ML and BJ prepared figures; ZQH take responsibility for the integrity of the work as a whole from inception to published article. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Institutional Review Board of Beijing Shijitan Hospital, Capital Medical University, Approval No: sjtkyll-lx-2023(010). This study is reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Peng, W., Ma, H., Zhao, R. et al. Role of intermittent hypoxic training combined with methazolamide in the prevention of high-altitude cerebral edema in rats. Sci Rep 14, 30252 (2024). https://doi.org/10.1038/s41598-024-81226-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81226-z