Abstract
Articular cartilage has a limited regenerative capacity, resulting in poor spontaneous healing of damaged tissue. Despite various scientific efforts to enhance cartilage repair, no single method has yielded satisfactory results. With rising drug development costs, drug repositioning has emerged as a viable alternative. This study aimed to identify a drug capable of improving cartilage defects by analyzing chondrogenesis-related microarray data from the Gene Expression Omnibus (GEO) public database. We utilized datasets GSE69110, GSE107649, GSE111822, and GSE116173 to identify genes associated with cartilage differentiation, employing StringTie for differential gene expression analysis and extracting drug data from the Drug-Gene Interaction database. Additionally, we aimed to verify the cartilage regeneration potential of the identified drug through experiments using cellular and animal models. We evaluated the effects of aripiprazole on adipose-derived mesenchymal stem cells (ADMSCs) and chondrocytes using qRT-PCR and a 3D pellet culture system. In vivo, we assessed cartilage restoration by combining aripiprazole with a scaffold and implanting it into artificially induced cartilage defects in Sprague-Dawley rats. Subsequent mRNA sequencing provided insights into the mechanistic pathways involved. Our results showed that aripiprazole significantly increased mRNA expression of COL2A1 and SOX9, markers of chondrogenesis, and promoted chondrogenic condensation in vitro. Furthermore, aripiprazole effectively enhanced cartilage regeneration in the rat model. KEGG pathway and Gene Ontology Biological Processes (GOBP) analyses of the mRNA sequencing data revealed that aripiprazole upregulated genes related to ribosomes and cytoplasmic translation, thereby facilitating chondrogenesis. In conclusion, our findings suggest that aripiprazole is a promising candidate for improving damaged cartilage, offering a novel approach to cartilage regeneration.
Similar content being viewed by others
Introduction
Cartilage regeneration is a challenging issue due to poor regenerative properties of tissue1,2. Damaged cartilage due to different factors, including trauma, metabolic, genetic, mechanical, and inflammatory factors, undergoes progressive degradation, leading to pain, stiffness, and loss of mobility, eventually resulting in osteoarthritis3.
Various scientific efforts have been made to restore articular cartilage damage, including cell-based therapies4,5, tissue engineering5,6,7, gene therapy7,8, and mechanical stimulation9. Some attempts have been proven to have an effect and have been utilized in clinical practice10,11,12. Nevertheless, given the limitations of current treatments, especially for older patients with larger, full-thickness defects, there’s a pressing need for innovative regenerative approaches13.
In recent years, drug repositioning—also known as drug repurposing, reprofiling, rescue, redirecting, switching, or retaking—has emerged as a promising strategy for developing new treatments for various diseases, including osteoarthritis14,15,16,17,18,19,20,21,22,23. Drug repositioning involves the identification of existing or abandoned drugs that may have therapeutic potential for new indications24. Compared with de novo drug manufacturing, this approach offers several advantages over traditional drug development, including reduced time and cost to market and a lower risk of side effects by utilizing existing data24.
In this study, we aimed to identify a drug that could improve cartilage defects by utilizing chondrogenesis microarray data collected from the Gene Expression Omnibus (GEO) public database. .We identified several drugs with the potential for cartilage regeneration among the commercially available drugs for other indications. Aripiprazole, an atypical antipsychotic, is primarily indicated for schizophrenia, bipolar disorder, and major depressive disorder. It functions as a partial agonist at dopamine D2 and serotonin 5-HT1A receptors and an antagonist at the 5-HT2A receptor, enhancing efficacy while minimizing side effects. Emerging studies suggest its role in modulating neuroinflammation, hinting at broader therapeutic applications. Our research explores its potential as a chondrogenic regenerative agent through drug repurposing. Finally, we verified the therapeutic effect of the repurposed drug for improving the healing treatment of cartilage defects using in vitro and in vivo models .
Materials and methods
Study outline
The general drug screening procedure is described in (Supplementary Fig. 1).
Chondrogenesis related public data gene analysis
For sequencing data quality control (QC), the quality scores and sequence composition of cDNA fragments were evaluated using the “fastp” program, according to base calling and demultiplexing steps. To ensure the accuracy of downstream analysis and data interpretation, raw sequence reads were trimmed to remove adapter sequences, tRNA and rRNA contamination, PCR duplicates, and poor-quality reads (error rate < 0.05). Reads shorter than 30 nucleotides were discarded after trimming and mapped to a reference genome using the STAR (version 2.7.5) aligner (Supplementary Fig. 2). StringTie assembles RNA-Seq alignments into potential transcripts. We used it to convert read counts from the STAR aligner into fragments per kilobase of transcript per million mapped reads (FPKM). After completing the gene quantification step, the data outputs were expressed as FPKM values (1_gene_expression). The minimum gene expression value was set to 0.5, and values below the cutoff were discarded. Genes exhibiting a two-fold or more increase or a two-fold or more decrease in expression [with |log2 (fold change)| ≥ 1] were identified as differentially expressed genes (DEGs).
Cell culture
Human adipose tissue-derived mesenchymal stem cells (ADMSCs) were obtained from PromoCell GmbH (Heidelberg, Germany), ADMSCs were cultured in standard culture and chondrogenic differentiation media (Gibco). Dulbecco’s Modified Eagle Medium High Glucose (DMEM, Welgen) supplemented with 1% penicillin/streptomycin (Gibco) and 10% fetal bovine serum (Gibco) was used as the standard culture medium. ADMSCs were induced to undergo chondrogenic differentiation for 21 days in a pellet culture (100,000 cells/pellet) in a chondrogenic medium consisting of high-glucose DMEM with 50 µg/mL ascorbate acid 2-phosphate (Sigma-Aldrich, MO, USA), 40 µg/mL proline (Sigma-Aldrich), and 1% ITS (Insulin-Transferrin-Selenium) Universal Culture Supplement Premix (BD Biosciences, CA, USA). All the cell lines tested negative for mycoplasma contamination and incubated at 37 °C in a 5% CO2 incubator.
Real-time reverse transcription-PCR analysis of mRNA
To prepare total RNA, cells were lysed using TRIzol Reagent (Invitrogen, Thermo Fisher Scientific) after treatment with each drug. Each drug was identified as being commonly regulated across two or more selected GSE datasets. The list of these drugs has been provided as supplementary data 1. Among them, 34 commercially available drugs are highlighted in Fig. 2A. Total RNA was synthesized into cDNA using a Maxime RT PreMix Kit (iNtRON, Seongnam, Korea). Briefly, 1 µg of total RNA and pure water were added into the Maxime RT PreMix tubes up to 20 µL, and the mixture was incubated according to the manufacturer’s protocol: cDNA synthesis, 45℃ for 60 min, and RTase inactivation step, 95℃ for 5 min. To quantify mRNA expression, qRT-PCR (quantitative Real-Time PCR) was performed using AMPIGENE qPCR Green Mix Lo-ROX (Enzo Life Sciences, Seoul, Korea). According to the manufacturer’s protocol, a mixture of cDNA, reagents, and specific primers was prepared and incubated as follows: one cycle, 95℃ for 2 min; 40 cycles, 95℃ for 5 s; and 60℃ for 30 s. The primers used are listed in Table 1. Data were analyzed using the 2-ΔΔCt method and normalized to glyceraldehyde-3-phosphate dehydrogenase expression. β-Actin was used as the endogenous control for mRNA determination. The sequences of the specific primers were as follows: COL2A1, forward, 5’‑ TGG ACG ATC AGG CGA AAC C-3’ and reverse, 5’- GCT GCG GAT GCT CTC AAT CT -3’; SOX9, forward, 5’‑ AGC GAA CGC ACA TCA AGAC-3’ and reverse, 5’-CTG TAG GCG ATC TGT TGG GG-3’; ACAN, forward, 5’‑ CCC CTG CTA TTT CAT CGA CCC-3’ and reverse, 5’- GAC ACA CGG CTC CAC TTG AT -3’; GAPDH, forward, 5’‑ GGA GCG AGA TCC CTC CAA AAT-3’ and reverse, 5’- GGC TGT TGT CAT ACT TCT CAT GG-3’.
Cartilage defect rat model
Eight-week-old Sprague-Dawley (SD) rats were purchased from ORIENT BIO. Animal experimental procedures were reviewed and approved by the CHA University Animal Care and Use Committee (IACUC220108), and conducted in accordance with ethical and procedural guidelines. Animals were maintained at 23 ± 1℃ and 50 ± 10% humidity under specific pathogen-free conditions. The light–dark period was cycled every 12 h, and food and water were provided ad libitum. After 1 week of adaptive breeding, SD rats were anesthetized and shaved, and the knee was disinfected. A medial temporal longitudinal incision was made to expose the synovium of the knee joint. Following lateral patellar luxation, the trochlear groove was further exposed. A defect, measuring 2 mm in diameter and 3 mm in depth, was drilled in the center of the trochlear groove. After irrigating the joint with sterile isotonic saline, the absorbable collagen sponge soaked in aripiprazole or DMEM were implanted. In the treatment group, 100 µL of 10 mM aripiprazole was applied with absorbable collagen sponge (n = 6), while 100 µL DMEM was treated in the control group (n = 6). After, patella was relocated, and the wound was sutured in layers. The progress of cartilage regeneration was evaluated after a 6-week period, and the mice were euthanized using a CO2 chamber with a fill rate of 30% volume per minute. Confirmation of euthanasia was based on pause of breathing and a loss of eye color.
Histologic analysis
The paraffin-embedded sections were prepared, dewaxed, and rehydrated. Five-micrometer sections were cut and stained with standard Hematoxylin and eosin (H&E), Safranin O, and Alcian blue. The criteria for the histology score and gross appearance have been provided in Tables 1 and 2.
mRNA sequencing
Library preparation, sequencing of DMSO or Aripiprazole-treated total RNAs, and library construction were performed using the QuantSeq 3’ mRNA-Seq Library Prep Kit (Lexogen, Inc., Austria) according to the manufacturer’s instructions. Briefly, 500 ng of total RNA was prepared for each sample, an oligo-dT primer containing an Illumina-compatible sequence at its 5’ end was hybridized to the RNA, and reverse transcription was performed. After degradation of the RNA template, second-strand synthesis was initiated by a random primer containing an Illumina-compatible linker sequence at its 5’ end. The double-stranded library was purified using magnetic beads to remove all the reaction components. The library was amplified to add complete adapter sequences required for cluster generation. The final library was purified from PCR components. High-throughput single-end 75 sequencing was performed using NextSeq 500 (Illumina, Inc., USA). Data analysis of QuantSeq 3 mRNA-Seq reads was aligned using Bowtie2. Bowtie2 indices were generated either from the genome assembly sequence or representative transcript sequences for alignment to the genome and transcriptome. The alignment file was used to assemble the transcripts, estimate their abundance, and detect differential gene expression. Gene classification was based on searches of the DAVID and Medline databases. Data mining and graphic visualization were performed using ExDEGA (Ebiogen Inc., Korea).
Statistical analysis
All the data were expressed as the mean ± standard error of the mean. All data were analyzed using the GraphPad Prism 8 software (GraphPad Software Inc., CA, USA) and were expressed as mean ± standard deviation. The Mann–Whitney U test was used to compare the means of two groups, and the Kruskal–Wallis test with Tukey’s post-hoc test was used to compare the means of two or more variables (induction and concentration).
Results
Cartilage repair enhancing drugs using public database
To identify drugs that enhance cartilage repair, we collected microarray data from chondrogenesis-related studies available in the GEO public database (https://www.ncbi.nlm.nih.gov/geo/)25. Utilizing gene sets from GSE69110, GSE107649, GSE111822, and GSE116173, we implemented a five-stage gene expression analysis pipeline. This pipeline included: (1) data preparation (including quality control), (2) read alignment, (3) expression quantification, (4) differential expression identification, and (5) biological function profiling. From the selected differentially expressed genes (DEGs), we specifically focused on the top 100 genes with the most significant fold changes in expression, visualizing them in a heatmap for each group (Fig. 1). Then, The Drug-Gene-Interaction (DGI) database was used to identify candidate drugs applicable to the identified genes. Furthermore, to select significant drug candidates, commonly tracked drugs from GSE studies were identified and organized (Table 3).
Aripiprazole as a novel drug to improve the cartilage defect
To identify novel compounds capable of enhancing the chondrogenic differentiation of ADMSCs, we curated a selection of 34 commercially available chondrogenic stimulators. Their effects on cartilage differentiation were evaluated, with particular emphasis on those that markedly upregulated the mRNA expression of SOX9 and COL2A1, two critical components essential for cartilage matrix formation. In the initial functional screening, seven compounds demonstrated a significant increase in the expression of both SOX9 and COL2A1. These selected candidates were subsequently subjected to a second round of screening, as illustrated in Fig. 2A. During the second screening, differential gene expression was assessed in chondrocyte differentiatopn media. Notably, treatment with aripiprazole and irinotecan significantly increased the expression of COL2A1, SOX9, and ACAN, which are established markers of the chondrocytic phenotype (Fig. 2B). To further evaluate the efficacy of these two drugs in enhancing chondrogenesis within a 3D pellet culture model, we conducted pellet cultures and performed gene expression analysis. Cells were cultured for three weeks in a chondrogenic differentiation medium, and it was observed that pellet size was effectively maintained in the aripiprazole-treated group (Fig. 2C). Histological analyses, including H&E staining, revealed increased cell proliferation, while Alcian blue staining confirmed higher levels of proteoglycan content in the aripiprazole-treated group, thus further promoting chondrogenic differentiation (Fig. 2D).
Functional screening of drugs promoting chondrogenic differentiation (A) First Screening of Cartilage Differentiation-Promoting Drugs: Adipose-derived mesenchymal stem cells (ADMSCs) were treated with 10 µM of each drug for 72 h. The expression levels of COL2A1 (top) and SOX9 (bottom) mRNA were assessed using qRT-PCR. Dotted lines indicate drugs that significantly increased SOX9 and COL2A1 expression compared to DMSO control. (B) Second Screening: The Venn diagram shows the overlap of drugs that increased COL2A1 and SOX9 expression. Bar graphs present relative mRNA expression levels with statistical significance indicated. The cartilage-differentiation effect of seven drugs selected from the first screening was re-evaluated by measuring changes in mRNA levels of COL2A1, SOX9, and aggrecan (ACAN).: *P < 0.05, **P < 0.01, ***P < 0.001 (n = 3). (C) ADMSCs were cultured in pellet form and treated with DMSO, aripiprazole, or irinotecan for three weeks. Aripiprazole treatment showed increased cartilage differentiation compared to DMSO and irinotecan. (D) Hematoxylin and Eosin (H&E) staining and Alcian blue staining of pellet cultures. Aripiprazole treatment resulted in larger pellet size and more intense staining, indicating enhanced cartilage matrix production. Scale bars represent 100 μm.
Aripiprazole significantly enhances cartilage regeneration in SD rat model
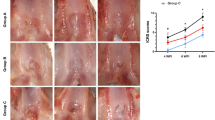
In the macroscopic appearance assessment of cartilage regeneration, the group treated with aripiprazole demonstrated a significantly higher advancement in cartilage regeneration compared to the control group treated with DMSO. The macroscopic images of the cartilage visually depict this difference, with the aripiprazole-treated samples showing more substantial restoration of cartilage tissue. Quantitative analysis revealed a marked increase in the macroscopic appearance score for the aripiprazole group, indicating superior cartilage healing. Specifically, the aripiprazole group achieved a gross appearance score significantly higher than both the defect and DMSO groups (p < 0.001), suggesting that aripiprazole plays a crucial role in enhancing cartilage regeneration (Fig. 3A). The histological evaluation supported the macroscopic findings. Safranin O and Alcian Blue staining, which target glycosaminoglycans and proteoglycans in cartilage matrix, were used. The defect group showed poor cartilage formation with sparse staining and disorganized structure. DMSO-treated group exhibited some cartilage regeneration, but it was limited in quality and extent. In contrast, the aripiprazole-treated group displayed extensive and well-organized cartilage regeneration. Safranin O staining indicated a dense, uniformly distributed cartilage matrix, while Alcian Blue staining showed significant glycosaminoglycan restoration. Histological scores for the aripiprazole group were significantly higher than DMSO groups (p < 0.001), highlighting aripiprazole’s effectiveness in promoting cartilage repair (Fig. 3B).
Restorative effect of aripiprazole in a rat model of articular cartilage defect. (A) Representative images of distal femur cartilage in the defect, DMSO, and aripiprazole groups taken at 8 weeks post-establishment of a cartilage defect model. The histology score and gross appearance were quantified and are presented as bar graphs. The histology score (left graph) and gross appearance (right graph) show significant improvement in the aripiprazole group compared to the defect and DMSO groups. Statistical significance is indicated as **p < 0.01, ***p < 0.001. (B) Histological analysis of cartilage defects in euthanized rats using H&E, Alcian blue, and safranin-O staining. The staining results demonstrate better cartilage regeneration and matrix formation in the aripiprazole-treated group compared to the defect and DMSO groups. Scale bars represent 100 μm.
Aripiprazole-induced gene expression changes
To validate the newly discovered mechanism of aripiprazole in promoting cartilage restoration, we conducted bulk mRNA sequencing. In order to gain insights into the associated biological functions, we performed GO biological process (GOBP) analyses on the genes that were upregulated and downregulated following treatment with aripiprazole, utilizing the DAVID bioinformatics tools. The KEGG pathway analysis identified several pathways significantly enriched in the aripiprazole-treated group. Notably, the ribosome pathway exhibited the highest fold enrichment with a significant p-value, indicating its potential role in cartilage restoration (Fig. 4A). In GOBP analysis, the most prominent processes included cytoplasmic translation, translation, tRNA processing, and ribosomal small subunit biogenesis (Fig. 4B). The KEGG pathway and GOBP analyses present strong evidence that aripiprazole affects a wide array of biological pathways and processes essential for cellular growth, metabolism, and protein synthesis. These results support the hypothesis that aripiprazole promotes cartilage restoration through complex mechanisms that include enhanced ribosome pathway and protein synthesis.
Gene expression changes in cartilage differentiation following aripiprazole treatment (A) KEGG pathway enrichment analysis of genes modulated in chondrocytes upon aripiprazole treatment. The pathways with significant enrichment are shown, with the horizontal axis representing fold enrichment and the size and color of the circles indicating the enrichment score (− log10(p-value)). (B) GO biological process (GOBP) enrichment analysis on upregulated and downregulated genes in chondrocytes treated with aripiprazole. The horizontal axis represents fold enrichment, and the size and color of the circles indicate the enrichment score (− log10(p-value)). Prominent biological processes include cytoplasmic translation, translation, tRNA processing, and ribosomal small subunit biogenesis.
Discussion
Our findings demonstrate the potential of drug repositioning for developing novel treatments for cartilage defects. By utilizing the GEO database, we identified chondrogenesis-associated genes and, through DGI analysis, selected drugs that promote chondrogenic differentiation. MSCs were treated with these drugs, and primary screening focused on the mRNA expression of SOX9 and COL2A1. Following further screening and pellet culture, aripiprazole emerged as a promising candidate. Its efficacy was validated in an SD rat cartilage defect model using a collagen sponge scaffold. Gene expression analysis of aripiprazole-treated chondrocytes showed a significant impact on ribosomal pathways and protein translation.
By repurposing existing drugs with known safety profiles and efficacy for other indications, researchers can accelerate the development of effective and safe therapies for osteoarthritis and improve the quality of life of millions of patients24. One example is metformin, commonly used to treat diabetes by controlling glucose levels. Metformin treatment (1 mM) inhibits micro RNA-34a while promoting SIRT 1 expression in osteoarthritic chondrocytes, regulating senescence and proliferation in human chondrocytes22. In a clinical comparison study, patients with type 2 diabetes treated with metformin demonstrated significantly reduced total joint replacement compared with patients in the propensity score-matched control group20. The major mechanisms involved in osteoarthritis include inflammation, oxidative stress, autophagy, adipokine levels, and microbiome modulation21. Nevertheless, metformin is likely beneficial in metabolic-type knee osteoarthritis, in which accompanying obesity and systemic low-grade inflammation are suggestive and is not currently recommended for primary arthritis treatment21.
Statin medications, such as simvastatin, have also been investigated for their potential effects on cartilage regeneration. Statins are commonly used to lower cholesterol levels by inhibiting 3-hydroxy-3-methylglutaryl-CoA. However, they also have anti-inflammatory and antioxidant properties, which may benefit cartilage repair and regeneration23,26,27. This mechanism is beneficial in preventing cartilage degradation. Han and Kim examined articular cartilage differentiation in a rabbit cartilage model using western blot analysis, RT-PCR, and immunohistochemical and immunofluorescence staining27. In a simvastatin-treated model, type II collagen loss was inhibited, and the ERK-1/2 and p38 kinase pathways regulated the simvastatin-induced differentiation of chondrocytes.
In addition, several repurposed drugs have been used for cartilage regeneration, including apremilast19 (primarily used for psoriatic arthritis), bevacizumab15 (antiangiogenic drug), and suramin (medication for African sleeping sickness)14; however, none of these drugs are primarily used for osteoarthritis treatment.
Aripiprazole, a third-generation antipsychotic, is used to treat schizophrenia, bipolar disorder, major depressive disorder, tic disorder, and autism-related irritability via oral, intramuscular, and intravenous routes28,29. It partially activates D2 and D3 receptors, stabilizing dopamine levels to address mental health conditions by modulating the mesolimbic and mesocortical pathways. Additionally, aripiprazole acts as a partial agonist at serotonin 5-HT1A receptors and an antagonist at 5-HT2A receptors, offering mood-regulating effects at lower doses. It also interacts with alpha-1 adrenergic and histamine H1 receptors, potentially contributing to its sedative and anxiolytic properties30,31.It also interacts with alpha-1 adrenergic and histamine H1 receptors, potentially contributing to its sedative and anxiolytic properties32.
Recently, aripiprazole was reported to have anticancer33 and radio-sensitizing effects in various cancers, including head and neck cancer34,35,36,37. Kim et al. argued that aripiprazole has antitumor effects via an important target molecule lined with Src, other than for psychotic purposes37.
SOX9 is regarded as the first transcription factor essential for chondrocyte differentiation and cartilage formation38. It also regulates the expression of many cartilage-specific genes, including COL2A1, which encodes type II collagen, a major component of cartilage extracellular matrix. Furthermore, the mRNA expression levels of SOX9 and COL2A1 have been used as indicators of the success of cartilage regeneration therapies and employed in cartilage regeneration experiments39,40,41. We also selected the final candidate drugs, DMSO and aripiprazole, through first and second screening, depending on the mRNA expression of SOX9 and COL2A1. Additionally, aripiprazole effectively promoted cartilage regeneration in both a 3D culture model (pellet culture) and a cartilage defect animal model.
Through mRNA sequencing, we confirmed that aripiprazole significantly upregulates genes involved in the ribosomal pathway and protein synthesis, critical components in cartilage regeneration. Ribosomes are central to producing the proteins required for chondrocyte proliferation, differentiation, and extracellular matrix (ECM) formation, including essential structural proteins like collagen and proteoglycans42,43,44,45.They also synthesize growth factors and cytokines (e.g., TGF-β, IGF, BMP) that regulate these processes. Additionally, ribosomes support cellular survival mechanisms under stress by producing antioxidant and stress-response proteins, ensuring chondrocyte functionality in damaged environments46,47,48,49. Efficient ribosomal activity, therefore, is vital for successful cartilage repair. Our findings suggest that aripiprazole enhances ribosomal function and cytoplasmic translation, facilitating cartilage regeneration and maintaining cellular health in compromised tissues.
Aripiprazole is administered orally, intramuscularly, and intravenously. While promising for cartilage regeneration, further studies are needed to optimize its dosing and delivery methods for this application. Any modifications in formulation or administration routes will necessitate safety reassessment. Future research should also validate its clinical efficacy and elucidate the mechanisms behind its chondrogenic effects. Given the variability in cartilage degeneration due to age and health, the treatment’s effectiveness may vary, underscoring the need for individualized approaches.
Conclusion
We discovered that aripiprazole can effectively improve damaged cartilage in a rat model, providing a potentially promising approach for cartilage regeneration also in other species.
Data availability
The datasets generated and/or analysed during the current study are available in the National Center for Biotechnology Information Gene Expression Omnibus database (NCBI GEO data base) with the accession number GSE GSE271026. Raw data are accessible at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE271026.
References
Charlier, E. et al. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 165, 49–65 (2019).
Shin, H. H. et al. Hydrophilic/hydrophobic janus nanofibers containing compound k for cartilage regeneration. Int. J. Nanomed. 19, 1683–1697 (2024).
Johnson, V. L. & Hunter, D. J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 28, 5–15 (2014).
Ismail, O. M., Said, U. N. & El-Omar, O. M. Adult stem cells for cartilage regeneration. Cureus 14, e32280 (2022).
Zelinka, A., Roelofs, A. J., Kandel, R. A. & De Bari, C. Cellular therapy and tissue engineering for cartilage repair. Osteoarthr. Cartil. 30, 1547–1560 (2022).
Stone, R. N., Reeck, J. C. & Oxford, J. T. Advances in cartilage tissue engineering using bioinks with decellularized cartilage and three-dimensional printing. Int. J. Mol. Sci. 24. (2023).
Guo, X. et al. Regeneration of articular cartilage defects: therapeutic strategies and perspectives. J. Tissue Eng. 14, 20417314231164765 (2023).
Evans, C. H., Ghivizzani, S. C. & Robbins, P. D. Osteoarthritis gene therapy in 2022. Curr. Opin. Rheumatol. 35, 37–43 (2023).
Taheri, S., Ghazali, H. S., Ghazali, Z. S., Bhattacharyya, A. & Noh, I. Progress in biomechanical stimuli on the cell-encapsulated hydrogels for cartilage tissue regeneration. Biomater. Res. 27, 22 (2023).
Colombini, A. et al. Autologous chondrocyte implantation provides good long-term clinical results in the treatment of knee osteoarthritis: a systematic review. Knee Surg Sports Traumatol. Arthrosc. (2022).
Xue, J., Qin, C. & Wu, C. 3D printing of cell-delivery scaffolds for tissue regeneration. Regen Biomater. 10, rbad032 (2023).
Roseti, L. & Grigolo, B. Current concepts and perspectives for articular cartilage regeneration. J. Exp. Orthop. 9, 61 (2022).
Emami, A., Namdari, H., Parvizpour, F. & Arabpour, Z. Challenges in osteoarthritis treatment. Tissue Cell. 80, 101992 (2023).
Guns, L. A. et al. Suramin increases cartilage proteoglycan accumulation in vitro and protects against joint damage triggered by papain injection in mouse knees in vivo. RMD Open. 3, e000604 (2017).
Lee, S., Nemeño, J. G. & Lee, J. I. Repositioning bevacizumab: a promising therapeutic strategy for cartilage regeneration. Tissue Eng. Part. B Rev. 22, 341–357 (2016).
Wang, L. et al. A discovery of clinically approved Panlongqi Tablet for repositioning to treat osteoarthritis by inhibiting PI3K/AKT activation. Phytomedicine 105, 154360 (2022).
Wang, K. D. et al. Digoxin targets low density lipoprotein receptor-related protein 4 and protects against osteoarthritis. Ann. Rheum. Dis. 81, 544–555 (2022).
Shi, S. et al. Identification of key regulators responsible for dysregulated networks in osteoarthritis by large-scale expression analysis. J. Orthop. Surg. Res. 16, 259 (2021).
Wang, B., Sun, W., Bi, K., Li, Y. & Li, F. Apremilast prevents IL–17–induced cellular senescence in ATDC5 chondrocytes mediated by SIRT1. Int. J. Mol. Med. 47 (2021).
Zhu, Z. et al. Metformin use and associated risk of total joint replacement in patients with type 2 diabetes: a population-based matched cohort study. Cmaj 194, E1672–e84 (2022).
Lambova, S. N. Pleiotropic effects of metformin in osteoarthritis. Life. 13 (2023).
Yan, S. et al. Metformin regulates chondrocyte senescence and proliferation through microRNA-34a/SIRT1 pathway in osteoarthritis. J. Orthop. Surg. Res. 18, 198 (2023).
Saberianpour, S. et al. Therapeutic effects of statins on osteoarthritis: a review. J. Cell. Biochem. 123, 1285–1297 (2022).
Jourdan, J. P., Bureau, R., Rochais, C. & Dallemagne, P. Drug repositioning: a brief overview. J. Pharm. Pharmacol. 72, 1145–1151 (2020).
Wang, Z., Lachmann, A. & Ma’ayan, A. Mining data and metadata from the gene expression omnibus. Biophys. Rev. 11, 103–110 (2019).
Riegger, J., Maurer, S., Pulasani, S. & Brenner, R. E. Simvastatin and fluvastatin attenuate trauma-induced cell death and catabolism in human cartilage. Front. Bioeng. Biotechnol. 10, 965302 (2022).
Han, Y. & Kim, S. J. Simvastatin induces differentiation of rabbit articular chondrocytes via the ERK-1/2 and p38 kinase pathways. Exp. Cell. Res. 346, 198–205 (2016).
Stelmach, A., Guzek, K., Rożnowska, A., Najbar, I. & Sadakierska-Chudy, A. Antipsychotic drug-aripiprazole against schizophrenia, its therapeutic and metabolic effects associated with gene polymorphisms. Pharmacol. Rep. 75, 19–31 (2023).
Prommer, E. & Aripiprazole Am. J. Hosp. Palliat. Care ;34, 180–185. (2017).
Rybakowski, J. K. Application of antipsychotic drugs in mood disorders. Brain Sci. 13. (2023).
Orzelska-Górka, J., Mikulska, J., Wiszniewska, A. & Biała, G. New atypical antipsychotics in the treatment of schizophrenia and depression. Int. J. Mol. Sci. 23 (2022).
Kumar, A., Singh, H., Mishra, A. & Mishra, A. K. Aripiprazole: an FDA approved bioactive compound to treat schizophrenia—a mini review. Curr. Drug Discov Technol. 17, 23–29 (2020).
Badran, A. et al. Antipsychotics drug aripiprazole as a lead against breast cancer cell line (MCF-7) in vitro. PLoS One. 15, e0235676 (2020).
Jeong, H. J. et al. Aripiprazole sensitizes head and neck cancer cells to ionizing radiation by enhancing the production of reactive oxygen species. Pharmacol. Res. Perspect. 10, e00989 (2022).
Zhuo, C. et al. Surprising anticancer activities of psychiatric medications: old drugs offer new hope for patients with brain cancer. Front. Pharmacol. 10, 1262 (2019).
Jiang, C. et al. A low dose of aripiprazole has the strongest sensitization effect among 19 repositioned bipolar drugs in P-gp-overexpressing drug-resistant cancer cells. Anticancer Res. 41, 687–697 (2021).
Kim, M. S. et al. Src is the primary target of aripiprazole, an atypical antipsychotic drug, in its anti-tumor action. Oncotarget 9, 5979–5992 (2018).
Bi, W., Deng, J. M., Zhang, Z., Behringer, R. R. & de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 22, 85–89 (1999).
Sun, Z. et al. Intraarticular injection of SHP2 inhibitor SHP099 promotes the repair of rabbit full-thickness cartilage defect. J. Orthop. Translat. 32, 112–120 (2022).
Wei, B. et al. Chondrogenic differentiation of marrow clots after microfracture with BMSC-derived ECM scaffold in vitro. Tissue Eng. Part. A. 20, 2646–2655 (2014).
Kavand, H., Haghighipour, N., Zeynali, B., Seyedjafari, E. & Abdemami, B. Extremely low frequency electromagnetic field in mesenchymal stem cells gene regulation: chondrogenic markers evaluation. Artif. Organs. 40, 929–937 (2016).
Turi, Z., Lacey, M., Mistrik, M. & Moudry, P. Impaired ribosome biogenesis: mechanisms and relevance to cancer and aging. Aging 11, 2512–2540 (2019).
Rellmann, Y., Eidhof, E., Dreier, R. & Review ER stress-induced cell death in osteoarthritic cartilage. Cell. Signal. 78, 109880 (2021).
Han, Z., Zhang, Q., Zhu, Y., Chen, J. & Li, W. Ribosomes: an exciting avenue in stem cell research. Stem Cells Int. 2020, 8863539 (2020).
Alcaide-Ruggiero, L., Cugat, R. & Domínguez, J. M. Proteoglycans in articular cartilage and their contribution to chondral injury and repair mechanisms. Int. J. Mol. Sci. 24 (2023).
Blanquer, S. B., Grijpma, D. W. & Poot, A. A. Delivery systems for the treatment of degenerated intervertebral discs. Adv. Drug Deliv Rev. 84, 172–187 (2015).
Chen, H. et al. Molecular mechanisms of chondrocyte proliferation and differentiation. Front. Cell. Dev. Biol. 9, 664168 (2021).
Mariani, E., Pulsatelli, L. & Facchini, A. Signaling pathways in cartilage repair. Int. J. Mol. Sci. 15, 8667–8698 (2014).
Riegger, J., Schoppa, A., Ruths, L., Haffner-Luntzer, M. & Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review. Cell. Mol. Biol. Lett. 28, 76 (2023).
Acknowledgements
The authors thank the members in the lab for their helpful comments, suggestions, and assistance with animal experiments.
Funding
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean government (No. RS-2023-00210067 and 2022R1A2C2005916).
Author information
Authors and Affiliations
Contributions
J.-K.L., project administration, data curation, and writing of original draft; H.Y., data curation, investigation, and formal analysis; S.C., data curation and formal analysis; K.K., manuscript review and editing and data curation; H.Kmanuscript review and editing and data curation; S.-S.L., data curation; H.L. formal analysis; Y.J., data curation; H.-J.A. and S.L., conceptualization, funding acquisition, investigation, methodology, and manuscript review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, JK., Yeo, H., Choi, S. et al. Therapeutic role of aripiprazole in cartilage defects explored through a drug repurposing approach. Sci Rep 14, 31006 (2024). https://doi.org/10.1038/s41598-024-82177-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82177-1