Abstract
This study compared the efficacy safety profiles of the Xen 63 and Preserflo MicroShunt devices, both standalone, in patients with primary open-angle glaucoma (POAG). It is a retrospective and single-center study conducted on consecutive on patients with medically uncontrolled POAG who underwent either a standalone Xen 63 or a standalone Preserflo and had a 12-month follow-up visit. The primary outcome was the mean IOP at month-12. Sixty eyes were included, 30 eyes in each Xen 63 and Preserflo groups, respectively. Preoperative IOP was significantly lowered from 20.8 ± 3.6 mmHg and 19.1 ± 3.8 mmHg to 14.2 ± 4.5 mmHg and 12.8 ± 2.3 mmHg in the Xen 63 and Preserflo groups, respectively (p < 0.0001 each, respectively); without significant differences between groups (p = 0.1346). Preoperative number of ocular-hypotensive drugs was significantly reduced from 2.3 ± 0.6 to 0.2 ± 06 drugs and from 2.3 ± 0.7 to 0.3 ± 0.6 drugs, in the Xen 63 and Preserflo groups, respectively (p < 0.0001 each, respectively); without significant differences between groups (p = 0.5212). Regarding safety, one (3.3%) eye in the Preserflo group required a device removal due to maculopathy. Three (10.0%) eyes in the Xen 63 group underwent needling. In conclusion, both the Xen 63 and the Preserflo devices effectively and safely reduced IOP and the requirement for IOP-lowering medications, exhibiting comparable IOP levels after 12 months.
Similar content being viewed by others
Introduction
The term open-angle glaucoma (OAG) includes a wide array of chronic and progressive optic neuropathies characterized by the degeneration of retinal ganglion cells and their axons, culminating in visual field impairment1. Lowering intraocular pressure (IOP) is currently considered the primary modifiable risk factor2. Initial treatment for OAG typically involves the administration of topical IOP-lowering agents3,4. The medical management often adheres to a stepwise protocol, initiating with a single topical medication and advancing to multidrug regimens or laser therapy if necessary3,4. Alternatively, the current European Glaucoma Society (EGS) guidelines advocate that selective laser trabeculoplasty (SLT) can be offered as a primary intervention4. In specific instances where a significantly lower target IOP is essential, surgical intervention may also be contemplated as a first-line option4.
However, some patients fail to attain adequate glaucoma control and, consequently, necessitate supplementary therapies and potentially surgical intervention3,4,5,6, such as trabeculectomy7, which regrettably has been linked with an elevated risk of sight-threatening complications8.
The limited efficacy of medical treatments in numerous patients, coupled with the complications linked to conventional surgery, has prompted the development of novel, effective, and safer surgical techniques that permit earlier intervention9. Substantial progress has been achieved in glaucoma surgery in recent years. One of the most remarkable advancements is the introduction of minimally or microinvasive glaucoma surgery (MIGS) and minimally invasive bleb surgery (MIBS) devices10,11. These innovations have been designed to offer safer and less traumatic approaches for reducing IOP in glaucoma patients10,11.
Although MIGS devices can be classified into trabecular procedures/devices and suprachoroidal devices, the European Glaucoma Society Guidelines strictly define MIGS as ab-interno, non-bleb-forming procedures11. Conversely, MIBS devices create an alternative outflow pathway for aqueous humor to the subconjunctival space, similar to trabeculectomy, and can be performed via either ab-interno or ab-externo approaches11.
The design of the Xen stent adheres to the Hagen–Poiseuille law of laminar flow, whereby the tube’s length and inner diameter determine the flow resistance and, consequently, the flow rate. Unlike the Xen 45 device, which has substantial clinical experience, the available evidence assessing the efficacy and safety of the Xen 63 is limited12,13,14,15,16. The new Xen 63 device utilizes the same needle injector as the Xen 45 to prevent early peri-implant flow and hypotony17. The stent is 6 mm in length, with an outer diameter of 250 μm and an inner diameter of 63 μm.
Similar to the Xen Gel Stent, the design of the Preserflo MicroShunt is based on the Hagen-Poiseuille equation. The device has a length of 8.5 mm and an internal lumen of 70 μm18. The Preserfl MicroShunt circumvents the conventional outflow pathway, directing aqueous humor from the anterior chamber to the subconjunctival and sub-Tenon spaces via a filtering bleb18. Studies by Beckers et al.19 and Bhayani et al.20 have demonstrated that the Preserflo MicroShunt was effective at lowering IOP). Additionally, Pillunat et al.21 and Jamke et al.22observed that the Preserflo MicroShunt necessitates fewer follow-up appointments compared to trabeculectomy. Moreover, previous investigations conducted by our group found the Preserflo MicroShunt, either alone or in combination with phacoemulsification, was an effective and safe treatment for lowering IOP and reducing the number of ocular hypotensive medications23,24.
Finally, the results of a meta-analysis, which assessed the effect of Preserflo MicroShunt over 12 months, found that IOP was significantly lowered by 9.07 mm Hg (95% CI: 7.88–10.25; p < 0.0001) and the number of ocular hypotensive medications was reduced by 2.37 drugs (95% CI: 2.15–2.60; p< 0.0001)25.
As far as we know, there are no studies comparing Xen 63 with Preserflo MicroShunt. Only two studies have compared the efficacy of Xen and Preserflo MicroShunt devices in OAG patients, and both studies were performed with the Xen 4526,27. While Scheres et al.26 reported similar IOP-lowering outcomes, Lüke et al.27 found that Preserflo MicroShunt was associated with a greater proportion of eyes with an IOP < 16 mmHg.
This study aimed to evaluate the efficacy, in terms of IOP-lowering and reduction of ocular hypotensive medication, and the safety profile of the Xen 63 and Preserflo MicroShunt devices, both standalone, in patients with primary OAG (POAG).
Methods
Study design
Retrospective and single-center study conducted on consecutive on patients with medically uncontrolled POAG who underwent either a standalone Xen 63 or a standalone MicroShunt and had a 12-month follow-up visit.
The study protocol received approval from the Ethics Committee of the San Carlos Clinical Hospital. This study adhered to the Good Clinical Practice/International Council for Harmonization Guidelines, the Declaration of Helsinki, and all relevant country-specific regulations governing the conduct of clinical research, whichever offered greater protection to the individual.
Any information that could potentially identify an individual has been encrypted or omitted as necessary to ensure their anonymity.
Study participants and Inclusion/exclusion criteria
This study included patients, aged ≥ 18 years, with insufficiently medically controlled early-to-moderate POAG4.
Patients were excluded if they had any form of glaucoma other than POAG; a history of incisional glaucoma surgery; any surgical procedure on the study eye within 3 months prior to the study; the presence of scars, previous surgeries, or other pathologies in the conjunctival superonasal quadrant; clinically significant inflammation and/or infection in the study eye within 30 days preceding the preoperative visit; or an allergy/sensitivity to any medication necessary for implantation (including anesthesia) or any of the device components.
Minimally invasive bleb surgery devices
Xen 63
The Xen gel stent (AbbVie Inc., Chicago, USA) is a hydrophilic, 6-mm long tube composed of porcine gelatin crosslinked with glutaraldehyde to prevent degradation following implantation17,28. The Xen 63 implant was positioned in the superior quadrant using an ab interno approach17. Mitomycin-C (MMC) at a subconjunctival concentration of 0.1 mg/mL, was administered during all surgeries.
Postoperative management included a regimen of antibiotic and anti-inflammatory therapy (topical combination of tobramycin and dexamethasone) administered every 2 h on the first postoperative day, gradually tapered over a period of 6–8 weeks.
Preserflo MicroShunt
The Preserflo MicroShunt (Santen former Innfocus, Miami, FL, USA) is manufactured from poly (styrene-block-isobutylene-block-styrene) (SIBS), a biocompatible material known for its resistance to biodegradation and biologically inert characteristics. Its softness and flexibility help minimize inflammation and scar tissue formation29. A conjunctival flap based on the fornix was fashioned either in the superonasal or superotemporal quadrant. A deep sub-Tenon’s pocket was created, and wet cautery was used to treat scleral vessels in the targeted area. MMC solution (0.4 mg/mL) was applied topically into the subconjunctival space using three LASIK Shields for a duration of 2 min23.
The sclera was marked 3 mm from the limbus. A scleral pocket was performed using a 1-mm triangular knife, followed by the insertion of a 25-gauge needle into this pocket to create a needle track extending into the anterior chamber. The Preserflo MicroShunt was then introduced into the anterior chamber through the needle track, securing the fin of the device snugly within the pocket After checking for flow, Tenon’s capsule was sutured using 10 − 0 Nylon and the conjunctiva with 9 − 0 Vicryl, ensuring a watertight closure23.
Postoperative care consisted of administering topical corticosteroids and antibiotics. The corticosteroid eye drops were gradually tapered off over a period of 3 months.
In cases where the IOP was deemed insufficient by the surgeon, postoperative topical therapy was resumed.
Study groups
The study sample was divided into two different groups, namely eyes who underwent a standalone Xen 63 device (Xen63 Group) and those who underwent a standalone Preserflo MicroShunt device (MicroShunt Group).
Definitions
Surgical success was defined according to three different criteria. Criterion I, defined as achieving a reduction in IOP of at least 20% from preoperative levels and an absolute IOP value between 6 and 18 mmHg, regardless of the use of ocular hypotensive medications; Criterion II, defined as achieving a reduction in IOP of at least 20% from preoperative levels and an absolute IOP value between 6 and 15 mmHg, regardless of the use of ocular hypotensive medications; and Criterion III, defined as achieving a reduction in IOP of at least 20% from preoperative levels and an absolute IOP value between 6 and 12 mmHg, regardless of the use of ocular hypotensive medications.
Outcomes
The primary endpoint was the mean IOP at month-12. The secondary end-points were the proportion of eyes considered as success at month-12 according to the three different criteria, the mean IOP lowering at month-12, the mean change in ocular hypotensive medications from preoperative values to month-12 and the incidence of adverse events (AEs).
Statistical analysis
The current analysis was carried out utilizing SPSS Inc software version 25 (PASW Statistics for Windows, Chicago: SPSS Inc. http://www.spss.com.hk/statistics/).
Data was tested for normal distribution using the Shapiro-Wilks test.
The independent sample Student’s t-test was used for testing preoperative differences between study groups.
Since both IOP and the number of ocular hypotensive medications followed a normal distribution, the paired samples Student’s t-test was used to assess the differences between preoperative and month-12 values.
Success rates for the study cohorts were estimated using Kaplan–Meier survival curves and statistically compared through the application of a log-rank test.
The Chi-square test was used to evaluate the differences in the proportion of eyes achieving success between groups.
P value of less than 0.05 was considered significant.
Results
The study includes a total of 60 eyes, 30 eyes in the Xen63 group and 30 eyes in the MicroShunt group. Their main demographic and clinical characteristics are shown in the Table 1.
There were no significant differences between the groups in any of the preoperative variables evaluated.
Intraocular pressure
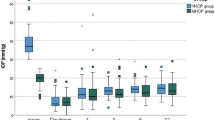
In the Xen63 Group, the mean preoperative IOP was significantly lowered from 20.8 ± 3.6 mmHg to 14.2 ± 4.5 mmHg at month-12 (Mean difference: −6.5 mmHg; 95%CI: −8.6 to −4.5 mmHg; p < 0.0001). In the MicroShunt Group there was a significant IOP lowering from 19.1 ± 3.8 mmHg at baseline to 12.8 ± 2.3 mmHg at month-12 (Mean difference: −6.3 mmHg; 95%CI: −8.0 to −4.6 mmHg; p < 0.00001).
Except for IOP at week 1 and month 1, which was significantly lower in the MicroShunt Group (p = 0.0352 and 0.0169, respectively), no significant differences were observed between the groups throughout the study follow-up.
The Fig. 1 shows the mean IOP at the different time points measured in both groups.
Mean intraocular pressure (IOP) in the Xen 63 and MicroShunt groups throughout study follow-up. Vertical bars represent 95% confidence interval. Intergroup statistical significance, at the different time point measurements, was determined using the independent sample Student’s t-test. As compared to preoperative values, the mean IOP was significantly reduced in both groups, at every time point measured, p < 0.0001.
Regarding IOP lowering, at month-12 there was no significant difference between the Xen63 (Mean IOP lowering: −6.6 ± 5.5 mmHg; 95%CI: −8.6 to −4.5 mmHg) and the Microshunt (−6.2 ± 4.2 mmHg; 95%CI: −7.9 to −4.5 mmHg) Groups (Mean difference: −0.4 ± 4.9 mmHg; 95%CI: −3.0 to 2.2 mmHg; p = 0.7743).
IOP was significantly reduced by 29.7 ± 23.5% and 32.9 ± 18.2% in the Xen63 and MicroShunt groups, respectively. No significant differences were observed between the groups (Mean difference: 3.2 ± 21.1%; 95%CI: −7.9–14.3%; p = 0.5646).
Ocular hypotensive medication
The mean preoperative number of ocular hypotensive medications were significantly reduced from 2.3 ± 0.6 and 2.3 ± 0.7 to 0.2 ± 0.6 and 0.3 ± 0.6 in the Xen63 and MicroShunt Groups, respectively (p < 0.0001 each, respectively).
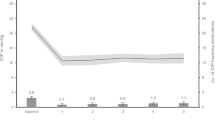
There were no significant differences between both groups throughout the study follow-up (Fig. 2).
Mean number of ocular hypotensive medications in the Xen 63 and MicroShunt groups throughout study follow-up. As compared to preoperative values, the mean number of ocular hypotensive drugs was significantly reduced in both groups, at every time point measured, p < 0.0001. No significant differences were observed between both groups throughout the study follow-up.
The mean number of ocular hypotensive medications, was reduced by −2.0 ± 0.9 and 1.9 ± 1.0 in the Xen63 and MicroShunt Groups, respectively (mean difference: −0.1; 95%CI: −0.6 to 0.4; p = 0.6786).
Proportion of eyes achieving a determining target IOP
At month 12, the proportion of eyes who were classified as success according to Criterion I (IOP lowering > 20% and a target IOP ≥ 6 mmHg and ≤ 18 mmHg) was 70.0% (21/30) and 70.0% (21/30) in the Xen63 and MicroShunt Groups, respectively (p = 1.000). According to Criterion II (IOP lowering > 20% and a target IOP ≥ 6 mmHg and ≤ 15 mmHg), the proportion of eyes considered as surgical success was 63.3% and 66.7% in the Xen63 and MicroShunt Groups, respectively (p = 0.7843); while the proportion of surgical success according to Criterion III (IOP lowering > 20% and a target IOP ≥ 6 mmHg and ≤ 12 mmHg) was 40.0% and 43.3%, respectively (p = 0.7971).
The Table 2 shows the number of eyes who achieved a predetermined target intraocular pressure in each group at the different time-point measured.
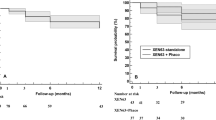
Kaplan–Meier survival analysis indicated no significant differences in the success rate between the Xen63 and the MicroShunt groups, regardless of the criteria used to define success (Fig. 3).
Kaplan-Meier survival analysis. A. According to the criterion I (IOP between 6 and 18 mmHg and IOP reduction ≥ 20% preoperative values). Mean Hazard ratio: 0.47; 95% CI: 0.14 to 1.54; p = 0.2109. B. According to the criterion II (IOP between 6 and 15 mmHg and IOP reduction ≥ 20% preoperative values). Mean Hazard ratio: 0.43; 95% CI: 0.16 to 1.18; p = 0.1023. C. According to the criterion III (IOP between 6 and 12 mmHg and IOP reduction ≥ 20% preoperative values). Mean Hazard ratio: 0.67; 95% CI: 0.29 to 1.55; p = 0.3468.
Safety
Needling was required in 3 eyes in the Xen63 group at months 3, 6 and 12, respectively. Two eyes underwent surgical bleb revision: one eye in the Xen63 group at month-1 and one eye in the MicroShunt group at month-6.
In the MicroShunt group, one eye underwent a reintervention at month 1 due to hypotony accompanied by choroidal detachment and hypotonic maculopathy. The hypotony persisted, requiring implant removal at month 3, after which both hypotony and maculopathy resolved.
In the MicroShunt group, one eye developed a Seidel leak requiring suturing on two occasions and subsequent amniotic membrane grafting.
Table 3 shows an overview of the different AEs reported throughout the study.
Discussion
This study compared the efficacy, measured in terms of both IOP lowering and reduction in the number of ocular hypotensive drugs, of the Xen63 and MicroShunt devices in patients with POAG.
Both devices demonstrated efficacy in lowering IOP and reducing the need for postoperative IOP-lowering medications. Our findings indicate that the Xen and MicroShunt implants exhibit comparable potential for IOP lowering and surgical effectiveness in patients with POAG. Regarding surgical success, this study did not detect significant differences between the two procedures, irrespective of the criteria employed. Specifically, 70% of eyes in the Xen63 Group and 70% of eyes who underwent MicroShunt implant were classified as surgical success (IOP lowering > 20% and a target IOP ≥ 6 mmHg and ≤ 18 mmHg), after 12 months of follow-up. In both cohorts, the mean IOP decreased to the low-mid teens at the 12-month. Additionally, the mean number of ocular hypotensive medications required was significantly reduced in both groups, with 26 (86.7%) and 20 (71.4%) eyes free of IOP-lowering medications at month-12 in the Xen63 and MicroShunt Groups, respectively (p = 0.5837).
Current evidence, including prospective and retrospective studies, demonstrated that Xen63 implant in patients with glaucoma is a safe and effective procedure, yielding a mean IOP in the mid-teens range (12–16 mmHg) after a 12-month follow-up period12,13,14,15,16. Particularly, in a prospective and multicenter study performed in Spanish population, Xen63 provided a significant IOP lowering (−6.9 mmHg) and reduction in the mean number of ocular hypotensive medications (from 2.3 ± 0.8 drugs at baseline to 0.3 ± 0.7 medications at month-12). The results of the current study agreed with the published evidence.
Regarding MicroShunt, the IOP-lowering observed in the current study (−6.2mmHg; 95%CI: −7.9 to −4.5 mmHg) was lower than that reported by the metanalysis published by Pietris & Casson (−9.07 mmHg; 95%CI: −10.25 to −7.88mmHg)25. These differences may be attributable to differences in the preoperative characteristics of the study populations. Notably, in our investigation, the mean preoperative intraocular pressure (IOP) was relatively low, averaging 19.1 ± 3.8 mmHg, with 22 eyes (73.3%) exhibiting a preoperative IOP of ≤ 20 mmHg.
To our knowledge, this is the first comparative study evaluating the Xen63 and MicroShunt devices. Prior research includes two studies that compared the efficacy and safety of the Xen45 and MicroShunt implants in patients with OAG26,27. These studies demonstrated that both devices significantly decreased IOP and reduced the need for IOP-lowering medications. However, Lüke et al.27 reported a greater proportion of eyes achieving an IOP of < 16 mmHg at 12 months in the MicroShunt group.
In agreement with these studies26,27, our study did not find significant differences between both devices in any of the outcomes analyzed. Moreover, unlike the study by Lüke et al.27, our study did not find differences in success rates, regardless of the criteria used.
Despite the similar drainage mechanisms of the two implants, they differ in several key aspects. Firstly, the Xen implant is introduced via an ab interno approach, avoiding the need to open the conjunctiva, whereas the MicroShunt is implanted using an ab externo approach, like trabeculectomy. Secondly, the two implants differ in their administration and the quantity of MMC used, which might influence surgery outcomes30. Thirdly, the implants are made of different materials and have distinct designs, which may impact their biocompatibility, foreign body reaction, and post-implantation migration17,28,29.
Regarding safety, 3 (10.0%) eyes in the Xen63 group required a needling procedure and 1 (3.3%) eye underwent surgical bleb revision. Interestingly, one (3.3%) eye in the MicroShunt group developed a hypotonic maculopathy, which required removal of the implant at month 1.
All other AEs reported in our study were successfully resolved within a maximum period of one month after the intervention.
The current study has several limitations, primarily due to its retrospective design. Consequently, results should be interpreted with this consideration in mind. Data were collected through routine clinical practice rather than a standardized reporting protocol. Despite this, gathering data from routine clinical practice provides a realistic depiction of current clinical methods. Additionally, since all participants were Caucasian, the study’s findings cannot be generalized to other ethnic populations regarding effectiveness and safety.
Conclusions
The results of this study suggested that both the Xen63 Gel Stent and the MicroShunt effectively and safely reduced IOP and the requirement for IOP-lowering medications, exhibiting comparable success rates after 12 months. Further prospective head-to-head trials and cost-effectiveness analyses are necessary to establish the optimal role of these new devices in the surgical management of glaucoma.
Data availability
Data not here published are obtainable on reasonable request from the corresponding author.
Abbreviations
- AEs:
-
Adverse events
- EGS:
-
European Glaucoma Society
- IOP:
-
Intraocular pressure
- MIBS:
-
Minimally invasive bleb surgery
- MIGS:
-
Minimally or microinvasive glaucoma surgery
- MMC:
-
Mitomycin-C
- OAG:
-
Open-angle glaucoma
- POAG:
-
Primary open-angle glaucoma
- SIBS:
-
Styrene-block-isobutylene-block-styrene
- SLT:
-
Selective laser trabeculoplasty
References
Weinreb, R. N. et al. Primary open-angle glaucoma. Nat. Rev. Dis. Primers. 2, 16067 (2016).
Heijl, A. et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest Glaucoma trial. Arch. Ophthalmol. 120(10), 1268–1279 (2002).
McKinnon, S. J., Goldberg, L. D., Peeples, P., Walt, J. G. & Bramley, T. J. Current management of glaucoma and the need for complete therapy. Am. J. Manag Care. 14(1 Suppl), S20–S27 (2008).
European Glaucoma Society Terminology and Guidelines for Glaucoma. ;105:1-169.5th Edition. Azuara Blanco A., Traverso C.E. (Eds); British Journal of Ophthalmology (2021).
Lichter, P. R. et al. Interim clinical outcomes in the collaborative initial Glaucoma treatment study comparing initial treatment randomized to medications or surgery. Ophthalmology 108(11), 1943–1953 (2001).
Newman-Casey, P. A. et al. The most common barriers to Glaucoma medication adherence: a cross-sectional survey. Ophthalmology 122(7), 1308–1316 (2015).
Landers, J., Martin, K., Sarkies, N., Bourne, R. & Watson, P. A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology 119(4), 694–702 (2012).
Jampel, H. D. et al. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am. J. Ophthalmol. 140(1), 16–22 (2005).
Cordeiro, M. F. et al. Prevalence of comorbidities with the potential to increase the risk of nonadherence to topical ocular hypotensive medication in patients with open-angle glaucoma. Curr. Med. Res. Opin. 40(4), 647–655 (2024).
Bar-David, L. & Blumenthal, E. Z. Evolution of Glaucoma surgery in the last 25 years. Rambam Maimonides Med. J. 9(3), e0024 (2018).
Abegao Pinto, L. et al. EGS surgery taskforce. European Glaucoma Society - A guide on surgical innovation for glaucoma. Br. J. Ophthalmol. 107(Suppl 1), 1–114 (2023).
Hussein, I. M., De Francesco, T. & Ahmed, I. I. K. Intermediate outcomes of the Novel 63-µm gelatin microstent versus the conventional 45-µm gelatin microstent. Ophthalmol. Glaucoma. 6(6), 580–591 (2023).
Fea, A. M. et al. Early experience with the New Xen63 Implant in Primary Open-Angle Glaucoma patients: clinical outcomes. J. Clin. Med. 10(8), 1628 (2021).
Fea, A. M. et al. Outcomes of Xen 63 device at 18-Month Follow-Up in Glaucoma patients: a two-Center Retrospective Study. J. Clin. Med. 11(13), 3801 (2022).
Evers, C. et al. Xen-63 compared to Xen-45 gel stents to reduce intraocular pressure in Glaucoma. J. Clin. Med. 12(15), 5043 (2023).
Martínez-de-la-Casa, J. M. et al. Effectiveness and safety of Xen63 in patients with primary-open-angle glaucoma. Sci. Rep. 14(1), 4561. https://doi.org/10.1038/s41598-024-55287-z (2024).
Lewis, R. A. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J. Cataract Refract. Surg. 40(8), 1301–1306 (2014).
Aghayeva, F. A., Chronopoulos, P., Schuster, A. K., Pfeiffer, N. & Hoffmann, E. M. Inter-eye relationship of intraocular pressure change after unilateral trabeculectomy, filtering canaloplasty, or PreserFlo microshunt implantation. Graefes Arch. Clin. Exp. Ophthalmol. 259(10), 3045–3053 (2021).
Beckers, H. J. M. et al. Safety and Effectiveness of the Preserflo MicroShunt in Primary Open-Angle Glaucoma: results from a 2-Year Multicenter Study. Ophthalmol. Glaucoma. 5(2), 195–209 (2022).
Bhayani, R. et al. Short-term safety and efficacy of Preserflo Microshunt in glaucoma patients: a multicentre retrospective cohort study. Eye (Lond). 37(4), 644–649 (2023).
Pillunat, K. R. et al. Preserflo MicroShunt versus trabeculectomy: first results on efficacy and safety. Acta Ophthalmol. 100(3), e779–e790 (2022).
Jamke, M. et al. Preserflo MicroShunt versus trabeculectomy: 1-year results on efficacy and safety. Graefes Arch. Clin. Exp. Ophthalmol. 261(10), 2901–2915 (2023).
Martínez-de-la-Casa, J. M. et al. Clinical outcomes of combined Preserflo Microshunt implantation and cataract surgery in open-angle glaucoma patients. Sci. Rep. 11(1), 15600 (2021).
Rojo-Arnao, M. et al. Preserflo MicroShunt implantation combined with Ologen TM in primary and secondary glaucoma patients in a clinical setting. Indian J. Ophthalmol. 72(3), 417–426 (2024).
Pietris, J. & Casson, R. One-year outcomes of Preserflo Microshunt for Primary Open Angle Glaucoma: a systematic review and Meta-analysis. J. Glaucoma. 33(7), e27–e34 (2024).
Scheres, L. M. J. et al. Xen Gel Stent compared to Preserflo MicroShunt implantation for primary open-angle glaucoma: two-year results. Acta Ophthalmol. 99(3), e433–e440 (2021).
Lüke, J. N. et al. Matched case-control comparison of surgical success after Xen45 gel stent and Preserflo MicroShunt implantation in a caucasian population. Clin. Exp. Ophthalmol. 6 https://doi.org/10.1111/ceo.14407 (2024 Jun).
Shute, T. S. et al. Biocompatibility of a Novel Microfistula Implant in Nonprimate mammals for the Surgical Treatment of Glaucoma. Invest. Ophthalmol. Vis. Sci. 57(8), 3594–3600 (2016).
Pinchuk, L. et al. Medical applications of poly(styrene-block-isobutylene-block-styrene) (SIBS). Biomaterials 29(4), 448–460 (2008).
Al Habash, A., Aljasim, L. A., Owaidhah, O. & Edward, D. P. A review of the efficacy of mitomycin C in glaucoma filtration surgery. Clin. Ophthalmol. 9, 1945–1951 (2015).
Author information
Authors and Affiliations
Contributions
Conceptualization: JMM, JGF; Methodology: JMM, FSF, CM; Formal analysis: APS, SGS, NGV; Investigation: JMM, APS, LMF, FSF, SGS, NGV, RSJ, CM; Supervision: JMM, FSF, JGF; Writing first draft: LMF, RSJ; Writing revision: JMM, CM, JGF. All authors met the ICMJE authorship criteria. All authors made substantial contributions to conception, design, analysis, and interpretation of data, contributed to writing the article, provided critical revision of the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethic Committee of the Centro Italiano Glaucoma Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards”.
Consent for publication
Not applicable.
Informed consent
Due to the characteristics of the study, the Ethic Committee of the San Carlos Clinical Hospital waived the need for written informed consent. Any information that could lead to an individual being identified has been encrypted or removed, as appropriate, to guarantee their anonymity.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Martínez-de-la-Casa, J.M., Pascual-Santiago, A., Morales-Fernandez, L. et al. Xen 63 versus Preserflo MicroShunt implant in patients with primary open-angle glaucoma. Sci Rep 15, 1634 (2025). https://doi.org/10.1038/s41598-024-81616-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81616-3
Keywords
This article is cited by
-
Update on XEN Gel Stent: A Narrative Review on Indications, Surgical Technique, and Postoperative Management
Ophthalmology and Therapy (2026)