Abstract
This study aims to explore the relationship between the Triglyceride Glucose Body Mass Index (TyG-BMI) and the risk of developing benign prostatic hyperplasia (BPH). Utilizing data from the China Health and Retirement Longitudinal Study (CHARLS), we included 3301 men aged 45 and above. We analyzed the association between TyG-BMI and the risk of developing BPH using multivariable logistic regression and restricted cubic spline models. During the 4-year follow-up period, 268 cases of BPH were identified. There was a positive correlation between TyG-BMI and the risk of BPH (OR 1.22, 95% CI 1.08–1.38, p < 0.001), with a significant dose-response relationship (P < 0.001). Stratified analyses indicated that the impact of TyG-BMI on BPH risk was consistent across various subgroups. There is a linear positive correlation between TyG-BMI and the risk of developing BPH. Managing TyG-BMI levels may help reduce the risk of BPH.
Similar content being viewed by others
Introduction
Benign prostatic hyperplasia (BPH) is a common urological condition among middle-aged and elderly men. Statistics in China indicate that the incidence of BPH exceeds 50% in men aged 60 and reaches as high as 83% in those aged 801. In 2019, there were 11.26 million new cases of BPH globally, resulting in 1.86 million disability-adjusted life years (DALYs)2. BPH occurs due to the proliferation of stromal and epithelial cells in the transitional zone of the prostate, leading to urethral narrowing and lower urinary tract symptoms (LUTS) such as urgency, frequency, nocturia, incomplete emptying, and weak stream. Although BPH is a benign condition, the associated LUTS can significantly reduce the quality of life, impair mental health, worsen overall health status, and increase the burden on healthcare resources3,4,5,6,7. Additionally, BPH can lead to complications such as increased residual urine, hydronephrosis, and urinary tract infections8,9. Therefore, early identification and intervention in high-risk populations for BPH are crucial.
The Triglyceride Glucose Body Mass Index (TyG-BMI) was first introduced in 201610. Researchers have used lipids, lipid factors, lipid-to-lipid factor ratios, visceral obesity indicators, TyG, and related parameters (TyG-WC, TyG-BMI) to identify insulin resistance (IR) early. They concluded that the TyG-BMI index is a more robust, simpler, and cost-effective measure for assessing IR. Previous studies have reported associations between benign prostatic hyperplasia (BPH) and obesity, dyslipidemia, as well as metabolic syndrome11,12. Is there an association between the TyG-BMI(a novel indicator of body lipid status)and BPH? Currently, there are no reported studies on the relationship between the TyG-BMI index and BPH. Therefore, we conducted a prospective study based on data from the China Health and Retirement Longitudinal Study (CHARLS) to explore the relationship between the TyG-BMI index and the risk of developing BPH.
Study population
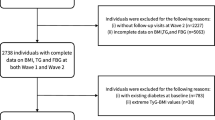
This study utilized baseline data from 2011 and follow-up data from 2015 from CHARLS database, which is freely accessible to the public. CHARLS is a large-scale, national, interdisciplinary survey covering participants aged 45 and above from 450 villages/communities across 28 provinces, autonomous regions, and municipalities. The survey encompasses a wide range of topics including demographic information, family structure, health status, healthcare and insurance, employment and pensions, income and expenditures, housing conditions, and laboratory test results. After fasting for over 12 h, venous blood samples were collected from participants and analyzed on-site for complete blood count. Whole blood samples were stored at 4 °C, with remaining samples transported to a central laboratory for further analysis of glucose and triglycerides (TG) using enzymatic colorimetric methods. BPH diagnosis information was also collected from the 2015 follow-up data. The CHARLS study received ethical approval from the Peking University Institutional Review Board in 2008 (Approval No: IRB00001052-11015). This study strictly adhered to CHARLS protocols and guidelines. All participants voluntarily signed informed consent forms prior to the survey. For this study, the inclusion criteria were: age ≥ 45 years; complete demographic information (including education level, marital status, and residence); and availability of both 2011 baseline and 2015 follow-up data. Ultimately, 3301 eligible participants were included in the study (Fig. 1).
Measurements
The TyG index is derived from fasting triglyceride and glucose levels, while BMI is a commonly used measure of weight relative to height.The TyG-BMI index combines the TyG index with the body mass index (BMI). The TyG-BMI is calculated as:
\({\text{TyG}} - {\text{BMI}}\,=\,{\text{ln}}({\text{fasting triglyceride level }}\left( {{\text{mg}}/{\text{dL}}} \right) \times {\text{fasting glucose level }}\left( {{\text{mg}}/{\text{dL}}} \right)/{\text{2}}) \times {\text{BMI}}.\)
BPH statistics
Men who were not diagnosed with BPH in the 2011 baseline survey but were newly diagnosed with BPH during the 2015 follow-up were defined as BPH cases. According to the investigation file DA029 questions13,14,15: Have you ever been diagnosed with a prostate illness, such as prostate hyperplasia(excluding prostatic cancer)? Have you ever been diagnosed with a prostate illness, such as prostate hyperplasia(excluding prostatic cancer)since we talked (in R’s LAST IW MONTH, YEAR/in the last two years)? Do you know if you had a prostate illness, such as prostate hyperplasia (excluding prostatic cancer) ? According to the answers to these three questions in the questionnaire, we can determine which part of the people suffer from prostate hyperplasia. This is the basis for the diagnosis of BPH in our study.
Covariate collection and definition
The analysis included sociodemographic characteristics, lifestyle factors, and physical examination indicators as covariates. Sociodemographic variables included age, education level (middle school or below/high school/college or above), residence (urban/rural), and marital status (married or unmarried/divorced/widowed). Sociodemographic variables included age, education level (middle school or below/high school/college or above), residence (urban/rural), and marital status (married/unmarried/divorced/widowed). This information was collected through self-reported questionnaires, completed under the guidance of trained interviewers. Physical examination indicators included systolic blood pressure (SBP) and diastolic blood pressure (DBP), measured three times using an Omron HEM-7200 electronic blood pressure monitor, with the average value recorded.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation if normally distributed, and as median (interquartile range) if skewed. Categorical variables were presented as counts (percentages). One-way ANOVA, Kruskal-Wallis H test, or Chi-square test was used to compare baseline characteristics and BPH incidence across TyG-BMI quartiles (Q1–Q4). Three logistic regression models were employed to assess the association between TyG-BMI and BPH risk, with TyG-BMI analyzed both as a continuous variable (per standard deviation) and as a categorical variable (quartiles). To assess the goodness of fit for our logistic regression model, we incorporated the Hosmer-Lemeshow test and Nagelkerke R2. The Hosmer-Lemeshow test evaluates model calibration by dividing predicted probabilities into groups and comparing observed versus expected outcomes within each group. For our model, we considered a p-value threshold of 0.05; a p-value greater than 0.05 would indicate an adequately calibrated model, while a p-value below this threshold would suggest potential calibration issues.In addition to the Hosmer-Lemeshow test, we calculated Nagelkerke R2, an adjusted pseudo-R2 suited for logistic regression models. Nagelkerke R2 approximates the proportion of variance in the outcome variable that the model explains, with values closer to 1 indicating stronger explanatory power. The strength of the associations was expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Interaction analyses were conducted by adding product terms of TyG-BMI and each covariate [TyG-BMI × (covariate)] to evaluate potential effect modification by sociodemographic and lifestyle factors on the TyG-BMI and BPH relationship. Additionally, restricted cubic spline curves were used to model the nonlinear dose-response relationship between TyG-BMI and BPH risk.
Restricted cubic splines were implemented using the “rms” package, with the rcs function defining the spline terms for the independent variable and fitting the regression model. We utilized 4 knots for the restricted cubic splines to effectively capture nonlinear relationships. The knots were placed at the 25th, 50th, and 75th percentiles of participants’ ages, as well as the boundary values of the data range. All statistical tests were two-sided, with p < 0.05 indicating statistical significance.
Results
Baseline characteristics
This study included 3,301 men with an average age of 59.4 ± 8.8 years, among whom 268 developed BPH during the follow-up period. The overall median TyG-BMI for the study population was 192.3 (170.6, 220.4). For those who did not develop BPH, the baseline median TyG-BMI was 191.6 (170.2, 219.3), whereas for those who developed BPH, the baseline median was 200.8 (175.6, 230.8). Baseline characteristics of BPH patients differed significantly from those without BPH. Specifically, BPH patients were older, had lower educational levels, were more likely to be from rural areas, and had higher body mass index (BMI) and waist circumference (WC) (Table 1).
Relationship between TyG-BMI and BPH
Three logistic regression models were constructed based on different sets of adjusted covariates. Model 1 was unadjusted. Model 2 adjusted for age, education level, residence, and marital status. Model 3 further adjusted for smoking status, alcohol consumption frequency, sleep duration, systolic blood pressure (SBP), and diastolic blood pressure (DBP) in addition to the covariates in Model 2. In Model 3, TyG-BMI was significantly associated with BPH risk, with an adjusted odds ratio (OR) of 1.77 (95% CI: 1.21–2.60). When TyG-BMI was included as a continuous variable (per standard deviation), it showed a positive association with BPH risk (OR = 1.22, 95% CI: 1.08–1.38, p < 0.001). Additionally, BPH risk increased with higher TyG-BMI quartiles (p = 0.039) (Table 2; Fig. 2). The Hosmer-Lemeshow goodness-of-fit test yielded a chi-square statistic of 3.123 with a p-value of 0.3, indicating that the model fits the data well with no significant calibration issues. The logistic regression model examining the association between TyG-BMI and BPH was significant, with a Nagelkerke R2 value of 0.639, indicating a good fit of the model to the data.
Restricted cubic spline of the association between TyG-BMI and the risk of BPH. The model was adjusted for age, education level, location and marital status, smoking status, drinking status, sleep time, SBP, and DBP. The plot shows a linear relationship between TyG-BMI and the risk of BPH. TyG-BMI triglyceride glucose body mass index, BPH benign prostatic hyperplasia. The reference point is the median of all the data.
Stratified analysis
To explore whether the association between TyG-BMI and BPH risk varies across different subgroups, we conducted stratified analyses based on age, education level, marital status, residence, smoking status, alcohol consumption, and sleep duration. The results of the stratified analysis indicated that the positive association between TyG-BMI and BPH risk was consistent across all subgroups, with no significant effect modification observed. In all subgroups, higher TyG-BMI values were associated with an increased risk of BPH (Fig. 3).
Discussion
This study, based on the CHARLS database, investigated the association between the TyG-BMI and the risk of developing BPH. The results revealed a significant positive correlation between TyG-BMI and the incidence of BPH, showing a clear dose-response relationship. As the TyG-BMI quartiles increased, the risk of developing BPH also increased in a stepwise manner, strongly suggesting that TyG-BMI may be an independent risk factor for BPH.
To further validate the generality of this association, we conducted detailed stratified analyses. The results indicated that the impact of TyG-BMI on BPH risk was particularly significant among individuals aged 45 and above. Additionally, the association remained consistent across subgroups with different education levels and residences, with no significant effect modification observed. These findings further enhance the reliability and generalizability of our conclusions. The significance of this study lies in its scientific confirmation of the positive association between TyG-BMI and BPH risk. This groundbreaking discovery not only enriches our understanding of the mechanisms underlying BPH but also provides new insights and directions for early prevention and intervention strategies. The results suggest that effectively managing triglyceride and glucose levels, as well as body mass index, may help reduce the risk of developing BPH.
The formation and progression of BPH is a complex process with mechanisms that are not yet fully understood. It may be caused by an imbalance between stromal cell proliferation and apoptosis. Among the confirmed risk factors, two conditions are necessary: aging and functional testes. Studies on ancient Chinese populations who underwent castration found that their prostates were either undetectable or significantly atrophied. Research indicates that obesity, including sarcopenic obesity and metabolic obesity, increases the risk of developing BPH16,17. Metabolic disturbances caused by obesity can elevate levels of various inflammatory factors and oxidative stress, leading to BPH18,19,20,21. Extensive infiltration of immune cells can cause prostate enlargement, which triggers and exacerbates lower urinary tract symptoms (LUTS)22. The conversion of circulating testosterone to estrogen, disrupting the balance between these hormones in prostate tissue, is one mechanism of BPH development21,23. Other studies highlight that steroid hormone levels and aging are also significant factors in prostate enlargement24. Furthermore, topological pathway analysis has shown that glycerophospholipid (GP) metabolism is a crucial metabolic pathway contributing to prostate enlargement25. Moreover, studies suggest that reducing body fat percentage in obese individuals may help alleviate LUTS in men26.
TyG-BMI was initially identified as a surrogate marker for early detection of IR. Our study results highlight the significant role of TyG-BMI in predicting the development of BPH. The findings indicate that higher TyG-BMI levels are associated with an increased risk of BPH. The pathways and mechanisms linking TyG-BMI to the risk of BPH include insulin resistance, chronic inflammation, and metabolic dysfunction. This association may stem from higher TyG-BMI levels leading to a higher incidence of metabolic syndrome, which in turn triggers increased levels of inflammatory factors and oxidative stress. The components of metabolic syndrome, as reflected in TyG-BMI, may impact prostatic growth through various mechanisms. For instance, obesity and dyslipidemia can lead to alterations in sex hormone levels, such as increased estrogen and decreased testosterone, which are known to influence prostatic health. Furthermore, metabolic dysfunction may induce oxidative stress and endoplasmic reticulum (ER) stress, contributing to cellular damage and promoting the development of BPH. A recent study indicated that patients with metabolic syndrome have higher International Prostate Symptom Scores compared to those without. Specifically, 59.3% of metabolic syndrome patients exhibited severe symptoms, compared to 36.2% of those without metabolic syndrome27. TyG-BMI serves as an indicator of insulin resistance, a condition that can lead to hormonal imbalances, particularly involving insulin and androgens. Elevated insulin levels may stimulate the growth of prostatic tissue by increasing the availability of circulating androgens and promoting the activity of growth factors such as insulin-like growth factor 1 (IGF-1). These hormonal alterations may contribute to the pathogenesis of BPH by fostering prostatic hyperplasia through enhanced cell proliferation and reduced apoptosis11. Higher levels of TyG-BMI are associated with systemic inflammation, which plays a significant role in the development of various chronic diseases, including BPH. Inflammatory markers, such as C-reactive protein (CRP) and cytokines, can influence prostatic tissue by promoting hyperplastic changes. The inflammatory microenvironment may lead to the activation of signaling pathways that further stimulate prostatic growth and development, exacerbating BPH28. Based on baseline data from CHARLS, we adjusted for confounding factors such as age, education level, residence, marital status, smoking status, alcohol consumption, and sleep duration, arriving at consistent conclusions. While it is important to include relevant confounders in regression models, the relationship between certain factors and BPH warrants consideration. Previous studies have indicated that education level can influence health literacy and access to healthcare, potentially affecting BPH diagnosis and management29. Geographic residence may also play a role, as regional differences can reflect variations in lifestyle, healthcare access, and environmental factors that contribute to BPH risk30,31. Additionally, alcohol consumption has been associated with hormonal alterations that could promote prostatic growth; excessive intake may lead to increased estrogen levels, which are linked to BPH development32. Diastolic pressure is another important factor, as hypertension is known to be associated with various metabolic disturbances that can affect prostate health33. Lastly, sleep duration is increasingly recognized for its role in metabolic health; inadequate sleep has been linked to hormonal imbalances and increased inflammation, both of which may contribute to the pathogenesis of BPH34. Therefore, it is essential to consider these factors when analyzing the associations with BPH, as they may serve as important confounders in the regression models. The study found that rural populations are more prone to BPH compared to urban populations, likely due to various factors such as access to medical resources, lifestyle, environmental factors, and hygiene practices. Lower education coverage in rural areas leads to insufficient awareness of prostate health, causing residents to often overlook early symptoms and delay seeking treatment. In contrast, urban areas have better health education, resulting in greater awareness of prostate diseases, enabling timely preventive measures and early treatment. Additionally, rural areas often have poorer economic conditions and lower medical insurance coverage, making it difficult for residents to afford regular check-ups and treatment. To enhance the accuracy of the results and ensure uniformity across different variables, we standardized the data. Following this standardization, the results further confirmed the significant positive role of TyG-BMI in promoting the development of BPH.
Research indicates that when TyG-BMI is high, the risk of BPH increases. However, rather than simply aiming to lower TyG-BMI, it is important to consider the positive roles of its components—triglycerides, glucose, and body mass index (BMI)—when they are within normal ranges. Triglycerides, as the primary form of energy storage, play a crucial role in energy supply, protecting internal organs, and aiding in the absorption of fat-soluble vitamins. Glucose is a major immediate energy source for the body, essential for the normal function of organs like the brain and muscles. Its stable levels, regulated by hormones, ensure overall energy balance in the body. An appropriate BMI reflects good nutritional status and helps maintain cardiovascular health and metabolic function. Therefore, we recommend maintaining TyG-BMI within an appropriate range in health management and disease prevention, rather than blindly aiming for the lowest possible value. This balanced approach can effectively reduce BPH risk while ensuring the normal functioning of various physiological processes. Future health interventions should be based on this comprehensive understanding, aiming to find the optimal balance between disease prevention and maintaining normal physiological functions, thus developing more scientific and rational health strategies.
This study has several key limitations. First, as an observational study, it cannot establish a causal relationship between TyG-BMI and BPH. Second, the four-year follow-up period may be insufficient to fully assess long-term risks. The study relies on the CHARLS database and self-reported BPH diagnoses, which may introduce selection bias and misclassification. The sample includes only Chinese men aged 45 and above, which may limit the generalizability of the results. Although multiple known confounding factors were adjusted for, there may still be unidentified or unmeasured confounders. Importantly, the study did not include measurements of androgen hormone levels, which are relevant to the development of BPH, nor did it explore the potential biological mechanisms underlying the association between TyG-BMI and BPH.
Conclusion
This study is the first to demonstrate a linear positive correlation between TyG-BMI and the risk of benign prostatic hyperplasia (BPH), showing a significant dose-response relationship. This suggests that managing the TyG-BMI index can help reduce the risk of BPH, offering new insights for early prevention and intervention.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
References
Gu, F. L., Xia, T. L. & Kong, X. T. Preliminary study of the frequency of benign prostatic hyperplasia and prostatic cancer in China. Urology 44, 688–691. https://doi.org/10.1016/s0090-4295(94)80207-6 (1994).
Devlin, C. M., Simms, M. S. & Maitland, N. J. Benign prostatic hyperplasia—what do we know? BJU Int. 127, 389–399. https://doi.org/10.1111/bju.15229 (2021).
Taub, D. A. & Wei, J. T. The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr. Urol. Rep. 7, 272–281. https://doi.org/10.1007/s11934-996-0006-0 (2006).
Xu, X. F. et al. Global, regional, and national incidence and year lived with disability for benign prostatic hyperplasia from 1990 to 2019. Am. J. Mens Health 15, 15579883211036786. https://doi.org/10.1177/15579883211036786 (2021).
Ottaiano, N., Shelton, T., Sanekommu, G. & Benson, C. R. Surgical complications in the management of benign prostatic hyperplasia treatment. Curr. Urol. Rep. 23, 83–92. https://doi.org/10.1007/s11934-022-01091-z (2022).
Lim, K. B. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 4, 148–151. https://doi.org/10.1016/j.ajur.2017.06.004 (2017).
Dornbier, R., Pahouja, G., Branch, J. & McVary, K. T. The New American Urological Association benign prostatic hyperplasia clinical guidelines: 2019 update. Curr. Urol. Rep. 21, 32. https://doi.org/10.1007/s11934-020-00985-0 (2020).
Gacci, M. et al. Male lower urinary tract symptoms and cardiovascular events: a systematic review and meta-analysis. Eur. Urol. 70, 788–796. https://doi.org/10.1016/j.eururo.2016.07.007 (2016).
Xia, M. F. et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci. Rep. 6, 38214. https://doi.org/10.1038/srep38214 (2016).
Er, L. K. et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE 11, e0149731 (2016).
Fu, X., Wang, Y., Lu, Y., Liu, J. & Li, H. Association between metabolic syndrome and benign prostatic hyperplasia: the underlying molecular connection. Life Sci. 1, 123192 (2024).
Xu, G. et al. The etiology and pathogenesis of benign prostatic hyperplasia: the roles of sex hormones and anatomy. Res. Rep. Urol. 1, 205–214 (2024).
Strauss, J. et al. Health outcomes and socio-economic status among the elderly in China: evidence from the CHARLS Pilot. J. Popul. Ageing 3, 111–142 (2010).
Smith, J. P., Shen, Y., Strauss, J., Zhe, Y. & Zhao, Y. The effects of childhood health on adult health and SES in China. Econ. Dev. Cult. Change 61, 127–156 (2012).
Lei, X., Hu, Y., McArdle, J. J., Smith, J. P. & Zhao, Y. Gender differences in cognition among older adults in China. J. Hum. Resour. 47, 951–971 (2012).
Rao, X. et al. The causal relationship between sarcopenic obesity factors and benign prostate hyperplasia. Front. Endocrinol. (Lausanne). 14, 1290639. https://doi.org/10.3389/fendo.2023.1290639 (2023).
Wang, Y. B. et al. Causal relationship between obesity, lifestyle factors and risk of benign prostatic hyperplasia: a univariable and multivariable Mendelian randomization study. J. Transl. Med. 20, 495. https://doi.org/10.1186/s12967-022-03722-y (2022).
Mraz, M. & Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 222, 113–127. https://doi.org/10.1530/joe-14-0283 (2014).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 114, 1752–1761. https://doi.org/10.1172/jci21625 (2004).
Sciarra, A. et al. Inflammation and chronic prostatic diseases: evidence for a link? Eur. Urol. 52, 964–972. https://doi.org/10.1016/j.eururo.2007.06.038 (2007).
Corona, G. et al. Benign prostatic hyperplasia: a new metabolic disease of the aging male and its correlation with sexual dysfunctions. Int. J. Endocrinol. 329456. https://doi.org/10.1155/2014/329456 (2014).
Chen, W., Man, S., Wang, B., Kadeerhan, G. & Huang, X. Metabolically healthy obesity is associated with increased risk of lower urinary tract symptoms secondary to benign prostatic hyperplasia: a cohort study of Chinese elderly males. Low Urin. Tract. Sympt. 14, 170–177. https://doi.org/10.1111/luts.12420 (2022).
Williams, G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-α and GPER signalling. Mol. Cell. Endocrinol. 351, 269–278. https://doi.org/10.1016/j.mce.2011.12.017 (2012).
Tubaro, A., De Nunzio, C., Puccini, F. & Presicce, F. The evolving picture of lower urinary tract symptom management. Eur. Urol. 67, 271–272. https://doi.org/10.1016/j.eururo.2014.10.016 (2015).
Fan, G. et al. Untargeted metabolomics reveals that declined PE and PC in obesity may be associated with prostate hyperplasia. PLoS ONE 19, e0301011. https://doi.org/10.1371/journal.pone.0301011 (2024).
Lee, J. et al. Lowering the percent body fat in the obese population might reduce male lower urinary tract symptoms. World J. Urol. 41, 1621–1627. https://doi.org/10.1007/s00345-023-04397-w (2023).
Daher, M., Saqer, T., Jabr, M. & Al-Mousa, S. Benign prostatic hyperplasia and metabolic syndrome; prevalence and association: a cross-sectional study in Syria. BMC Urol. 23, 187. https://doi.org/10.1186/s12894-023-01365-9 (2023).
Cao, H. et al. Association between circulating inflammatory proteins and benign prostatic disease: a Mendelian randomization study. Sci. Rep. 14, 23667 (2024).
Rana, P. et al. Unveiling the potential of novel 5α-reductase inhibitors via ligand based drug design, molecular docking and ADME predictions to manage BPH. J. Mol. Struct. 1320, 139547 (2025).
Xiong, Y., Zhang, Y., Li, X., Qin, F. & Yuan, J. The prevalence and associated factors of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males. Aging Male 23, 1432–1439 (2020).
Nagakura, Y., Hayashi, M. & Kajioka, S. Lifestyle habits to prevent the development of benign prostatic hyperplasia: analysis of Japanese nationwide datasets. Prostate Int. 10, 200–206 (2022).
Liao, G. et al. Joint effect between bisphenol A and alcohol consumption on benign prostatic hyperplasia: a case–control study in Hong Kong Chinese males. Prostate 81, 1214–1224 (2021).
Zeng, L. P., Li, Y., Li, X. Y. & Yin, Z. Incidence of comorbid ED with LUTS and its risk factors in patients with BPH. Natl. J. Androl. 30, 139–144 (2024).
Ma, K. & Dong, Q. Association between sleep quality and benign prostate hyperplasia among middle-aged and older men in India. BMC Public Health 23, 1147 (2023).
Author information
Authors and Affiliations
Contributions
BL and ZZ: Conceptualization and design of the study, data acquisition and interpretation, drafting the manuscript. CS and YS: Conceptualization and design of the study, formal analysis, and methodology. XL, and JL: Preparation of figures and tables, drafting the manuscript. All authors contributed to the final version of the manuscript and approved it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, B., Zhang, Z., Sun, C. et al. Association between TyG-BMI and BPH in a national prospective cohort study. Sci Rep 15, 8743 (2025). https://doi.org/10.1038/s41598-024-81629-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81629-y