Abstract
Renorrhaphy is often performed after tumor resection during robotic-assisted laparoscopic partial nephrectomy (RAPN). This study aimed to investigate the association between renorrhaphy performance and inflammatory markers. A retrospective cohort study was conducted including patients with renal cell carcinoma who underwent RAPN at eight institutions in Japan between April 2016 and November 2023. The primary endpoint was the association between the renorrhaphy performance in RAPN and the postoperative inflammatory markers. The secondary endpoints were perioperative outcomes in patients with and without renorrhaphy. The patients were divided into two groups at the time of RAPN: those who underwent renorrhaphy (renorrhaphy group) and those who did not (omitted group). In total, 934 patients were enrolled in this study. After propensity score matching, the rate of change in C-reactive protein and neutrophil-lymphocyte ratio on postoperative day 28 were not significant difference between the two groups. In contrast, the rate of change in platelet-lymphocyte ratio (PLR) on postoperative day 28 was significantly higher in renorrhaphy group than omitted group. Regarding surgical outcomes, the renorrhaphy group had a significantly longer hospital stay, operative time, and warm ischemia time (P = 0.038, P = 0.022, and P = 0.009, respectively) than the omitted group did. Furthermore, the omitted group had a significantly higher rate of Trifecta achievement than the renorrhaphy group did. This study demonstrated that renorrhaphy performance in RAPN was significantly associated with the higher value of postoperative PLR.
Similar content being viewed by others
Introduction
In Japan, approximately 21,000 patients were newly diagnosed with RCC in 2019 according to the National Cancer Institute database1. The majority of RCCs are identified incidentally on abdominal imaging studies, including magnetic resonance imaging or computed tomography, and the frequency of asymptomatic small RCC has been increasing2. Nephron-sparing surgery, which preserves normal renal parenchyma to avoid the risk of chronic renal disease, is recommended in several guidelines for the treatment of small RCCs for which surgery is indicated3,4. In the past, open partial nephrectomy was widely performed; however, minimally invasive laparoscopic partial nephrectomy (LPN) or robotic-assisted laparoscopic partial nephrectomy (RAPN) have recently become the standard of treatment for small RCCs5,6,7,8.
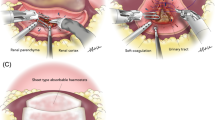
With regard to the RAPN technique, renorrhaphy using a sliding clip technique with two layers of sutures in the medulla and cortex has been widely used9. Reconstruction of the renal parenchyma by renorrhaphy may damage the normal renal vessels and affect renal blood flow, causing ischemic changes in the remaining renal parenchyma8. Single-layer renorrhaphy, in which the cortical suture is omitted and only the medullary suture is performed, is also being widely adopted9. Single-layer renorrhaphy is considered more advantageous than double-layer renorrhaphy, not only in avoiding postoperative complications, such as pseudoaneurysm, but also in preserving normal renal parenchymal volume and postoperative renal function8,10. We hypothesized that renorrhaphy may induce excessive inflammation of the renal parenchyma, resulting in decreased renal function. In terms of evaluating inflammatory status, C-reactive protein, neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) are known as easily measurable markers that reflect the general inflammatory status11. The relationship between renorrhaphy and inflammatory markers has not yet been elucidated. In addition, we considered the possibility that damage to the normal renal parenchyma caused by renorrhaphy could lead to persistent chronic inflammation and future deterioration of renal function. This study aimed to investigate the association between renorrhaphy performance and these inflammatory markers which explain inflammatory status of the body, and their influence on postoperative renal function.
Materials and methods
Patients
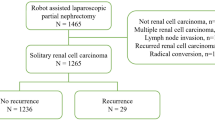
We conducted a retrospective multicenter cohort study including patients with RCC who underwent RAPN at eight institutions in Japan between April 2016 and November 2023. Eligible patients were those with RCC < 7 cm in diameter and without lymph node or distant metastases, based on the classification of clinical stage according to the 8th edition of the American Joint Committee on Cancer Staging Manual12. We excluded patients younger than 20 years, those who did not consent to participate in the study, and those with missing data for the analysis of this study. Patients whose renal vein was clamped during RAPN and patients with an open urinary tract during procedures were also excluded, because these factors could affect the postoperative inflammatory response.
Variables
The preoperative characteristics of the patients enrolled in the study included age, sex, body mass index (BMI), Eastern Cooperative Oncology Group Performance Status (ECOG-PS), and the presence of comorbidities, such as hypertension and diabetes. The following preoperative clinical characteristics were investigated: tumor size, R.E.N.A.L. nephrometry score13, preoperative hemoglobin level, preoperative CRP level, preoperative NLR, and preoperative PLR. Tumor location, depth, and other characteristics were assessed using the R.E.N.A.L. nephrometry score, which consists of (R)adius (maximum diameter of the tumor), (E)xophytic/endophytic features of the tumor, (N)earness of the tumor deepest (A)anterior/posterior descriptors, and (L)ocation relationship to the polar line13. Surgical outcomes of RAPN were investigated in terms of operative time, warm ischemia time (WIT), estimated blood loss (EBL), presence of renal revascularization, and achievement rate of Trifecta. Trifecta criteria were defined as negative tumor resection margins, WIT within 25 min, and no perioperative complications14. Perioperative outcomes were evaluated with regard to postoperative hospital stay, the rate of change in estimated glomerular filtration rate (eGFR; eGFR-r), The rate of change in CRP at 28 days postoperatively (CRP-r 28d), the rate of change in NLR at 28 days postoperatively (NLR-r 28d), and the rate of change in PLR at 28 days postoperatively (PLR-28d). The rate of change in eGFR at 28 days and 1 year postoperatively (eGFR-r 28d and eGFR-r 1y, respectively) was the ratio of preoperative to postoperative eGFR. CRP-r 28d, NLR-r 28d, and PLR-r 28d were also the ratio of preoperative to postoperative value. eGFR was calculated using the Modification of Diet in Renal Disease 2 formula modified for Japanese patients by the Japanese Society of Nephrology: eGFR = 1.94 × serum creatinine level × 1.094 × age × (0.739 for women)15.
Statistical analysis
The primary endpoint of this study was the association between cortical renorrhaphy performance and postoperative inflammatory markers. Secondary endpoints were perioperative outcomes and postoperative changes in renal function, with or without cortical renorrhaphy, at the time of RAPN. The enrolled patients were divided into two groups: those who underwent cortical renorrhaphy (renorrhaphy group) and those who did not (omitted group). The patients with only medullary renorrhaphy were divided into omitted group. All statistical analyses were performed using EZ-R software version 1.61 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Patient characteristics were described as median and interquartile range for continuous variables or frequency (percentage) for categorical variables. Continuous variables were analyzed using the Mann-Whitney U test, and categorical variables were analyzed using Fisher’s exact test. As this was a multicenter study, the Mann-Whitney U test was used to statistically assess the enrolled patients because the data did not follow a normal distribution. A 1:1 propensity score matching by logistic regression analysis using a caliper width of 0.2 was performed to minimize imbalances between the two groups for variables potentially affecting perioperative outcomes, including age, sex, BMI, ECOG-PS, clinical Tumor stage, tumor size, R.E.N.A.L. nephrometry score, preoperative hemoglobin, preoperative CRP, preoperative NLR, and preoperative PLR. Statistical significance for all comparisons was set at a two-sided P-value < 0.05.
Institutional Review Board approval
This study was conducted with the approval of the Institutional Review Board (IRB) of Mie University (approval number: H2022-114). As this study was conducted using a retrospective cohort approach, instead of obtaining informed consent from each eligible patient, consent was assumed to have been obtained from the patients by the opt-out method. According to Japanese ethics committees and ethical guidelines, written consent is not required for retrospective cohort studies that use existing data because the research information is publicly available. Need for informed consent to participate was waived by an IRB of Mie University. The details of this retrospective study are available in Japanese at the following URL.: https://mie.bvits.com/rinri/publish_document.aspx?ID=3184 (accessed June 16, 2024).
Results
Patient characteristics
A total of 934 patients were enrolled in this study. Table 1 shows the patient characteristics and clinical data of the renorrhaphy and omitted groups. The median age, BMI, and tumor diameter of all enrolled patients were 64.0 years, 24.4 kg/m2, and 22.0 mm, respectively. The renorrhaphy group had a significantly younger age, better ECOG-PS, higher T-stage, higher R.E.N.A.L nephrometry score, and higher preoperative hemoglobin levels than the omitted group did.
Surgical outcomes, postoperative renal function, and inflammatory markers
Table 2 compares surgical outcomes, postoperative renal function, and inflammatory markers after RAPN with and without cortical renorrhaphy. The omitted group had a significantly shorter hospital stay and shorter WIT than the renorrhaphy group did. The eGFR-r 28d and eGFR-r 1y in the renorrhaphy group were significantly worse than those in the omitted group. The RAPN-related complication rates were not significantly different between the groups (P = 0.198; data not shown). In terms of inflammatory markers, the PLR-r 28d in the renorrhaphy group was significantly higher than omitted group.
Patient characteristics after propensity score matching
The patient characteristics after propensity score matching are presented in Table 3. After matching, each group was assigned 253 patients, and no significant differences in any comparison were observed between two groups.
Surgical outcomes, postoperative renal function, and inflammatory markers after propensity score matching
Table 4 shows parameters of surgical outcomes and postoperative outcomes with and without cortical renorrhaphy after propensity score matching. The omitted group had a significantly shorter hospital stay, shorter operative time, shorter WIT than the renorrhaphy group did. Furthermore, the omitted group had a significantly higher rate of Trifecta achievement than the renorrhaphy group did. The reason for the lower trifecta achievement rate in the renorrhaphy group was that significantly more patients had a WIT of more than 25 min. The incidence of other complications was not significantly different between the two groups. CRP-r 28d and NLR-r 28d were not significant difference in the two groups. In contrast to the other two inflammatory markers, PLR-r 28d was significantly higher in renorrhaphy group than omitted group. There was a trend toward less eGFR-r 28d and eGFR-r 1y in omitted group than renorrhaphy group, but no significant difference between the two groups.
Discussion
Various current guidelines recommend partial nephrectomy by open, laparoscopic, or robotic-assisted approaches depending on the skill and experience of each surgeon and the availability of instruments3,4. Several studies have shown that LPN has lower EBL and shorter WIT, compared with open partial nephrectomy, while having similar oncologic outcomes16,17. Furthermore, RAPN was superior to LPN in terms of fewer complications and shorter WIT and operative time18. With regard to renal function, reduction of renal function is more dependent upon reduced renal volume rather than upon WIT19. A study using propensity score-matched analysis revealed that the renal parenchymal volume preserved was greater with RAPN than LPN (89% vs. 77%)20. We hypothesized that spillover of renal parenchymal inflammation caused by cortical suture may be a possible trigger for the development of renal dysfunction after RAPN surgery. In general, renorrhaphy is performed to prevent postoperative hemorrhage and urine leakage8. Most cases of renorrhaphy use a two-layer surgical suture with a sliding clip technique8. The advantage of this technique is understood to be a significant reduction in overall operative time and a shorter WIT8. However, the disadvantage of this technique is that it may decrease the amount of residual renal parenchyma due to decreased renal blood flow by tightening the renal parenchyma more than necessary, and consequently, it has been suggested that it may affect postoperative renal function8. Therefore, single-layer renorrhaphy with only medullary sutures has recently been reported8,21. Compared with single-layer renorrhaphy, double-layer renorrhaphy has been reported to result in a longer operative time and WIT as well as poorer postoperative renal function8,21. In prior studies regarding two renorrhaphy techniques, it has also been stated that double-layer renorrhaphy has a longer WIT and operative time and greater parenchymal damage, compared with single-layer renorrhaphy9,19,22. Therefore, when performing RAPN, it may be advisable to avoid suturing the renal parenchyma to preserve renal function after surgery. This study showed renorrhaphy group has not only a longer operative time and longer WIT, but a longer hospital stay than omitted group. The operative time was statistically shorter in the omitted group than in the renorrhaphy group because renorrhaphy was not performed in the omitted group. In contrast, the volume of bleeding was small in both groups because adequate hemostasis was performed before the renal artery was released.
In the present study, PLR-r 28d was significantly high in patients with cortical renorrhaphy after RAPN. Platelets are widely known as one of the blood cells that work on both hemostasis and inflammatory reactions11. As a biomarker of inflammatory status due to various factors, PLR as well as CRP and NLR are easily measurable markers that reflect the inflammatory status of the body11. In addition to platelet-derived growth factors promoting tumor growth and invasion, cytokines and chemokines produced by platelets have also been shown to contribute to inflammation in the body23,24. With regard to the association between PLR and oncologic outcomes of RCC, a meta-analysis of 44 studies including 15,193 patients with RCC showed that high PLR was associated with poor overall, cancer-specific, recurrence-free, disease-free, and metastasis-free survival24. With respect to inflammatory markers, PLR has been reported to be superior to NLR in predicting chronic inflammation25,26. Similar reports have been made in other diseases, showing that NLR increases and PLR decreases in the early stages of diabetes but PLR increases compared with NLR in the chronic phase of the disease27. For this reason, PLR may reflect inflammation in a more chronic phase, whereas NLR reflects inflammation in a relatively early phase27,28. Therefore, we considered the possibility that renorrhaphy may induce chronic inflammation of the renal parenchyma, which subsequently affects renal function. Regarding the association between performance of renorrhaphy and inflammatory markers in this study, there was no significant difference between CRP-r 28d and NLR-r 28d, while PLR-r 28d showed a significant difference in two groups. This suggests that PLR elevation in renorrhaphy group may reflect a renorrhaphy-induced chronic inflammation of the renal parenchyma, which may have further affected long-term renal function after RAPN. As for renal function in patients with immunoglobulin A nephropathy, PLR has been reported to be a prognostic factor that may predict long-term deterioration of renal function28. The previous study showed the higher PLR group began to decline after 1 year of the observation compared to the lower PLR group, and the difference continued to widen, described in Kaplan-Meier curve28. Because the present study investigated renal function only until 1 year after RAPN, the association between PLR after RAPN and long-term deterioration of renal function could not be shown. However, a significant decrease in e-GFR was observed in the renorrhaphy group when patients were enrolled without matching backgrounds, as shown in Table 2, although there was no statistically significant difference in e-GFR between the two groups at 1 year after propensity score matching. Therefore, it is necessary to further extend the observation period and monitor the evolution of renal function over time, although high PLR levels may lead to a decline in renal function.
This study has some limitations. First, this was a retrospective study using data from multiple institutions. Therefore, differences in the clinical diagnosis and surgical methods at each institution could have biased this study. Because various surgeons performed the RAPN, the technical skills of each surgeon may have affected the results of this study. We conducted propensity score matching with the total RENAL nephrometry score. This may cause bias depending on each factor of RENAL nephrometry score. Second, the number of patients enrolled in this study was relatively small and the follow-up period was relatively short. Therefore, the long-term oncological outcomes and course of renal function remain unclear. Third, the volume of the remaining kidney after RAPN was not evaluated in this study. Therefore, the effect on renal function could not be assessed. Finally, we could not ignore the possibility that surgery-related factors, such as the type of robot and instruments used, timing of arterial clamping, and use of renal protection material, may have influenced the study results because these factors varied among institutions.
Conclusions
To the best of our knowledge, this is the first study to evaluate the association between PLR, a marker of inflammation, and renorrhaphy performance after RAPN. In the present study, renorrhaphy was significantly associated with PLR-r 28d after RAPN. The results of this study suggest that renorrhaphy may affect chronic inflammation of the renal parenchyma. Further studies on the associations among chronic renal inflammation, renorrhaphy, and long-term renal dysfunction are warranted.
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Sasaki, T. et al. Urological cancer statistics on incidence from 1975 to 2019 and mortality from 1958 to 2022 in Japan. Int. J. Clin. Oncol. 29, 1088–1095 (2024).
Padala, S. A. et al. Epidemiology of renal cell carcinoma. World J. Oncol. 11, 79–87 (2020).
Ljungberg, B. et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur. Urol. 82, 399–410 (2022).
Campbell, S. C. et al. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline: part I. J. Urol. 206, 199–208 (2021).
Porpiglia, F. et al. Partial nephrectomy in clinical T1b renal tumors: multicenter comparative study of open, laparoscopic and robot-assisted approach (the RECORd Project). Urology 89, 45–53 (2016).
Calpin, G. G., Ryan, F. R., McHugh, F. T. & McGuire, B. B. Comparing the outcomes of open, laparoscopic and robot-assisted partial nephrectomy: a network meta-analysis. BJU Int. 132, 353–364 (2023).
Novara, G. et al. Robot-assisted partial nephrectomy. Int. J. Surg. 36, 554–559 (2016).
Ito, H. et al. Impact of robotic-assisted partial nephrectomy with single layer versus double layer renorrhaphy on postoperative renal function. Curr. Oncol. 31, 2758–2768 (2024).
Benway, B. M., Wang, A. J., Cabello, J. M. & Bhayani, S. B. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur. Urol. 55, 592–599 (2009).
Bertolo, R. et al. Suture techniques during laparoscopic and robot-assisted partial nephrectomy: a systematic review and quantitative synthesis of peri‐operative outcomes. BJU Int. 123, 923–946 (2019).
Wagner, D. D. New links between inflammation and thrombosis. Arterioscler. Thromb. Vasc Biol. 25, 1321–1324 (2005).
Paner, G. P. et al. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur. Urol. 73, 560–569 (2018).
Kutikov, A. & Uzzo, R. G. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 182, 844–853 (2009).
Khalifeh, A. et al. Comparative outcomes and assessment of trifecta in 500 robotic and laparoscopic partial nephrectomy cases: a single surgeon experience. J. Urol. 189, 1236–1242 (2013).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992 (2009).
Lane, B. R. & Gill, I. S. 5-Year outcomes of laparoscopic partial nephrectomy. J. Urol. 177, 70–74 (2007).
Lane, B. R. & Gill, I. S. 7-Year oncological outcomes after laparoscopic and open partial nephrectomy. J. Urol. 183, 473–479 (2010).
Masson-Lecomte, A. et al. A prospective comparison of surgical and pathological outcomes obtained after robot‐assisted or pure laparoscopic partial nephrectomy in moderate to complex renal tumours: results from a French multicentre collaborative study. BJU Int. 111, 256–263 (2013).
Shatagopam, K., Bahler, C. D. & Sundaram, C. P. Renorrhaphy techniques and effect on renal function with robotic partial nephrectomy. World J. Urol. 38, 1109–1112 (2020).
Tachibana, H. et al. Robot-assisted laparoscopic partial nephrectomy versus laparoscopic partial nephrectomy: a propensity score‐matched comparative analysis of surgical outcomes and preserved renal parenchymal volume. Int. J. Urol. 25, 359–364 (2018).
Bahler, C. D. & Sundaram, C. P. Effect of renal reconstruction on renal function after partial nephrectomy. J. Endourol. 30, S37–S41 (2016).
Moreno Cortes, J. C., Garcia, J. G., Velasco, J. C., Chamizo, J. A. & Rios, D. S. Reconstruction techniques after partial nephrectomy: classic vs. sutureless approach-A narrative review. Curr. Urol. Rep. 25, 49–54 (2024).
Yamamoto, T., Kawada, K. & Obama, K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int. J. Mol. Sci. 22, 8002 (2021).
Zhou, X. & Luo, G. A meta-analysis of the platelet-lymphocyte ratio: a notable prognostic factor in renal cell carcinoma. Int. J. Biol. Mark. 37, 123–133 (2022).
Meng, X. et al. The platelet-to-lymphocyte ratio, superior to the neutrophil-to-lymphocyte ratio, correlates with hepatitis C virus infection. Int. J. Infect. Dis. 45, 72–77 (2016).
Turkmen, K. et al. Platelet-to‐lymphocyte ratio better predicts inflammation than neutrophil‐to‐lymphocyte ratio in end‐stage renal disease patients. Hemodial. Int. 17, 391–396 (2013).
Mertoglu, C. & Gunay, M. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Meab Syndr. 11, S127–S131 (2017).
Chang, D. et al. The prognostic value of platelet-to-lymphocyte ratio on the long-term renal survival in patients with IgA nephropathy. Int. Urol. Nephrol. 53, 523–530 (2021).
Author information
Authors and Affiliations
Contributions
Tomoki Taniguchi: Data analysis, manuscript writing/editing, data collection and management; Kentaro Muraoka: data collection and management; Kohei Nishikawa: data collection and management; Yoshinori Ikehata: data collection and management; Kiyoshi Setoguchi: data collection and management; Suguru Oka: data collection and management; Shin Ebara: data collection and management; Akira Fujisaki: data collection and management; Kazuhide Makiyama: data collection and management; Takahiro Inoue: data collection and management, review; Hiroshi Kitamura: data collection and management; Kazutaka Saito: data collection and management; Shinji Urakami: data collection and management; Tatsuaki Yoneda: data collection and management; Takuya Koie: Protocol/project development, data management, manuscript writing/editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Mie University (January 4, 2023 / H2022-114).
Consent to participate
Owing to the retrospective observational nature of this study, the need for informed consent was waived.
Consent for publication
Patient consents were not applicable for this retrospective study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Taniguchi, T., Muraoka, K., Nishikawa, K. et al. Impact of platelet-lymphocyte ratio after robot-assisted partial nephrectomy with renorrhaphy. Sci Rep 14, 30986 (2024). https://doi.org/10.1038/s41598-024-82197-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82197-x