Abstract
The triglyceride to high density lipoprotein cholesterol (TG/HDL-C) ratio has been consistently linked with the risk of coronary heart disease (CHD). Nevertheless, there is a paucity of studies focusing on acute coronary syndrome (ACS) patients undergoing percutaneous coronary intervention (PCI) or experiencing bleeding events. The study encompassed 17,643 ACS participants who underwent PCI. Survival analysis, Cox regression analysis and restricted cubic spline (RCS) were employed to assess the associations between TG/HDL-C ratio and the risk of major adverse cardiovascular events (MACE), all-cause death, cardiac death and all-cause bleeding events. Over a 12-month follow-up period, 638 (3.9%) patients experienced MACE while 2837 (16.1%) patients experienced bleeding events. The TG/HDL-C ratio exhibited significant positive correlations with the incidence of MACE, all-cause death and cardiac death; conversely it displayed significant negative correlations with the incidence of all-cause bleeding. Patients in the high quartile TG/HDL-C category demonstrated significantly higher risks for MACE compared to those in the low quartile category, with hazard ratio (HR) [95%confidence interval (CI)] of 1.46 (1.17–1.83); conversely, they showed significantly lower risks for all-cause bleeding compared to their counterparts in the low quartile group, with HR (95%CI) of 0.72 (0.65–0.81). The structure of subgroup analyses remained robust and consistent, with gender being the sole factor interacting with TG/HDL-C specifically in relation to MACE events (P for interaction = 0.037). A higher baseline TG/HDL-C ratio was associated with an elevated risk of MACE but a reduced risk of bleeding events in ACS patients undergoing PCI.
Similar content being viewed by others
Introduction
Cardiovascular disease leads to 4 million deaths per year and accounts for over 40% of total mortality in China and acute coronary syndrome (ACS) is the most serious type1. After undergoing standardized percutaneous coronary intervention (PCI) treatment, it is imperative for patients with ACS to receive guideline-directed pharmacotherapy in order to optimize their prognosis. However, due to variations in demographic parameters, hematological markers, medication utilization and medical history among individuals, the prognostic outcomes of ACS patients undergoing PCI exhibit significant heterogeneity. This is particularly evident in those with comorbidities such as hypertension, hyperglycemia and hyperlipidemia, who are at heightened risk for recurrent adverse cardiovascular events. Consequently, early risk stratification plays a pivotal role in the secondary prevention of coronary heart disease (CHD) for such patient cohorts2,3,4,5,6.
Insulin resistance (IR) is defined as a pathophysiological state characterized by reduced insulin sensitivity in peripheral target organs, necessitating higher-than-normal levels of insulin to elicit normal responses. IR is typically associated with metabolic syndrome and plays a pivotal role in the pathogenesis of CHD. The structural and functional vascular disease linked to IR serves as a highly predictive factor for cardiovascular morbidity and mortality7,8,9,10. While the hyperinsulinemic euglycemic clamp (HEC) remains the gold standard for evaluating IR11, its clinical application is limited due to complexity and expense, thus hindering widespread use in assessing the risk of adverse cardiovascular events among patients. Nevertheless, evidence suggests that the triglyceride to high density lipoprotein cholesterol (TG/HDL-C) ratio can serve as a simple and reliable alternative indicator of IR, significantly associated with the risk of metabolic syndrome and CHD while also predicting severity and recurrence risk of CHD to some extent12,13,14,15. However, there remains a lack of reports on bleeding events despite associations between IR and adverse cardiovascular event risks in both healthy populations and CHD patients being primarily related to ischemic events. The ACS population undergoing PCI requires long-term antiplatelet therapy and lipid-lowering therapy for improved prognosis and reduced risk of recurrent adverse cardiovascular events16,17, yet studies on IR within this population are scarce. The objective of this study was to predict the risks of ischemic and bleeding events in patients with ACS after PCI through the TG/HDL-C ratio, and potentially aid in the decision-making of antiplatelet therapy and assist patients in preventing cardiovascular adverse events to a certain extent.
Materials and methods
Study design and participants
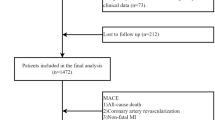
The data utilized in the study were derived from patients in the General Hospital of Northern Theater Command, China. The study protocol conforms to the guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the Ethics Committee of the General Hospital of Northern Theater Command and this study with a waiver of informed consent was approved. A total of 17,643 ACS patients who underwent PCI between March 2016 and March 2019 were enrolled in the study, encompassing those with unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI). To mitigate result bias, patients not receiving dual antiplatelet therapy were excluded (Fig. 1). Participants were followed up for a period of 12 months. Experienced cardiology physicians performed PCI surgery and administered antiplatelet drugs according to standard procedures. Post-discharge drug usage adhered to current guidelines. Demographic data, medical history, and clinical manifestations were documented upon admission. Fasting peripheral venous blood was collected before PCI for routine blood tests, blood biochemistry, and coagulation function assessment using a standardized spreadsheet for retrospective data collection.
Definition of covariates and outcomes
The TG/HDL-C ratio was computed as the quotient of TG (mg/dL) divided by HDL-C (mg/dL). The estimated glomerular filtration rate (eGFR) was derived using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation based on baseline creatinine measurements. ST change is defined as abnormal elevation or depression of the ST segment on an electrocardiogram (ECG). Discharge P2Y12 inhibitors consisted of clopidogrel or ticagrelor. The primary clinical outcome was major adverse cardiovascular events (MACE), encompassing cardiac death, nonfatal myocardial infarction (MI) stroke and target vessel revascularization (TVR) within 12 months post-discharge. Secondary outcomes included all-cause mortality, cardiac death, and all-cause bleeding events within 1 year, with bleeding scores categorized according to the bleeding academic research consortium (BARC) bleeding classification. Upon the selection of covariates, we carried out univariate Cox regression analyses on all common clinical indicators, screening out those associated with the outcome event MACE with a P < 0.05. Then, these indicators were incorporated into the multivariate Cox regression analysis. If the P value remained < 0.05, they were included in the final statistical model. Besides, we also referred to the articles previously published by our center and determined the variables in this study based on the covariates in the OPT-CAD score that is more applicable to the Chinese population18. Covariates associated with MACE included TG/HDL-C ratio, gender, age, previous MI or stroke, hypertension, hemoglobin levels, plasma glucose levels, TnT and eGFR. Covariates linked to all-cause bleeding were TG/HDL-C ratio, gender, age, diabetes, previous MI, ECG ST segment changes, TnT levels and use of discharge P2Y12 inhibitors.
Statistical analyses
Data analysis was executed utilizing R 4.2.1 and SPSS 25.0. Continuous variables were expressed as the mean (SD), while categorical variables were represented as frequency (percentage). Data comparison was conducted through one-way ANOVA analysis and Chi-square test. Covariates underwent screening via univariate and multivariate Cox regression. Restricted cubic spline (RCS) fitting was employed to evaluate the linear or nonlinear association between TG/HDL-C ratio and outcomes (threshold, P < 0.05). TG/HDL-C ratio was categorized into four subgroups based on quartiles, with the first subgroup designated as the reference group. Kaplan‒Meier cumulative incidence maps were generated for quartiles of TG/HDL-C ratio, tested by log-rank test to assess associations with outcome events during follow-up. Multivariate adjusted Cox regression model was used to calculate hazard ratio (HR) and 95% confidence interval (CI) for higher quartiles versus lowest quartiles of hazard ratios in ACS patients and subgroups. A two-sided P < 0.05 denoted statistical significance.
Results
Baseline data of the included participants
Overall and stratified by quartiles of the TG/HDL-C ratio, as delineated in Table 1. Out of the total 17,643 participants enrolled, 12,872 (73.0%) were male, with a mean (SD) age at baseline of 60.77 (10.24) years. The prevalence of hypertension and diabetes was 62.0% and 31.1%. Elevated TG/HDL-C ratio levels were associated with younger age and male gender, higher prevalence of current smoking, prior MI, hypertension and diabetes; elevated hemoglobin, glucose, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), creatinine levels; increased platelet count and adenosine diphosphate (ADP); as well as lower rates of previous stroke occurrence along with reduced levels of troponin T (TnT), international normalized ratio (INR), D-dimer and fibrinogen. Higher TG/HDL-C ratio were linked to a higher incidence of NSTEMI and greater usage of angiotensin-converting enzyme inhibitors (ACEIs) /angiotensin receptor blockers (ARBs) and beta-blockers but a lower incidence rate for STEMI. Over the course of the one-year follow-up period, MACE occurred in 638 patients (3.9%), including cardiac death in 188 patients (1.1%), nonfatal MI in 85 patients (0.5%), stroke in 109 patients (0.6%) and TVR in 339 patients (1.9%). Additionally, 2837 patients experienced bleeding events during this time frame. Amongst observed outcome events, individuals with higher TG/HDL-C ratio had an increased likelihood for TVR while experiencing decreased incidences of all-cause bleeding events.
Associations between TG/HDL-C ratio and outcome events
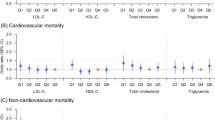
The study investigated the association between TG/HDL-C ratio and outcome events. Univariate Cox regression analysis demonstrated a significant correlation between continuous TG/HDL-C ratio and an elevated risk of MACE, all-cause death and cardiac death, as well as a decreased risk of all-cause bleeding. Following multivariate adjustment, the Cox regression analysis and RCS curve illustrated a nonlinear positive correlation between TG/HDL-C ratio and MACE risk (HR = 1.06, 95%CI = 1.03–1.09, P < 0.001) and all-cause death (HR = 1.09, 95%CI = 1.05–1.13, P < 0.001), a linear positive correlation with cardiac death (HR = 1.09, 95%CI = 1.05–1.14, P < 0.001), and a nonlinear negative correlation with all-cause bleeding (HR = 0.93, 95%CI = 0.90–0.95, P < 0.001) (Fig. 2; Table 2; Supplementary Table 1, Additional File 1; Supplementary Table 2, Additional File 1). Univariate Cox regression analysis of TG showed similar significance, while HDL-C was only significantly associated with an increased risk of all-cause bleeding (Supplementary Table 3, Additional File 1).
After stratifying TG/HDL-C ratio into four categorical variables, the Kaplan-Meier survival curve revealed a significantly elevated cumulative risk of MACE in the fourth quartile compared to the first quartile, despite the lack of statistical significance in the overall survival curve as determined by the log-rank test (P = 0.064). Conversely, the cumulative risk of all-cause bleeding was notably lower in the fourth quartile than the first quartile (P < 0.001) (Fig. 3). These results exhibited enhanced robustness following multi-factor adjustment and demonstrated a more pronounced differentiation in risk between groups (Supplementary Fig. 1, Additional File 2). The multivariate Cox regression analysis demonstrated that patients in the fourth quartile exhibited a heightened incidence of MACE (HR = 1.46, 95%CI = 1.17–1.83, P for trend = 0.009) and a reduced incidence of all-cause bleeding (HR = 0.72, 95%CI = 0.65–0.81, P for trend < 0.001) compared to those in the first quartile. Meanwhile, there was no significant disparity in the risk of all-cause death and cardiac death events following TG/HDL-C ratio grouping (Table 3).
Subgroup analyses
Subgroup analyses were conducted to ascertain whether demographic characteristics and comorbidities accounted for the association between TG/HDL-C ratio and the incidence of MACE and all-cause bleeding. Consistent findings were observed in stratified analyses by age, hypertension and eGFR for MACE events (all P for interaction > 0.05). TG/HDL-C ratio exhibited a significant interaction with gender (P for interaction = 0.037), with the fourth quartile increasing the risk of MACE by 46% compared to the first quartile in the general population, but by 84% in women (Fig. 4). Similarly, consistent results were found for all interactions in all-cause bleeding events when stratified by gender, age, diabetes, and discharge P2Y12 inhibitor use (all P for interaction > 0.05) (Supplementary Fig. 2, Additional File 2).
Discussion
Previous studies have demonstrated that reliable surrogate markers of IR exhibit varying degrees of prognostic ability for adverse cardiovascular events in both healthy controls and CHD patients. Our real-world data analysis revealed that high IR is not only associated with an increased risk of MACE, all-cause death and cardiac death, but also closely linked to a decreased risk of all-cause bleeding events.
IR is frequently concomitant with metabolic syndrome, encompassing obesity, hypertriglyceridemia, reduced concentrations of HDL-C, hypertension, microalbuminuria and dysglycemia. These factors are pivotal risk elements for CHD. Consequently, the TG/HDL-C ratio, linked to hyperlipidemia, exhibits a significant correlation with adverse cardiovascular events. The impact of insulin on blood vessels is intricate and may exert either protective or deleterious effects on the vascular system19,20,21,22. For instance, insulin resistance may attenuate nitric oxide (NO) production by endothelial cells while enhancing procoagulant factor release, thereby precipitating platelet aggregation and impairing endothelial function23,24. Additionally, it can directly induce inflammation and oxidative stress in vascular endothelial cells25,26. Hyperinsulinemia has been shown to instigate the proliferation of diverse vascular smooth muscle and endothelial cells, fostering atherogenic processes via activation of mitogen-activated protein kinase (MAPK) signaling pathways27. This mechanistic insight elucidates why individuals with elevated IR are predisposed to developing NSTEMI associated with atherosclerosis as per the foundational data presented in this study.
Hyperinsulinemic ECT is considered the gold standard for evaluating insulin resistance (IR). However, due to its high cost and time-consuming nature, alternative IR indicators such as the triglyceride and glucose (TyG) index, TyG × body mass index (BMI), TG/HDL-C ratio and metabolic score for insulin resistance (METS-IR) have emerged. A study of 2533 participants with a history of CHD undergoing PCI confirmed that these IR indicators were significantly associated with major adverse cardiovascular and cerebrovascular events (MACCEs) in females, with METS-IR demonstrating the strongest predictive power28. Nevertheless, incorporating these IR indicators did not enhance the discriminative ability of the basic risk model. Among them, the TyG×BMI index was not only significantly linked to CHD severity but also an independent risk factor for multivessel CHD29. In a separate study involving 47,270 participants without a history of CHD over a 4-year period revealed that maintaining optimal METS-IR throughout life is crucial for cardiovascular health as increasing METS-IR correlated with elevated CHD risk over time30. Furthermore, an analysis of 403,335 UK participants without prior CHD history indicated that elevated baseline TyG index and TG/HDL-C ratio were associated with increased CHD risk primarily mediated by higher incidences of dyslipidemia, type 2 diabetes and hypertension31. Consistent with previous research findings, this study also affirmed the association between IR indicators and heightened risk of adverse cardiovascular events. Inconsistently, in the ACS patients undergoing PCI, the TG/HDL-C ratio can serve as a predictor for both ischemic events and bleeding events which may guide adjustments in antiplatelet therapy regimens.
In a study of 17,643 ACS patients undergoing PCI, we constructed Cox proportional hazards models, which showed that the TG/HDL-C ratio was significantly positively correlated with MACE and death events but significantly negatively correlated with bleeding events. Patients in the higher quartiles of the TG/HDL-C ratio had a greater risk of MACE than did those in the lower quartiles within 12 months after PCI, while these patients had a significantly reduced risk of all-cause bleeding events. In the subgroup analysis, a pronounced interaction between TG/HDL-C ratio and gender was evident in predicting MACE risk, indicating that TG/HDL-C ratio may exert a more robust predictive influence on MACE risk in female patients. Moreover, the discriminative capacity of TG/HDL-C ratio for all-cause bleeding risk was notably higher in the clopidogrel group compared to the ticagrelor group. This disparity could be attributed to variances in the utilization of these two anticoagulants or discrepancies in long-term bleeding risks associated with each medication.
IR is strongly associated not only with the occurrence and fatality of ischemic CHD, but also with bleeding events. A study involving 274 patients with STEMI found that the highest tertile of the TyG index independently and significantly increased the risk of major bleeding events in patients with PE32. Another study involving 240 patients confirmed that IR is a potential marker for predicting intracranial microbleeds in patients with intracranial small vessel disease33. Both studies suggested that high IR increased the risk of both thrombosis and hemorrhage, but limited evidence due to small sample sizes was noted34,35. In contrast to these findings, a case‒control study examining both ischemic and hemorrhagic stroke patients revealed that among the 1555 included patients, higher total HDL cholesterol levels as well as lower HDL cholesterol levels were associated with an increased risk of ischemic stroke, while a lower total cholesterol level was linked to a greater risk of hemorrhagic stroke36,37. Our analysis of 17,643 patients also found that high IR significantly reduced the risk of all-cause bleeding events-both major and minor. TG, LDL, and HDL-C are associated with coagulation function. High TG is positively correlated with coagulation factor VII and fibrinogen while being negatively correlated with plasminogen activator inhibitor. High LDL is positively correlated with vitamin K-dependent coagulation factors and fibrinogen. High HDL-C inhibits platelet aggregation as well as red blood cell aggregation; it inhibits the coagulation cascade and stimulates fibrinolysis38,39. Therefore, higher TG and LDL levels along with lower HDL-C levels promote thrombosis; conversely lower TG or LDL levels alongwith higher HDL-C levels increase likelihoods for bleeding. The PRACTICE study, which included 15,250 patients from China, also revealed that a low level of HDL cholesterol was associated with a decreased bleeding risk in PCI patients40. Additionally, a brain autopsy study on 750 male hospital deaths over nearly two decades in Japan revealed that low TG/TC levels can degrade vascular smooth muscle cells and collagen fibers, damage vascular endothelium, increase arterial fragility and cause blood leakage and vascular wall rupture41. In our study, patients with a low TG/HDL-C ratio not only presented lower platelet counts and ADP levels but also exhibited elevated INR and D-dimer levels. This suggests that the heightened risk of bleeding events is likely attributed to impaired platelet aggregation, reduced coagulation function and capillary structure damage associated with low IR itself. A Mendelian randomized study combining the UK Biobank and China-PAR project involving nearly 400,000 individuals indicated a linear relationship between LDL-C and CHD risk. However, when LDL-C levels were < 1.8 mmol/L, the risk of cerebral hemorrhage and dementia increased as LDL-C levels decreased42. The RCS curves in this study suggested that when TG/HDL-C ratio was low, there was a more significant change in the risk of MACE and all-cause bleeding events. In clinical work, when maintaining the normal levels of TG and HDL-C in patients, it is necessary to take into account the occurrence risks of both ischemic and bleeding events43,44.
Our results indicate that TG/HDL-C ratio can serve as a robust and reliable risk predictor of MACE, all-cause death, cardiac death and all-cause bleeding events in ACS patients undergoing PCI. These findings have significant implications for the secondary prevention and risk stratification of this patient cohort. Although we are unable to provide a specific range of the optimal TG/HDL-C ratio, in this study, as indicated by the RCS curve, to minimize the occurrence risk of MACE, the ratio of TG/HDL-C ratio is preferably maintained below 1.46. However, at this point, the risk of bleeding increases as the risk of bleeding begins to rise rapidly when TG/HDL-C ratio is less than 1.40. It should be noted that the bleeding events with an increased risk identified in this study are mainly minor bleeding and do not affect the use of antiplatelet drugs. Based on clinical experience, they would still exert a certain degree of influence on the patients’ lives. It would be best if they could be avoided. Therefore, based on the evidence from this study, it can be concluded that to minimize the risk of MACE as much as possible, TG/HDL-C ratio is preferably maintained below 1.46. While maintaining the normal range of TG and HDL-C, it is advisable not to reduce TG/HDL-C ratio too much, otherwise, the risk of bleeding would increase. This can be accomplished through the modulation of lifestyle and the administration of lipid-lowering drugs, and the variations in the risks of ischemia and bleeding reflected by the numerical values provide reference values for the use of antiplatelet drugs. However, our study has several limitations. Firstly, we only observed outcome events during follow-up without conducting any follow-up on hematological indicators, leading to a lack of dynamic data on the TG/HDL-C ratio. Secondly, despite adjusting for numerous potential confounding factors in the Cox proportional hazards regression analyses, there are still many unknown factors influencing the observed correlations such as BMI, lifestyle, physical activity, economic level and genetic testing. Among them, evidently, BMI plays a pivotal role in the influence of IR on MACE. However, in this study, due to the fact that the total number of missing BMI in our database is too large, we were compelled to eliminate it. Undoubtedly, the omission of this data would lead to a possible bias in the results of this study. Thirdly, while vascular endothelial degradation explains the relationship between low IR and bleeding events, it remains unclear how low TG and TC lead to the degradation of smooth muscle cells and collagen fibers. Fourthly, although a systematic review and meta-analysis encompassing studies from Asia, Europe and America demonstrated that an elevated TG/HDL-C ratio might be independently associated with an increased risk of cardiovascular events in the general population45, and its subgroup analyses also indicated that such factors as population gender, geographical region, follow-up duration, adjustment for other lipid parameters, adjustment for diabetes, and classification did not significantly modify this relationship. However, its applicability among Westerners with ACS undergoing PCI remains unknown. In future studies, these limitations should be considered with an aim to address them comprehensively.
Conclusion
In this analysis of 17,643 ACS patients undergoing PCI, the TG/HDL-C ratio, a reliable proxy for IR, was associated with an increased risk of MACE, all-cause death and cardiac death events and a decreased risk of all-cause bleeding events at 12 months.
Data availability
The datasets analysed during the current study are not publicly available because of the need to protect patient privacy but are available from the corresponding author on reasonable request.
References
Hu, D. Y., Han, Y. L., Guang, N. & Ma, C. S. Chinese guideline on the primary prevention of cardiovascular diseases. Cardiol. Discov.. 1(2), 70–104 (2021).
Tao, L. C., Xu, J. N., Wang, T. T., Hua, F. & Li, J. J. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc. Diabetol. 21(1), 68 (2022).
Khambhati, J. et al. The art of cardiovascular risk assessment. Clin. Cardiol. 41(5), 677–684 (2018).
Hageman, S. et al. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 42(25), 2439–2454 (2021).
Damen, J. A. A. G. et al. Prediction models for cardiovascular disease risk in the general population: Systematic review. BMJ 353, i2416 (2016).
Aalto-Setala, K. et al. Cardiovascular disease risk prediction using automated machine learning: A prospective study of 423,604 UK Biobank participants. PLoS ONE. 14(5), e0213653 (2019).
Vázquez., M. C. & Sobrevia, L. Insulin therapy, insulin resistance and vascular dysfunction. Curr. Vascul. Pharmacol. 17(5), 429–431 (2019).
Adeva-Andany, M. M., Ameneiros-Rodriguez, E., Fernandez-Fernandez, C., Dominguez-Montero, A. & Funcasta-Calderon, R. Insulin resistance is associated with subclinical vascular disease in humans. World J. Diabetes. 10(2), 63–77 (2019).
Ladeira, L. L. C. et al. Obesity, insulin resistance, caries, and periodontitis: Syndemic framework. Nutrients 15(16), 3512 (2023).
Dallak, M., Bin-Jaliah, I., Sakr, H. F., Al-Ani, B. & Haidara, M. A. Swim exercise inhibits hemostatic abnormalities in a rat model of obesity and insulin resistance. Arch. Physiol. Biochem. 125(1), 79–84 (2019).
Matsuda, M. & Defronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care. 22(9), 1462–1470 (1999).
Wu, Z. et al. Comparison of three non-insulin-based insulin resistance indexes in predicting the presence and severity of coronary artery disease. Front. Cardiovasc. Med. 9, 918359 (2022).
Nur Zati Iwani, A. K. et al. TG: HDL-C ratio as insulin resistance marker for metabolic syndrome in children with obesity. Front. Endocrinol. 13, 852290 (2022).
Che, B. et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: An analysis of UK biobank data. Cardiovasc. Diabetol. 22(1), 34 (2023).
Huang, Z. et al. Triglyceride-glucose index trajectory and stroke incidence in patients with hypertension: A prospective cohort study. Cardiovasc. Diabetol. 21(1), 141 (2022).
Kamran, H. et al. Oral antiplatelet therapy after acute coronary syndrome. Rev. JAMA. 325(15), 1545–1555 (2021).
Cayla, G., Silvain, J., O’Connor, S. A., Collet, J. P. & Montalescot, G. Current antiplatelet options for NSTE-ACS patients. QJM 105(10), 935–948 (2012).
Han, Y. et al. Predicting long-term ischemic events using routine clinical parameters in patients with coronary artery disease: The OPT-CAD risk score. Cardiovasc. Ther. 36(5), e12441 (2018).
Tanno, M. & Osanami, A. Insulin resistance-beginning of the road to coronary microvascular dysfunction and beyond. Circ. J. 86(5), 874–876 (2022).
Madonna, R. & De Caterina, R. Cellular and molecular mechanisms of vascular injury in diabetes–part I: Pathways of vascular disease in diabetes. Vascul. Pharmacol. 54(3–6), 68–74 (2011).
Madonna, R. & De Caterina, R. Cellular and molecular mechanisms of vascular injury in diabetes–part II: Cellular mechanisms and therapeutic targets. Vascul. Pharmacol. 54(3–6), 75–79 (2011).
Georgakis, M. K. et al. Diabetes Mellitus, glycemic traits, and Cerebrovascular Disease. Neurology 96(13), e1732–e1742 (2021).
Wu, G. & Meininger, C. J. Nitric oxide and vascular insulin resistance. Biofactors 35(1), 21–27 (2009).
Muniyappa, R. & Sowers, J. R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 14(1), 5–12 (2013).
Shoelson, S. E. Inflammation and insulin resistance. J. Clin. Invest. 116(7), 1793–1801 (2006).
Yaribeygi, H., Farrokhi, F. R., Butler, A. E. & Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 234(6), 8152–8161 (2018).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17(1), 122 (2018).
Zhang, Z., Zhao, L., Lu, Y., Meng, X. & Zhou, X. Association between non-insulin-based insulin resistance indices and cardiovascular events in patients undergoing percutaneous coronary intervention: A retrospective study. Cardiovasc. Diabetol. 22(1), 161 (2023).
Zhang, Y., Wang, R., Fu, X. & Song, H. Non-insulin-based insulin resistance indexes in predicting severity for coronary artery disease. Diabetol. Metab. Syndr. 14(1), 191 (2022).
Tian, X. et al. Magnitude and time course of insulin resistance accumulation with the risk of cardiovascular disease: An 11-years cohort study. Cardiovasc. Diabetol. 22(1), 339 (2023).
Wu, Z. et al. The impact of the metabolic score for insulin resistance on cardiovascular disease: A 10-year follow-up cohort study. J. Endocrinol. Invest. 46(3), 523–533 (2023).
Zhao, X. et al. Triglyceride glucose index combined with plaque characteristics as a novel biomarker for cardiovascular outcomes after percutaneous coronary intervention in ST-elevated myocardial infarction patients: An intravascular optical coherence tomography study. Cardiovasc. Diabetol. 20(1), 131 (2021).
Chen, S. et al. A novel metabolic score for insulin resistance and symptomatic intracranial hemorrhage in ischemic stroke patients after endovascular thrombectomy. Neuropsychiatr. Dis. Treat. 19, 321–328 (2023).
Ye, X. H. et al. Association between Insulin Resistance and remote diffusion-weighted imaging lesions in primary intracerebral hemorrhage. Front. Immunol. 12, 719462 (2021).
Li, D., Li, Y., Wang, T. & Zhu, X. Correlation between insulin resistance and cerebral microbleeds among Chinese patients with cerebral small vessel disease. J. Clin. Neurosci. 111, 1–5 (2023).
Tirschwell, D. L., Smith. N. L., Heckbert, S. R., Lemaitre, R. N., Longstreth, W. T., & Psaty, B. M. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology 63(10), 1868–1875 (2004).
Li, J. et al. Impact of lipid profiles on parenchymal hemorrhage and early outcome after mechanical thrombectomy. Ann. Clin. Transl Neurol. 10(10), 1714–1724 (2023).
Rosenson, R. S. & Lowe, G. D. Effects of lipids and lipoproteins on thrombosis and rheology. Atherosclerosis 140(2), 271–280 (1998).
Qie, R. et al. Dose-response association between high-density lipoprotein cholesterol and stroke: A systematic review and meta-analysis of prospective cohort studies. Prev. Chronic Dis. 18, E45 (2021).
Zheng, Y. Y. et al. Low HDL cholesterol is associated with reduced bleeding risk in patients who underwent PCI: Findings from the PRACTICE study. Thromb. Haemost. https://doi.org/10.1055/a-2104-1693 (2023).
Iso., K. M. & Komachi., H. Associations of serum total cholesterol, different types of stroke, and stenosis distribution of cerebral arteries. The Akita pathology study. Stroke 24(7), 954–964 (1993).
Liu, H. et al. Efficacy and safety of low levels of low-density lipoprotein cholesterol: Trans-ancestry linear and non-linear mendelian randomization analyses. Eur. J. Prev. Cardiol. 30(12), 1207–1215 (2023).
Duprez, D. & Jacobs, D. R. LDL-cholesterol lowering: To be or not to be too low. Eur. J. Prev. Cardiol. 30(12), 1205–1206 (2023).
Drexel, H. et al. Triglycerides revisited: Is hypertriglyceridaemia a necessary therapeutic target in cardiovascular disease? Eur. Heart J. Cardiovasc. Pharmacother. 9(6), 570–582 (2023).
Chen, Y. et al. Triglyceride to high-density lipoprotein cholesterol ratio and cardiovascular events in the general population: A systematic review and meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 32(2), 318–329 (2022).
Acknowledgements
We gratefully thank staff at Northern Theater General Hospital for collecting and collating these clinical data and creating the database.
Funding
The study was supported by the National Key Research and Development Program of China (2022YFC2503500 and 2022YFC2503504).
Author information
Authors and Affiliations
Contributions
Shangxun Zhou designed this study. Miaohan Qiu and Kexin Wang performed the statistical analysis. Shangxun Zhou wrote the first draft of the manuscript. Jing Li and Yi Li wrote some sections of the manuscript. Yaling Han revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study with a waiver of informed consent were approved by the ethics committee of the General Hospital of Northern Theater Command and conducted in line with the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, S., Qiu, M., Wang, K. et al. Triglyceride to high density lipoprotein cholesterol ratio and major adverse cardiovascular events in ACS patients undergoing PCI. Sci Rep 14, 31752 (2024). https://doi.org/10.1038/s41598-024-82064-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82064-9
Keywords
This article is cited by
-
Genomic and clinical predictors of cardiovascular disease in Familial dyslipidemia: risk stratification in Egyptian adolescents and young adults
Lipids in Health and Disease (2025)
-
Association between atherogenic index of plasma and various metabolic conditions: an umbrella review on meta-analyses
BMC Cardiovascular Disorders (2025)