Abstract
The evidence for the association between the triglyceride (TG) to high-density lipoprotein cholesterol (HDL-c) ratio and the risk of developing microalbuminuria is still limited in the Chinese population. Therefore, our research will endeavor to explore the relationship between the two. The cross-sectional survey enrolled 32,877 general population from eight regional centers in China. If the urinary albumin-creatinine ratio (UACR) ≥ 30 mg/g, it was considered microalbuminuria. The dependent variable in the study was microalbuminuria, while the independent variable was the TG/HDL-c ratio. A binary logistic regression model was utilized to examine the independent association between the likelihood of microalbuminuria and the TG/HDL-c ratio. A generalized additive model (GAM) and spline smoothing were employed to investigate non-linear relationships between these two variables. To confirm the findings and pinpoint important turning points, subgroup and threshold effect tests were performed. The average age of the study participants was 57.67 ± 9.29 years, with 22,052 (67%) females and 10,825 (33%) males. The ratio of TG/HDL-c had a median value of 1.06, while the UACR had a median value of 9.92 mg/g. The prevalence of microalbuminuria was 14.4%. Following covariate adjustment, the prevalence of microalbuminuria and the TG/HDL-c ratio showed a significant positive connection (OR = 1.17,95% CI 1.13–1.21). Interestingly, we discovered a non-linear relationship between the occurrence of microalbuminuria and the TG/HDL-c ratio. At a TG/HDL-c ratio of 0.911, we detected an inflection point, with ORs of 1.12 (95% CI: 1.08, 1.17) on the right side of the point and 1.89 (95% CI: 1.51, 2.36) on the left. Subgroup analyses further validated the results. Our research revealed a positive and non-linear connection between the TG/HDL-c ratio and the prevalence of microalbumin in Chinese individuals. The connection was especially robust when the TG/HDL-c ratio was below 0.911. Reducing TG or raising HDL-c levels in order to lower the TG/HDL-c ratio may significantly mitigate the risk of microalbuminuria.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) presents a substantial and increasing worldwide burden, given its widespread occurrence and high rates of mortality1. According to estimates, CKD will rank as the world’s fifth most common cause of death by 20402. Increased excretion of albuminuria is considered a sign of kidney damage. The UACR is regarded as a reliable marker of albuminuria excretion3, which can reliably reflect the 24-hour urinary protein content. Moreover, it is rapid, simple, and accurate4. Previous research has demonstrated that UACR not only enables the early detection of kidney injury5, but also predicts the probability of cardiovascular events in individuals with CKD, diabetes, and the general population6,7,8,9. In addition, UACR can be used to forecast mortality risk in elderly individuals suffering from CKD10. Given that the leading cause of death for persons with CKD is cardiovascular disease (CVD)11, timely identification of individuals at high risk of microalbuminuria assumes paramount importance in both CKD and CVD prevention and management.
The TG/HDL-c ratio has attracted the attention of researchers as an innovative index for lipid studies. The research findings suggest an autonomous association between the TG/HDL-c ratio and a higher occurrence of CKD12. Numerous experiments and research in the existing literature have identified the TG/HDL-c ratio as a new indicator for forecasting the likelihood of cerebrovascular disease (CCVD), hypertension, CVD, diabetes, and hyperuricemia13. In addition, recent research has shown that the ratio is a trustworthy substitute for insulin resistance (IR)14. Atherosclerosis, hypertension, diabetes, lipid abnormalities, and other illnesses are all mediated by IR and are recognized as indicators of CVD and CKD11,15,16,17.
Researchers conducted a cross-sectional study in a rural Korean population and found an independent relationship between the incidence of proteinuria and the TG/HDL-c ratio in normotensive individuals18. In the general Japanese population, elevated TG/HDL-c levels were associated with a significant increase in the prevalence of CKD, low estimated glomerular filtration rate (eGFR), and proteinuria19. Furthermore, a longitudinal study involving the Japanese has revealed that individuals in higher baseline TG/HDL-c quartiles are substantially linked with decreased eGFR and increased proteinuria excretion20. Elevated TG/HDL-c levels were substantially linked to aberrant UACR when compared to other lipid markers, according to a second cross-sectional investigation carried out among the general population in China21. The TG/HDL-c ratio has been determined to be a potential predictor of increased excretion of low eGFR and UACR in non-diabetic patients22.
This series of studies provides new perspectives for the prevention of proteinuria. Nevertheless, there are few studies on the correlation between the TG/HDL-c ratio and the likelihood of microalbuminuria in the Chinese population. As far as we are aware, the previous literature has not examined the non-linear correlation between these two variables. Taking into account the aforementioned factors, we designed a cross-sectional study targeting a large sample of the general Chinese population. The main objectives of this investigation were as follows: (1) to examine the linear correlation between TG/HDL-c ratio and microalbuminuria; (2) to investigate any potential non-linear correlation; and (3) to perform subgroup analyses to assess differences in different populations.
Methods
Study design and participants
This study utilized a dataset established by Ye et al. (2019) from the PLOS ONE database (https://journals.plos.org/plosone/)23, which was freely available for non-profit use under the terms of service of the database. The Multicenter Longitudinal Study of Diabetes Cancer Risk Assessment in China provided the data used in this investigation. It comprised 33,850 individuals from 8 different regional centers across China (Wuhan, Guangxi, Guangzhou, Shanghai, Lanzhou, Luzhou, Zhengzhou, and Dalian). The Human Research Committee at Shanghai Jiaotong University School of Medicine’s Ruijin Hospital approved the initial study. Before data collection, each subject provided written informed consent. Informed permission or institutional ethics committee approval was unnecessary for this retrospective study24.
The initial study employed the following criteria for exclusion: (1) Individuals with primary kidney disease; (2) Participants on daily ACEI or ARB medications; (3) Participants who provided inaccurate reports of their sleep duration (< 4 h or > 12 h). Ultimately, the initial research involved 33,850 individuals in the analysis.
In this current study, following the original research, we initially removed individuals with exceptionally high or low TG/HDL-c levels that exceeded or fell below three standard deviations from the average (n = 549)25. Individuals with incomplete covariate information, specifically regarding smoking and alcohol intake, were excluded from the analysis (n = 424). 32,877 people in total participated in this study at the end. Figure 1 shows illustrate the process of selecting participants.
Schematic diagram of study population selection. Figure 1 shows the participant selection process. The original study included 33,850 individuals in the analysis. After excluding 973 individuals, the final total number of participants in this study was 32,877.
Trained medical professionals employed standardized surveys to collect essential demographic information, including gender, age, medical history, and patterns of tobacco and alcohol use. Measurements of the participants’ weight, hip circumference (HC), height, and waist circumference (WC) were taken without their outer clothing, shoes, or headgear. The calculation of WC involved taking a measurement of the circumference of the abdomen at the midpoint between the anterior superior iliac spine and the lower edge of the 12th rib. The horizontal length of the buttocks’ most noticeable portion was designated as HC. To one decimal place, all measurements were recorded. Urine samples obtained in the morning were used to calculate the UACR. The subjects were given 5 min of sedentary rest before having their blood pressure recorded 3 times with an electronic sphygmomanometer. The average reading was computed from the three readings. Following an overnight fast of eight hours, blood samples were taken in the morning. Individuals without a prior diagnosis of diabetes were administered a 75-gram oral glucose tolerance test, whereas those with diabetes underwent a 100-gram test. Venous blood samples were collected at the 0 and 120-minute marks for all participants. TG, Serum creatinine (Scr), alanine aminotransferase (ALT), Postprandial blood glucose (PBG), total cholesterol (TC), Aspartate aminotransferase (AST), low-density lipoprotein cholesterol (LDL-c), Serum urea nitrogen (BUN), HDL, γ-Glutamyl transpeptidase (GGT), Fasting blood glucose (FBG), and Hemoglobin A1c (HbA1c) were the biochemical markers.
Definitions and calculations
The eGFR (ml/min*1.73 m2) was calculated using the Modification of Diet in Renal Disease (MDRD) formula: eGFR (ml/min*1.73 m2) = 186*Scr (mg/dl)−1.154*Age (years)−0.203(female*0.742). The definition of CKD was eGFR less than 60 ml/min. FBG ≥ 7.0 mmol/L or PBG ≥ 11.1 mmol/L or a definitive medical history of diabetes was used to identify diabetes mellitus (DM). An average systolic blood pressure (SBP) ≥ 130 mmHg or Diastolic blood pressure (DBP) ≥ 80 mmHg or a verified medical diagnosis of hypertension was used to define hypertension. Body mass index (BMI) = weight divided by height2.
Assessment of outcome
The outcome variable was the occurrence of microalbuminuria, (dichotomous variable: 1 = microalbuminuria, 0 = non-microalbuminuria). A UACR of ≥ 30 mg/g was used to define microalbuminuria. A value of “0” indicated that UACR was < 30 mg/g, while “1” indicated UACR ≥ 30 mg/g.
Assessment of covariates
The selection of variables in our research was guided by primary investigation, clinical expertise, and previous studies on the risk of lipid and proteinuria. We took into account both categorical and continuous factors as covariates. The categorical variables included basic information (gender), disease history (diabetes and tumor), smoking behaviors, and drinking behaviors. The continuous variables included DBP, age, BMI, SBP, AST, eGFR, and ALT.
Statistical analysis
The TG/HDL-c ratio’s quartiles were used to stratify the participants. The mean ± standard deviation (SD) was the representation used for continuous parameters with a Gaussian distribution. The interquartile range was used to indicate the median for variables having skewed distributions. To test for differences between the different prevalence groups, the study used a range of statistical methods. These included the χ2 test for categorical variables, the one-way ANOVA test for normally distributed data, and the Kruskal-Wallis test for non-normally distributed variables.
To assess the likelihood of covariate covariance, we computed the variance inflation factor (VIF)26. VIF is an indicator used to detect the degree of covariance between the independent variables in a multiple regression model and is calculated as VIF = 1 / (1 - R2), where R2 is the value of the r-squared obtained from the linear regression equation. When performing a regression analysis, we usually consider the variable of interest as the dependent variable, while the remaining variables are considered as independent variables. Considering that the VIF indicates the presence of covariance between the independent variables, if the VIF corresponding to an independent variable is greater than 5, it is recommended not to include that independent variable in the multiple regression model (Table S1).
After screening for covariance by calculating VIF values, we used three distinct models for univariate and multivariate logistic regression analysis. Including non-adjusted model (non-adjusted covariates), partially adjusted model (SBP, DBP, BMI, drinking and smoking status, age, gender, diabetes history, tumor history), fully adjusted model (SBP, DBP, BMI, drinking and smoking status, age, gender, diabetes history, tumor history, ALT, AST, eGFR). We noted the OR and the associated 95% confidence intervals (CI). Confounding variables were adjusted for using clinical expertise, relevant literature, and covariance screening, and there was no covariance between covariates.
Due to the limitations of binary logistic regression models in handling non-linear associations, we employed GAM and spline smoothing. Upon detecting nonlinearity, we developed a two-piece binary logistic regression model on each side of the inflection point using a recursive technique to identify it. To find the best model to explain the relationship between the TG/HDL-c ratio and microalbuminuria, a log-likelihood ratio test was utilized.
To verify the stability of the results, we performed subgroup analyses for different subgroups (drinking status, BMI, age, SBP, DBP, gender, tumor history, smoking status, and diabetic history) using hierarchical binary logistic regression models. Continuous variables like BMI, SBP, DBP, and age were categorized as follows: BMI (< 18.5, ≥ 18.5 to < 24, ≥24 to < 28, ≥28 kg/m2); SBP (< 130 mmHg or ≥ 130 mmHg); DBP (< 80 mmHg or ≥ 80 mmHg); age (< 60 years or ≥ 60 years)27,28,29. In addition to the stratification factors themselves, each stratification is adjusted according to gender, AST, BMI, drinking status, DBP, diabetes history, SBP, tumor history, age, ALT, eGFR, and smoking status. Finally, we used likelihood ratio tests to examine interactions by comparing models with and without interaction terms.
Every result was written using the STROBE statement as a guide. Empower Stats (X&Y Solutions, Inc., Boston, MA, http://www.empowerstats.com; version: 4.1) and the R statistical software packages (http://www.r-project.org, The R Foundation) were utilized for all statistical analyses. P values (two-sided) less than 0.05 were deemed statistically significant.
Results
Characteristics of participants
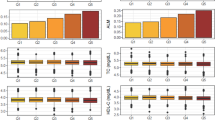
Table 1 displays the clinical and demographic features of the subjects. The mean age of participants was 57.67 ± 9.29 years, including 10,825 males (33%) and 22,052 females (67%). The distribution of levels for the TG/HDL-c ratio is shown in Fig. 2. TG/HDL-c showed a skewed fractional distribution with a median of 1.06 and an interquartile spacing of (0.14–5.73). The UACR had a median value of 9.92 mg/g. The prevalence of microalbuminuria was 14.4% (4734/32877). The mean eGFR was 94.00 ± 18.98 ml/min*1.73 m2. Participants were grouped according to TG/HDL-c quartiles (< 0.69, ≥ 0.69 to < 1.06, ≥ 1.06 to < 1.65, ≥ 1.65). Compared to participants in the Q1 group (TG/HDL-c < 0.69), a higher proportion of microalbuminuria, males, alcohol drinkers, smokers, obesity, and diabetes mellitus were observed among participants in the Q4 group (1.65 ≤ TG/HDL-c < 5.73). In addition, significant increases in AGE, DBP, SBP, HC, WC, Scr, ALT, AST, GGT, HbA1C, FBG, PBG, BMI, and UACR were also observed. In contrast, females, non-smokers, non-drinkers, HDL, and eGFR showed opposite trends.
Values are n (%), mean ± SD or medians (quartiles).
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; FBG, fasting blood glucose; PBG, postprandial blood glucose; eGFR, estimated glomerular filtration rate; Scr, serum creatinine; BUN, blood urea nitrogen; LDL-c, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GGT, gamma-glutamyltransferase;
Individuals were split into 2 groups based on the absence or presence of microalbuminuria. Figure 3 displays the findings of the TG/HDL-c ratio in both groups. The findings indicate that the microalbuminuria group had a higher TG/HDL-c ratio distribution level. In comparison, the TG/HDL-c ratio was significantly lower in the non-microalbuminuria group. Participants with higher TG/HDL-c ratios also had higher incidences of microalbuminuria (P < 0.001), as Fig. 4 demonstrates. As shown in Fig. 5, across all age groups in the 10-interval age stratification, the incidence of microalbuminuria was greater in female participants compared to male subjects, except those under 40 years of age. Additionally, it was shown that in both males (with the exception of those under 40) and females, the incidence of microalbuminuria increased with age.
The TG/HDL-c ratio levels of all participants from the Microalbuminuria and non-Microalbuminuria groups. Figure 3 indicated that the distribution level of the TG/HDL-c ratio in the Microalbuminuria group was higher. In contrast, the TG/HDL-c ratio level in the non-Microalbuminuria group was relatively lower.
The rate of microalbuminuria by quartiles of TG/HDL-c ratio. Figure 4 shows that participants with higher TG/HDL-c ratios had higher rates of microalbuminuria (P < 0.001).
Prevalence of microalbuminuria stratified by age in 10 intervals. Figure 5 shows that in age stratification by 10 intervals, except for age < 40 years, the female subjects had a higher prevalence of microalbuminuria than the male subjects no matter what age group they were in. Furthermore, It could also be found that the prevalence of microalbuminuria increased with age, both in male (except for age < 40) and female participants.
The prevalence rate of microalbuminuria
A total of 4734 individuals had microalbuminuria, according to Table 2. All individuals had a prevalence percentage of 14.40% (14.00–14.79%) overall. The four TG/HDL-c ratio groups had relative prevalence rates of 10.12% (9.47–10.83%), 13.53% (12.80–14.24%), 15.71% (14.89–16.50%), and 18.23% (17.43–19.12%). Those with higher TG/HDL-c ratios were more likely to have microalbuminuria compared with those with lower ratios (p < 0.001 for trend).
TG/HDL-c ratio, triglycerides/high-density lipoprotein cholesterol ratio, CI confidence.
Univariate logistic regression
Univariate analysis indicated that HbA1C, the TG/HDL-c ratio, FBG, PBG, BMI, TG, AST, ALT, GGT, SBP, DBP, WC, HC, and Age had a positive association with the risk of microalbuminuria. HDL, LDL, and TC were adversely correlated with the risk of microalbuminuria, while tumor history did not exhibit a significant association. Women, individuals who are obese, hypertensive, or diabetic, exhibit a higher prevalence of microalbuminuria compared to men, non-obese, non-hypertensive, and non-diabetic individuals. A higher proportion of non-smokers and non-drinkers developed microalbuminuria compared to smokers and drinkers. There was a 25.4% increase in the incidence of microalbuminuria for every unit increase in the TG/HDL-c ratio (p < 0.001, Table 3).
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; FBG, fasting blood glucose; PBG, postprandial blood glucose; eGFR, estimated glomerular filtration rate; Scr, serum creatinine; BUN, blood urea nitrogen; LDL-c, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GGT, gamma-glutamyltransferase;
Multivariate logistic regression analysis
Table 4 shows the results of the multifactorial analysis. In Model I (unadjusted), the incidence of microalbuminuria arose by 25% (OR = 1.25, 95% CI: 1.22–1.29) for each unit increase in the TG/HDL-c ratio. After controlling for relevant confounders, in partially adjusted model II, the prevalence of microalbuminuria rose by 18% (OR = 1.18,95% CI 1.14–1.22) for each unit increase in the TG/HDL-c ratio. For every unit rise in the TG/HDL-c ratio, the incidence of microalbuminuria elevated by 17% (OR = 1.17, 95% CI 1.13–1.21) in the fully adjusted model III. The distribution of CIs indicated that the results obtained by the models are reliable.
Furthermore, we categorized the individuals depending on their TG/HDL-c ratio and incorporated these groups into the multivariate adjustment model. Those with TG/HDL-c ratios in the first quartile comprised the reference group in the fully adjusted model. In comparison to the reference group, there was a 25% increase in the incidence of microalbuminuria for individuals in the second quartile, as indicated by the OR of 1.25 (95% CI 1.13–1.38). The third quartile group’s OR was 1.39 (95% CI 1.26–1.53), which indicates a 39% increase in the probability of microalbuminuria. For the reference group, there was a 57% increase in risk, as indicated by the OR for the fourth quartile, which was 1.57 (95% CI 1.43–1.74). (Table 4, Model III).
The authors computed an E value of 1.61, which was higher than the relative risk linked to unmeasured confounders and the TG/HDL-c ratio. This implies that the results are unaffected by unmeasured or unidentified factors.
Non-linear relationship between TG/HDL-c and microalbuminuria
We discovered a non-linear association between the likelihood of microalbuminuria and the TG/HDL-c ratio employing GAM and smoothed curve fitting (Fig. 6). We fitted a segmented binary logistic regression model to the data to explain these two distinct slopes. The log-likelihood ratio test was utilized to select the best-fitting model (Table 5) with P < 0.001.
We determined that the TG/HDL-c ratio’s inflection point was 0.911 by using a recursive technique. Next, we determined the effect magnitude and CI surrounding the inflection point using the binary logistic regression model. At a TG/HDL-c ratio of 0.911, we detected an inflection point, with ORs of 1.12 (95% CI: 1.08, 1.17) on the right side of the point and 1.89 (95% CI: 1.51, 2.36) on the left.
The results of subgroup analyses
We evaluated the association between the likelihood of microalbuminuria and the TG/HDL-c ratio, as well as the interactions between variables, in both specified and exploratory subgroups (Table 6). According to our research, there were no significant interactions between gender, BMI, SBP, DBP, diabetes history, tumor history, smoking history, age, or alcohol consumption (All P-values for interaction > 0.05).
Discussion
In order to examine the relationship between the TG/HDL-c ratio and the risk of microalbuminuria in the general Chinese population, we performed a cross-sectional analysis in this study. We discovered a significant rise in the risk of microalbuminuria linked with a rise in the TG/HDL-c ratio. This association persisted regardless of controlling for confounders such as age, DBP, diabetes mellitus, tumor history, BMI, alcohol consumption, smoking, sex, SBP, eGFR, ALT, and AST. This indicates that there is an independent contribution of the TG/HDL-c ratio to the risk of microalbuminuria.
In addition, a threshold effect curve was discovered, which shows that there are variable relationships between TG/HDL-c and microalbuminuria on either side of an inflection point. Subsequent subgroup analyses revealed that gender, SBP, DBP, BMI, diabetes history, tumor history, smoking history, age, and alcohol consumption did not substantially affect the outcome. Specifically, in exploratory analyses, the TG/HDL-c ratio was consistent with the risk of microalbuminuria in all 9 pre-specified subgroups.
Several studies have demonstrated that proteinuria and the TG/HDL-c ratio are positively correlated. A cross-sectional study carried out in Japan observed that proteinuria significantly increased with higher TG/HDL-c quartiles after adjusting for various confounding factors (hypertension, obesity, age, WC, daily alcohol consumption, diabetes, smoking status, history of stroke and heart disease, dyslipidemia medication, regular exercise habits, and eGFR) (P < 0.001). The ORs for quartiles 2, 3, and 4 in men were 0.96 (95% CI 0.89–1.03), 1.03 (95% CI 0.95–1.11) and 1.13 (95% CI 1.04–1.21), respectively. The ORs for quartiles 2, 3, and 4 in women were 0.94 (95% CI 0.86–1.03), 0.96 (95% CI 0.88–1.05) and 1.23 (95% CI 1.13–1.34)19. Unlike our study, the Japanese study’s definition of proteinuria was a urine protein level of 1+ as measured by a test paper test. In a subsequent longitudinal study by Kazuhiko Tsuruya involving the Japanese population, adjusted multivariate analyses revealed a significant increase in proteinuria with higher TG/HDL-c quartiles (Q4: OR = 1.18, 95% CI 1.12–1.25)20. Similarly, a cross-sectional study in Korea found that adjusting for BMI, FBG, age, smoking status, energy intake per day, exercise status, daily alcohol consumption, and logarithm-transformed hsCRP, higher TG/HDL-c ratios were associated with higher UACR in both males (Q4: OR = 1.38, 95% CI 1.03–1.85) and females (Q4: OR = 1.45, 95% CI 1.10–1.90). However, this correlation lost statistical significance in men (p = 0.240) after further adjustment for SBP, though it remained significant in women (Q4: OR = 1.36, 95% CI: 1.03–1.79). Among participants with hypertension, there existed no meaningful change in OR regardless of the adjustments made18. In contrast, our study found a positive correlation between TG/HDL-c and microalbuminuria in both normotensive and hypertensive populations. In the same study in the Chinese general population21, factors including age, ALT, smoking status, SBP, γ-GGT, drinking status, CVD, BMI, eGFR, HbA1c, center, DBP, pulse and hypoglycemic drugs, and HOMA-IR were adjusted. For women, the link between TG/HDL-c and UACR remained significant (Q2: OR = 1.20, 95% CI: 1.07–1.35; Q3: OR = 1.13, 95% CI: 1.00-1.27; Q4: OR = 1.28, 95% CI: 1.13–1.44), while in men, the correlation was weakened but still significant in Q4 (Q4: OR = 1.24, 95% CI: 1.02–1.50). Stratified analyses from this study showed that in women, the correlation was present in the prehypertensive and hypertensive strata, the overweight stratum, the normal BMI stratum, the prediabetic stratum, and the age > 55 and age ≤ 55 strata. In men, this association was present only in the overweight. The TG/HDL-c ratio was independently linked to the likelihood of microalbuminuria, according to our multifactorial regression analysis.
From the above literature, we know that the level of TG/HDL-c ratio is positively linked to the likelihood of proteinuria. Elevated levels of TG/HDL-c ratio usually occur in three situations: an increase in the level of TG, which we describe as hypertriglyceridemia (HTG); a decrease in HDL-c levels; and an elevation of the ratio even though both TG and HDL-c are within normal limits. HTG prevalence is particularly high in Chinese populations, reported in 2019 up to 15%30. A number of literature reports that HTG is a risk factor for obesity, DM, CKD, atherosclerotic disease, acute pancreatitis, and hyperuricemia31,32,33,34. A substantial correlation exists between low HDL-c levels and cardiovascular events as well as metabolic syndrome35,36. Our study explored that elevated TG/HDL-c ratio was positively and independently correlated with increased UACR. Measuring TG/HDL-c levels may assist in determining the likelihood of microalbuminuria and early renal insufficiency. The beginning and development of CKD are insidious. Tight management of TG and HDL-C levels facilitates timely intervention in the emergence of proteinuria and the progression of CKD. Maintaining lower TG/HDL-c levels may reduce the likelihood of obesity, metabolic syndrome, diabetes, CKD, and cardiovascular events.
Additionally, a non-linear correlation between the TG/HDL-c ratio and microalbuminuria was discovered initially in the present research. The inflection point of the curve, determined through likelihood ratio analysis, was found to be 0.911. As illustrated in Fig. 6, there was an 89% increase in the risk of microalbuminuria for each 1-unit elevation in TG/HDL-c to the left of the curve’s inflection point. In contrast, to the right of the inflection point, a 1-unit increase in TG/HDL-c was associated with a 12% rise in the likelihood of microalbuminuria. It can be found that compared with TG/HDL-c less than 0.911, individuals with TG/HDL-c greater than 0.911 have higher age, BMI, FBG, PBG, SBP, DBP, HbA1c, WC, level or proportion of smoking and drinking (Table S2). Microalbuminuria and these markers are tightly associated37,38,39,40,41,42. When TG/HDL-c > 0.911, the effect of TG/HDL-c on the risk of microalbuminuria was relatively weak because of the existence of the risk elements mentioned above. In contrast, when TG/HDL-c < 0.911, the incidence of risk elements for microalbuminuria decreased, and the impacts on microalbuminuria were lessened. Thus, the ratio of TG/HDL-c had a relatively higher influence.
Non-linear relationship between the TG/HDL-c ratio and microalbuminuria risks. The red line represents the estimated relationship, while the blue lines indicate the 95% confidence intervals. The inflection point, identified at a TG/HDL-c ratio of 0.911, marks a change in the slope of the association, suggesting different risk dynamics on either side of this value.
These results offer a crucial foundation for TG/HDL-c ratio level interventions in the clinical context that lower the likelihood of microalbuminuria. We noticed that TG/HDL-c levels were consistently positively linked to microalbuminuria, but the degree of positive correlation varied. From a preventive point of view, it is recommended to monitor TG/HDL-c levels and intervene promptly to reduce the likelihood of microalbuminuria. From a therapeutic point of view, in patients who already have microalbumin, lowering TG/HDL-c levels while treating proteinuria may be helpful in reducing proteinuria levels and improving CKD prognosis. As a result, there are significant clinical implications for our work. The findings of this study might assist future predictive modeling efforts to evaluate the likelihood of microalbuminuria.
The connection between TG/HDL-c and UACR has several explanations. Firstly, this ratio serves as a reliable indicator of IR14, which is closely linked to the development of CKD and CVD43,44. IR is associated with elevated blood pressure and BMI45. Given a rise in glomerular pressure caused by high blood pressure, proteinuria increases46. Similarly, overweight status may lead to persistent renal hemodynamic abnormalities46. Hyperinsulinemia caused by IR may lead to endothelial dysfunction by affecting intracellular calcium and magnesium metabolism and increasing the expression of endothelin-147.In addition, it may increase the anti-transport activity of sodium lithium and stimulate renal sodium reabsorption, increasing glomerular hydrostatic pressure48. These variables worsen glomerular hyperfiltration and endothelial dysfunction, raise renal vascular permeability, and raise the excretion of proteinuria49. Podocytes are an important part of the glomerular filtration barrier. Lipid metabolism abnormalities affect podocyte biology by inducing posterior cytoskeletal arrangement, mitochondrial dysfunction, and decreased ATP, leading to decreased glomerular osmoselectivity and increased excretion of proteinuria50. Furthermore, prior research indicates that in the context of CKD, glomerular lipid and protein excretion increases, which further worsens dyslipidemia51. Therefore, in CKD patients, a vicious cycle is formed between chronic renal insufficiency and dyslipidemia, which also accelerates the occurrence of CVD to some extent52. An increased TG/HDL-c ratio is closely linked to raised LDL-c levels19,20,53. Low-density LDL-c can be transformed into oxidized LDL-c, which damages podocyte function, podocyte structure, and renal tubule interstitial tissue through different pathogenesis54.
Our study has several advantages worthy of recognition. Firstly, it is a multi-center analysis with a substantial sample size, ensuring the representativeness of the results. Secondly, we have pioneered the examination of the non-linear association between TG/HDL-c and microalbuminuria, pinpointing its inflection point, which marks significant progress. Thirdly, by using subgroup analysis and target independent variable transformation, we ensure the validity of the results in this study. However, our research also has some limitations. Firstly, being a cross-sectional study, it can only show the correlation between the two but cannot explain the causal relationship. Future large-scale cohort investigations will be essential to elucidate the causal connection between TG/HDL-c and microalbuminuria. Secondly, UACR measurements were based on a single assessment, and methods varied across the eight centers. Standardizing measurement protocols and employing multiple measurements would yield more accurate values. Thirdly, this study was focused on the association between the TG/HDL-c ratio and the risk of microalbuminuria in the Chinese population, and the generalisability to non-Chinese populations is not known. In the future, the authors will endeavor to collaborate with other researchers to investigate the relationship between TG/HDL-c ratio and microalbuminuria in different populations. Finally, uncontrolled or unmeasured variables including lipid-lowering drugs, physical activity/exercise, nutrition, and insulin concentrations may exist, as with all observational research. Nevertheless, in order to estimate the possible impact of unrecorded covariates, the authors calculated the E-value and discovered that these factors were unable to account for the results. In future studies, the authors will strive to obtain more comprehensive data to fully analyze the connection between TG/HDL-c and microalbuminuria.
Conclusions
This research demonstrated a positive and non-linear association between the TG/HDL-c ratio and the risk of microalbuminuria in the general Chinese population. Specifically, when TG/HDL-c < 0.911, the risk of microalbuminuria significantly increases as the TG/HDL-c values increase. These findings have implications for future clinical practice. Even among populations without risk factors for microalbuminuria, maintaining lower TG/HDL-c values is crucial for preventing proteinuria, CKD, and CVD.
Data availability
Data can be downloaded from the PLOS ONE database (https://journals.plos.org/plosone/).
Abbreviations
- TG/HDL-c ratio:
-
Triglycerides to high-density lipoprotein cholesterol ratio
- BMI:
-
Body mass index
- Scr:
-
Serum creatinine
- TC:
-
Total cholesterol
- BUN:
-
Serum urea nitrogen
- TG:
-
Triglyceride
- ALT:
-
Alanine aminotransferase
- HDL-c:
-
High-density lipoprotein cholesterol
- AST:
-
Aspartate aminotransferase
- LDL-c:
-
Low-density lipoprotein cholesterol
- GGT:
-
γ-Glutamyl transpeptidase
- WC:
-
Waist circumference
- OR:
-
Odds ratio
- Ref:
-
Reference
- CI:
-
Confidence intervals
- DM:
-
Diabetes mellitus
- CVD:
-
Cardiovascular disease
- GAM:
-
Generalized additive model
- CKD:
-
Chronic kidney disease
- SD:
-
Standard deviation
- MDRD:
-
Modification of Diet in Renal Disease
- TG:
-
Triglycerides
- UACR:
-
Urinary albumin-creatinine ratio
- eGFR:
-
Estimated glomerular filtration rate
- PBG:
-
Postprandial blood glucose
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Hemoglobin A1c
- IR:
-
Insulin resistance
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- CCVD:
-
Cerebrovascular disease
- HC:
-
Hip circumference
- HTG:
-
Hypertriglyceridemia
References
Kalantar-Zadeh, K., Jafar, T. H., Nitsch, D., Neuen, B. L. & Perkovic, V. Chronic kidney disease. Lancet 398, 786–802. https://doi.org/10.1016/S0140-6736(21)00519-5 (2021).
Foreman, K. J. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 392, 2052–2090. https://doi.org/10.1016/S0140-6736(18)31694-5 (2018).
Ren, F., Li, M., Xu, H., Qin, X. & Teng, Y. Urine albumin-to-creatinine ratio within the normal range and risk of hypertension in the general population: Ameta-analysis. J. Clin. Hypertens. (Greenwich). 23, 1284–1290. https://doi.org/10.1111/jch.14263 (2021).
Zhang, A. et al. The relationship between urinary albumin to creatinine ratio and all-cause mortality in the elderly population in the Chinese community: A 10-year follow-up study. BMC Nephrol. 23, 16. https://doi.org/10.1186/s12882-021-02644-z (2022).
Koeda, Y. et al. Comparison between urine albumin-to-creatinine ratio and urine protein dipstick testing for prevalence and ability to predict the risk for chronic kidney disease in the general population (Iwate-KENCO study): A prospective community-based cohort study. BMC Nephrol. 17, 46. https://doi.org/10.1186/s12882-016-0261-3 (2016).
Nochioka, K. et al. Impaired glucose tolerance and albuminuria in patients with chronic heart failure: A subanalysis of the SUPPORT trial. ESC Heart Fail. 6, 1252–1261. https://doi.org/10.1002/ehf2.12516 (2019).
Tao, J. et al. Elevated urine albumin-to-creatinine ratio increases the risk of new-onset heart failure in patients with type 2 diabetes. Cardiovasc. Diabetol. 22 https://doi.org/10.1186/s12933-023-01796-6 (2023).
Fung, C. S., Wan, E. Y., Chan, A. K. & Lam, C. L. Association of estimated glomerular filtration rate and urine albumin-to-creatinine ratio with incidence of cardiovascular diseases and mortality in Chinese patients with type 2 diabetes mellitus - a population-based retrospective cohort study. BMC Nephrol. 18, 47. https://doi.org/10.1186/s12882-017-0468-y (2017).
Huan, L., Yuezhong, L., Chao, W. & HaiTao, T. The urine albumin-to-creatinine ratio is a reliable indicator for evaluating complications of chronic kidney disease and progression in IgA nephropathy in China. Clin. (Sao Paulo). 71, 243–250. https://doi.org/10.6061/clinics/2016(05)01 (2016).
Bansal, N. et al. Development and validation of a model to predict 5-year risk of death without ESRD among older adults with CKD. Clin. J. Am. Soc. Nephrol. 10, 363–371. https://doi.org/10.2215/CJN.04650514 (2015).
Kim, J. Y., Kang, H. T., Lee, H. R., Lee, Y. J. & Shim, J. Y. Comparison of lipid-related ratios for prediction of chronic kidney disease stage 3 or more in Korean adults. J. Korean Med. Sci. 27, 1524–1529. https://doi.org/10.3346/jkms.2012.27.12.1524 (2012).
Kang, H. T. et al. Association between the ratio of triglycerides to high-density lipoprotein cholesterol and chronic kidney disease in Korean adults: the 2005 Korean National Health and Nutrition Examination Survey. Kidney Blood Press. Res. 34, 173–179. https://doi.org/10.1159/000323895 (2011).
Kosmas, C. E. et al. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and Cardiovascular Disease. Diagnostics 13 https://doi.org/10.3390/diagnostics13050929 (2023).
Mu, X., Wu, A., Hu, H., Yang, M. & Zhou, H. Correlation between alternative insulin resistance indexes and diabetic kidney disease: A retrospective study. Endocrine https://doi.org/10.1007/s12020-023-03574-6 (2023).
Bindlish, S., Presswala, L. S., & Schwartz, F. Lipodystrophy syndrome of severe insulin resistance. Postgrad. Med. 127, 511–516. https://doi.org/10.1080/00325481.2015.1015927 (2015).
Wondmkun, Y. T. Obesity Insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 13, 3611–3616. https://doi.org/10.2147/DMSO.S275898 (2020).
Reaven, G. M. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: Similarities and differences. J. Clin. Hypertens. (Greenwich). 13, 238–243. https://doi.org/10.1111/j.1751-7176.2011.00439.x (2011).
Kang, H. T. et al. Independent association of TG/HDL-C with urinary albumin excretion in normotensive subjects in a rural Korean population. Clin. Chim. Acta. 413, 319–324. https://doi.org/10.1016/j.cca.2011.10.021 (2012).
Tsuruya, K. et al. Association of the triglycerides to high-density lipoprotein cholesterol ratio with the risk of chronic kidney disease: Analysis in a large Japanese population. Atherosclerosis 233, 260–267. https://doi.org/10.1016/j.atherosclerosis.2013.12.037 (2014).
Tsuruya, K. et al. Impact of the triglycerides to high-density lipoprotein cholesterol ratio on the incidence and progression of CKD: A longitudinal study in a large Japanese Population. Am. J. Kidney Dis. 66, 972–983. https://doi.org/10.1053/j.ajkd.2015.05.011 (2015).
Xue, J. et al. Triglycerides to high-density lipoprotein cholesterol ratio is superior to triglycerides and other lipid ratios as an indicator of increased urinary albumin-to-creatinine ratio in the general population of China: A cross-sectional study. Lipids Health Dis. 20, 13. https://doi.org/10.1186/s12944-021-01442-8 (2021).
Raikou, V. D., Kyriaki, D. & Gavriil, S. Triglycerides to high-density lipoprotein cholesterol ratio predicts chronic renal disease in patients without diabetes Mellitus (STELLA Study). J. Cardiovasc. Dev. Dis. 7 https://doi.org/10.3390/jcdd7030028 (2020).
Ye, Y. et al. Self-reported sleep duration and daytime napping are associated with renal hyperfiltration and microalbuminuria in an apparently healthy Chinese population. PLoS One. 14, e0214776. https://doi.org/10.1371/journal.pone.0214776 (2019).
Chen, Y. et al. Association of body mass index and age with incident diabetes in Chinese adults: A population-based cohort study. BMJ Open. 8 https://doi.org/10.1136/bmjopen-2018-021768 (2018).
Zhang, N. et al. Non-high-density lipoprotein cholesterol: high-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: Results from a population-based cohort study. J. Diabetes. 10, 708–714. https://doi.org/10.1111/1753-0407.12650 (2018).
Wax, Y. Collinearity diagnosis for a relative risk regression analysis: An application to assessment of diet-cancer relationship in epidemiological studies. Stat. Med. 11, 1273–1287. https://doi.org/10.1002/sim.4780111003 (1992).
Poulter, N. R., Prabhakaran, D. & Caulfield, M. Hypertens. Lancet 386, 801–812, doi:https://doi.org/10.1016/S0140-6736(14)61468-9 (2015).
Yuan, Y. et al. Body mass index and mild cognitive impairment among rural older adults in China: the moderating roles of gender and age. BMC Psychiatry. 21, 54. https://doi.org/10.1186/s12888-021-03059-8 (2021).
Tu, L. et al. Association between estimated glomerular filtration rate and reversion to normoglycemia in people with impaired fasting glucose: A 5-year retrospective cohort study. Eur. J. Med. Res. 29, 140. https://doi.org/10.1186/s40001-024-01669-y (2024).
Song, P. K. et al. Trends in lipids level and dyslipidemia among Chinese adults, 2002–2015. Biomed. Environ. Sci. 32, 559–570. https://doi.org/10.3967/bes2019.074 (2019).
Miura, Y. & Suzuki, H. Hypertriglyceridemia and atherosclerotic carotid artery stenosis. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms232416224 (2022).
Yang, A. L. & McNabb-Baltar, J. Hypertriglyceridemia and acute pancreatitis. Pancreatology 20, 795–800. https://doi.org/10.1016/j.pan.2020.06.005 (2020).
Boren, J., Matikainen, N., Adiels, M. & Taskinen, M. R. Postprandial hypertriglyceridemia as a coronary risk factor. Clin. Chim. Acta. 431, 131–142. https://doi.org/10.1016/j.cca.2014.01.015 (2014).
Lee, P. H. et al. Hypertriglyceridemia: An independent risk factor of chronic kidney disease in Taiwanese adults. Am. J. Med. Sci. 338, 185–189. https://doi.org/10.1097/MAJ.0b013e3181a92804 (2009).
Cholesterol, H. D. L. Very Low Levels of LDL Cholesterol, and Cardiovascular Events.
Yamashita, S., Sakai, N., Tsujii, K., Nakamura, T. & Matsuzawa, Y. Prevalence of low HDL-cholesterol and the metabolic syndrome. Int. Congr. Ser. 1262, 273–276. https://doi.org/10.1016/j.ics.2004.01.015 (2004).
Liu, X. et al. Body mass index (BMI) is associated with microalbuminuria in Chinese hypertensive patients. Int. J. Environ. Res. Public. Health. 12, 1998–2008. https://doi.org/10.3390/ijerph120201998 (2015).
Lin, C. C. et al. Microalbuminuria and the metabolic syndrome and its components in the Chinese population. Eur. J. Clin. Invest. 37, 783–790. https://doi.org/10.1111/j.1365-2362.2007.01865.x (2007).
Li, Y. et al. Glycated Hemoglobin Is Independently Associated with Albuminuria in Young nondiabetic people with obesity: A cross-sectional study. Med. Sci. Monit. 23, 2612–2618. https://doi.org/10.12659/msm.902450 (2017).
Hao, G. et al. Prevalence of microalbuminuria among middle-aged population of China: A multiple center cardiovascular epidemiological study. Angiology 66, 49–56. https://doi.org/10.1177/0003319713513144 (2015).
Ukena, C. et al. Smoking is associated with a high prevalence of microalbuminuria in hypertensive high-risk patients: Data from I-SEARCH. Clin. Res. Cardiol. 99, 825–832. https://doi.org/10.1007/s00392-010-0194-y (2010).
Jiang, Y. et al. Impaired fasting blood glucose is associated with incident albuminuria: data from a Chinese community-based cohort. J. Diabetes Complications. 36, 108125. https://doi.org/10.1016/j.jdiacomp.2022.108125 (2022).
McLaughlin, T. et al. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann. Intern. Med. 139, 802–809. https://doi.org/10.7326/0003-4819-139-10-200311180-00007 (2003).
Wang, T. et al. Association between Insulin Resistance and Cardiovascular Disease Risk varies according to glucose tolerance status: A nationwide prospective cohort study. Diabetes Care. 45, 1863–1872. https://doi.org/10.2337/dc22-0202 (2022).
DeFronzo, R. A. & Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 14, 173–194. https://doi.org/10.2337/diacare.14.3.173 (1991).
Matsumoto, H. et al. Insulin resistance contributes to obesity-related proteinuria. Intern. Med. 44, 548–553. https://doi.org/10.2169/internalmedicine.44.548 (2005).
Esteghamati, A. et al. Insulin resistance is an independent correlate of increased urine albumin excretion: A cross-sectional study in Iranian type 2 diabetic patients. Diabet. Med. 26, 177–181. https://doi.org/10.1111/j.1464-5491.2008.02653.x (2009).
Suzuki, M., Kanazawa, A., Shiba, M., Kojima, H. & Harano, Y. Insulin resistance in diabetic microangiopathies. J. Diabetes Complications. 14, 40–45. https://doi.org/10.1016/s1056-8727(00)00052-0 (2000).
Sun, X. et al. Associations of pancreatic beta-cell function and insulin resistance with microalbuminuria in type 2 diabetes. Diabetes Res. Clin. Pract. 110, e22–26. https://doi.org/10.1016/j.diabres.2015.09.012 (2015).
Luo, Z., Chen, Z., Hu, J. & Ding, G. Interplay of lipid metabolism and inflammation in podocyte injury. Metabolism 150, 155718. https://doi.org/10.1016/j.metabol.2023.155718 (2024).
Ferro, C. J. et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 14, 727–749. https://doi.org/10.1038/s41581-018-0072-9 (2018).
Sahadevan, M. & Kasiske, B. L. Hyperlipidemia in kidney disease: Causes and consequences. Curr. Opin. Nephrol. Hypertens. 11, 323–329. https://doi.org/10.1097/00041552-200205000-00009 (2002).
Quispe, R. et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the very large database of Lipids-4 (VLDL-4) study. Atherosclerosis 242, 243–250. https://doi.org/10.1016/j.atherosclerosis.2015.06.057 (2015).
Nosadini, R. & Tonolo, G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 21, 79–85. https://doi.org/10.1016/j.numecd.2010.10.002 (2011).
Acknowledgements
As this is a secondary analysis, the data and method description are mainly derived from the following research: Ye Y, Zhang L, Yan W, Wang A, Wang W, Gao Z, Tang X, Yan L, Wan Q, Luo Z, Qin G, Chen L, Wang S, Wang Y, Mu Y. Self-reported sleep duration and daytime napping are associated with renal hyperfiltration and microalbuminuria in an apparently healthy Chinese population. PLoS One. 2019 Aug 30;14(8):e0214776. doi: https://doi.org/10.1371/journal.pone.0214776. PMID: 31469836; PMCID: PMC6716775. We are grateful to all the authors of the study.
Funding
This study was supported by the Shenzhen Science and Technology Innovation Committee (JCYJ20210324133412033) and the Discipline Construction Ability Enhancement Project of the Shenzhen Municipal Health Commission (SZXJ2017031).
Author information
Authors and Affiliations
Contributions
Xue Zhang, Lishu He, and Haofei Hu designed the study and wrote the first draft of the manuscript. Xue Zhang, Xia Huang, and Zhichao Zhang conducted the statistical analyses, and Lirong Tu, Heping Zhang, and Feiyuan Liu collaborated in conducting the study and interpreting the data; they also ensured that the analyses were accurate and comprehensive. Yongcheng He and Xiaohui Liu took part in the talks and offered insightful feedback throughout the evaluation procedure. Yongcheng He, Haofei Hu, and Xiaohui Liu revised the manuscript and contributed to the overall design of the study. All authors carefully reviewed the final draft of the manuscript and agreed with it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The original study followed guidelines outlined by the Helsinki Declaration. The original study was approved by the Committee on Human Research at Rui-Jin Hospital affiliated with the School of Medicine, Shanghai Jiao Tong University. Written informed consents were obtained from all participants before data collection. All methods were performed by the relevant Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Hu, H., He, L. et al. Association between triglyceride to high-density lipoprotein cholesterol ratio and microalbuminuria in the Chinese population. Sci Rep 14, 30960 (2024). https://doi.org/10.1038/s41598-024-82084-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82084-5