Abstract
Relative anterior microphthalmos (RAM) is a rare ocular condition characterized by disproportionately small anterior segments but normal axial length (corneal diameter < 11 mm and axial length > 20 mm). This study aimed to determine the prevalence of RAM and its association with glaucoma utilizing IOL Master 700 data (Carl Zeiss Meditec, Jena, Germany). A retrospective analysis was conducted of the biometric parameters of 6,407 eyes, and 115 cases of RAM were identified. The incidence of glaucoma was assessed, together with the outcomes of cataract surgery in cases of RAM with glaucoma. RAM prevalence was 1.8%. RAM patients had a higher incidence of glaucoma (26.1%), notably of the angle-closure subtype. Cataract surgery significantly reduced intraocular pressure in cases of RAM with glaucoma; however, RAM patients experienced a higher rate of surgical complications. RAM poses clinical challenges due to its association with glaucoma and increased surgical risks. This study emphasizes the importance of advanced diagnostic tools such as the IOL Master in tailoring interventions to optimize patient outcomes.
Similar content being viewed by others
Introduction
Small eye spectrum is a type of eye derangement resulting from developmental arrests during embryogenesis. Although uncommon, it can cause serious ophthalmic issues, ranging from developmental visual problems to frequent occurrences of associated conditions such as glaucoma and some surgery-related complications following refractive correction and cataract surgery1,2. There are diverse “small eye” variants, ranging from pan-ocular size reduction, such as nanophthalmos and microphthalmos, to disproportionate smallness of specific parts of the eye, either in the anterior or posterior segment, such as relative anterior microphthalmos (RAM) and posterior microphthalmos (PM)3.
The term relative anterior microphthalmos was first used by Naumann in 1980 to define an eye with normal axial length (≥ 21 mm) but disproportionately smaller anterior segment (corneal diameter ≤11 mm)4. Auffarth et al. conducted a study of 2000 patients with RAM morphometry and characterized RAM as an eye with horizontal corneal diameters ≤ 11 mm, axial length of > 20 mm, but no other morphologic malformations5. These characteristics have resulted in increased surgical difficulty and complications, including postoperative transient corneal edema (up to 75%), uveal inflammation (12%) associated with surgical trauma and presence of small pupil, Descemet’s membrane detachment (5.95%), and posterior capsule ruptures (2.38%)6. Over the years, there has been a scarcity of studies focused on this condition, leading to its potential damage being overlooked, especially with regard to its association with glaucoma and related management.
In recent years, there has been a shift in the standard measurement of intraocular lens (IOL) power calculation for cataract patients from the ultrasonic biometry (A-scan) to non-contact optical biometry. This change eliminates ocular discomfort associated with the contact A-scan technique, whether through applanation or immersion, and is a more convenient method7,8. IOL Master (Carl Zeiss Meditec, Jena, Germany) applies a non-contact coherence inferred diode laser via partial coherence interferometry to measure particular ocular parameters such as keratometry (K), lens thickness (LT), anterior chamber depth (ACD), and axial length (AL)9. This advancement has allowed us to measure ocular parameters accurately. Digital collection of all pertinent data over an extended period is potentially beneficial for research10,11.
The current study utilized automated devices (in particular the IOL Master) alongside optical biometry to determine the prevalence of RAM and to analyze its correlation with various subtypes of glaucoma. Additionally, we investigated cataract surgery and its complications in RAM patients. This study aims to provide valuable insights into the clinical importance of RAM in both glaucoma and cataract surgery, with the potential to shape tailored treatment approaches for individuals managing both conditions concurrently.
Materials/Subjects and methods
A retrospective study of RAM identified with IOL Master was conducted in the tertiary eye care centre of Rajavithi Hospital, Bangkok, Thailand. The research protocol was approved by the hospital’s Ethics Committee (Document number 151/2022), and the study was performed between December 2022 and December 2023, following all of the guidelines for experimental investigation in human subjects required by the Ethics Committee, with informed consent secured from every subject. All investigations were carried out in accordance with the Declaration of Helsinki.
Inclusion criteria were cataract patients requiring lens removal and IOL implantation who underwent IOL Master700 scanning by experienced technicians. Exclusion criteria were patients who had opaque optical media, dense cataract, anterior and/or posterior segment diseases such as corneal scar, advanced pterygium, proliferative diabetic retinopathy, or maculopathy. Participants were also excluded if they had any previous history of eye injury; ocular surgery, such as refractive surgery, cataract, glaucoma (trabeculectomy and/or glaucoma tube shunt); or vitreoretinal surgery.
Ocular parameters, including anterior chamber depth (ACD), lens thickness (LT), total axial length (AL), and white to white (WTW), were assessed by the biometer. Raw data from the IOL Master 700, comprising 15,727 entries, was initially exported to an MS Excel spreadsheet. Following deidentification, pseudophakic eyes and those with inaccurate or unsuccessful measurements were excluded from the dataset. This left a total of 4,385 patients with accurate axial length (AL) and white-to-white (WTW) measurements, corresponding to 8,267 eyes. After eliminating duplicate entries, the final dataset consisted of 4,163 patients, leaving 6,407 eyes for analysis.
Definition of RAM
RAM is defined when ocular parameters from IOL Master 700 measurement meet all of the following criteria:
Definition of glaucoma
Primary open-angle glaucoma is defined as an eye with glaucomatous optic neuropathy with or without visual field defect, gonioscopic open angle, and IOP > 21 mmHg. Normal-tension glaucoma is defined as POAG with IOP ≤ 21 mmHg.
Primary angle-closure disease (PACD) included primary angle-closure suspect (PACS), primary angle-closure (PAC), and primary angle-closure glaucoma (PACG) as classified by Foster et al.13 PACS was defined as Shaffer’s gonioscopic grading ≤ 2 in at least 2 quadrants, with normal IOP and without glaucomatous optic disc and visual field defect; PAC was classified as PACS with IOP > 21 mmHg, presence of PAS and without any glaucomatous changes; and PACG was defined as PAC with glaucomatous changes.
Using retrospective chart review, data of the RAM group were extracted, including biographic data, measured ocular parameters, and other associated ocular conditions such as corneal guttata and glaucoma. For patients who underwent cataract surgery, we collected additional information such as age, sex, preoperative and postoperative intraocular pressure (IOP), and antiglaucoma medication usage, as well as surgical complications.
Glaucoma-related parameters were taken from the 3 months preceding the date of IOL Master measurement. If there were more than 2 values, we chose the most recent one prior to IOL measurement. Intraocular pressure measurements were performed using Goldmann applanation if it was accessible. Preoperative data were extracted from the latest outpatient department visit to the surgery day or admission day, while postoperative data were collected during the 1-month postoperative period. Surgeons were categorized as senior (ophthalmologists with more than 5 years of experience) and junior (resident, fellow or ophthalmologists with 1 to 5 years of experience). Additionally, complications from surgery and their potential associated risks were evaluated. Corneal decompensation, suprachoroidal hemorrhage, retinal detachment, and endophthalmitis were considered to be serious surgical complications14,15.
Statistical analysis
Statistical analysis was performed with SPSS software version 28 (SPSS Inc., Chicago, IL, USA). Continuous variables (ocular parameters, age, IOP, and number of medications) were reported as mean with standard deviation while categorical variables (gender, type of glaucoma, presence of corneal guttata) were presented as percentages. Differences in ocular parameters in two different groups were assessed by independent T-test and Mann-Whitney U Test. Paired Sample T-test was used to evaluate the change in IOP and the number of medications needed before and after cataract surgery. Factors associated with complication risk were calculated using logistic regression analysis and presented as Odds Ratio (OR). P values \(\:\le\:\) 0.05 were considered to be statistically significant.
Results
Out of the 6,407 eyes of 4,163 patients, 115 eyes of 105 patients met the criteria for RAM, indicating a prevalence of 1.8% (115 from 6,407 eyes). Eighty-one cases (77.14%) were female, and 21 (17.4%) were bilateral.
Among the 115 eyes examined, 3 (2.6%) had corneal guttata, and one of these developed corneal decompensation after cataract surgery. Glaucoma was recorded in 30 eyes (26.09%). Detailed demographic data of patients with RAM are presented in Table 1.
Among the glaucoma patients, females were predominantly affected, with 22 eyes in 20 females, while males accounted for only 8 cases. The most frequently encountered type of glaucoma was PACD, succeeded by POAG, secondary glaucoma, and NTG. Nineteen eyes (16.67%) with RAM presented with acute angle-closure crisis (AACC), and 15 (10%) were identified as fellow eyes following a previous AACC attack.
Glaucoma surgery was performed on 6 eyes, involving 1 use of phacoemulsification with intraocular lens implantation (PE-IOL) with goniosynechialysis (PE-GSL) and 5 of trabeculectomy. Laser peripheral iridotomy was performed in 17 eyes with RAM: 9 with PACG, 7 with PAC, and 1 with neovascular glaucoma (NVG).
A comparative analysis of ocular parameters between individuals with and without relative anterior microphthalmos (RAM) revealed significant disparities. In a study comprising 115 eyes with RAM and 6291 without it, median values (interquartile range) were scrutinized for various parameters. RAM eyes, defined by a WTW measurement of ≤ 11 mm and an AL exceeding 20 mm, exhibited notably shallower ACD, shorter AL, and slightly thicker lens compared to non-RAM eyes; however, there was no statistically significant difference between CCT in the two groups. Furthermore, RAM eyes displayed a higher lens-axial length factor (LAF) than non-RAM eyes (2.14 vs. 1.96, p < 0.01). Detailed findings are presented in Table 2. Notably, among the eyes with RAM, 23 (20%) exhibited a LAF greater than 2.3. (ref. 16).
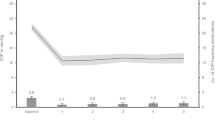
From baseline data in RAM, the mean IOP was 23.43 ± 12.71 mmHg, ranging from 6 to 52 mmHg. The vertical cup-disc ratio was 0.74 ± 0.18, varying from 0.3 to 1.0. On average, patients were using 2.7 ± 1.46 IOP-lowering medications, with the number of medications ranging from 0 to 5.
A comparative analysis of RAM with and without glaucoma is shown in Table 3. ACD and WTW in the glaucoma group was significantly lower than in the non-glaucoma patients.
Cataract surgery and its complications in RAM
Forty-four eyes in the RAM group underwent cataract removal by multiple surgeons, either senior (24 eyes) or junior ophthalmologists (20 eyes). The procedures encompassed different types of cataract surgery: 9 underwent PE-IOL; 7 received PE-GSL; 1 had PE combined with trabeculectomy; 4 underwent PE with pars plana vitrectomy; and 3 extracapsular cataract extractions (ECCE) were performed.
IOP reduction and number of IOP-lowering medications
Cataract surgery resulted in a notable reduction in intraocular pressure (IOP), with a mean preoperative IOP of 18.11 ± 7.82 mmHg decreasing to 14.42 ± 3.26 mmHg postoperatively (p = 0.004). Specifically, in the subgroup of patients with coexisting glaucoma, there was a significant decrease in IOP from 22.47 ± 11.70 mmHg before surgery to 13.40 ± 3.46 mmHg afterwards (p = 0.009). Additionally, surgery led to a significant decrease in the number of IOP-lowering medications required, dropping from 1.07 ± 1.634 preoperatively to 0.48 ± 1.0 at one month postoperatively (p < 0.001).
Surgical complications
Among the 44 eyes with RAM that underwent cataract surgery, a total of 18 complications (40.9%) were observed. These included 14 cases of corneal edema at 1-week post-surgery, 1 posterior capsule rupture, 1 hyphema, 1 retained lens cortex, and 1 case of corneal decompensation, which was identified as the only serious complication in our study. Comparing biometric parameters in cases with and without complications, we found statistically significant differences between ACD and LAF in these groups: ACD in cases with complications was significantly shallower (mean 2.34 ± 0.41 mm vs. 2.62 ± 0.35 mm, p = 0.023) and LAF was significantly higher (2.23 ± 0.20 vs. 2.07 ± 0.26, p = 0.05). No other sight-threatening complications (such as expulsive choroidal hemorrhage or endophthalmitis) occurred.
Several risk factors were identified in association with surgical complications. An ACD of ≤ 2.2 mm (p = 0.023), LAF ≥ 2.3 (p = 0.030), and the involvement of a junior surgeon (p = 0.011) were pinpointed as significant contributors, elevating the likelihood of complications by 5.5, 5.8, and 6.1 times respectively. Interestingly, glaucoma and gender did not exhibit any discernible risk based on crude odds ratios in our analysis. (see Table 4)
Discussion
In this clinical study of RAM, a large dataset of 6,407 eyes was investigated. We identified a lower prevalence of RAM in Thai patients than found in a previous study of Indian people (1.8% versus 6%). This difference in prevalence could stem from various factors, including type of measurement technique used. Nihalani et al. employed manual calipers and A-scan ultrasound to determine RAM, whereas we utilised an optical biometer for measurement, and this may have resulted in variations in parameter values6. For instance, Ang et al. and Baumeister et al. revealed that the IOL Master generally produced larger WTW values than those identified using calipers (12.14 vs. 11.45 and 12.02 vs. 11.91 mm respectively)17,18. Anterior chamber depth and AL can also exhibit discrepancies. Two studies conducted in the same country have shown contrasting results: one demonstrated differing ACD values obtained by the IOL Master and ultrasound in both normal and short eye groups, with the IOL Master tending to yield shallower ACD measurements19, while the other found that the IOL Master obtained deeper ACD measurements than ultrasound20. Additionally, some studies have shown significantly longer AL values measured by the IOL Master than those found using ultrasound, while other research has found no significant difference21,22,23,24. With the newer technology of the ocular biometry that we used, we believe that the prevalence of RAM in the present study is more accurate than previously reported.
In addition, differences in study populations and demographics, such as our focus on a Southeast Asian population compared to studies examining populations from South Asia, may contribute to variations in reported prevalence rates. While there has so far been no direct head-to-head comparison in terms of this parameter, based on our latest understanding, we believe there is a high potential for such differences to exist25. Factors such as genetic predispositions, environmental influences, and socioeconomic factors specific to each population could influence the prevalence rates of conditions like relative anterior microphthalmos. Further research focusing on comparative studies across different populations with this optical biometer could provide valuable insights into these potential variations.
Microphthalmos manifests either as a unilateral or bilateral disease and may be associated with other ocular deformations such as coloboma, or persistent hyperplastic primary vitreous. Fahnehjelm et al. reported that 26 of 35 (74.3%) instances of anophthalmos/microphthalmos were unilateral26. In the present study, RAM presented as a unilateral disease in 82.6% of cases, while 17.4% were bilateral. There was no association with ocular coloboma in those 115 RAM eyes.
Glaucoma association
Our findings reveal a remarkably higher rate of glaucoma within the RAM group compared to that of the general population, 26.1%, vs. 3.54% respectively27,28. Focusing on the closed-angle subtype, it is noteworthy that RAM patients exhibited a higher incidence, with 6.9% of RAM cases presenting with acute angle-closure attacks, whether in the RAM eye or fellow eyes. This incidence is higher than that in the general population in Asia, which typically ranges from 6 to 12 per 100,00029,30. The increased occurrence can be attributed to shallower ACD and a higher LAF in RAM eyes. This raises concerns regarding access to the angle by gonioscopy technique or earlier laser iridotomy in high-risk patients, as indicated by data from the IOL Master. This finding is corroborated by the fact that 16 out of 115 RAM cases necessitated laser peripheral iridotomy based on clinical indications. Consequently, given the significant IOP reduction following cataract extraction, this procedure may also be considered the best treatment for lowering IOP in RAM. Notably, 20% (6 out of 30) of glaucoma cases require glaucoma surgical intervention due to maximally tolerated medical treatment, indicating a higher severity and the necessity for vigilant monitoring.
In our study, we also compared findings between microphthalmos and RAM, emphasizing the distinction based on axial length. By definition, microphthalmos is characterized by an axial length ≤ 20 mm. Among our cohort, six eyes from three patients met this criterion, two of whom had primary angle-closure glaucoma (PACG). The first patient, a 29-year-old woman, had axial lengths of 17.02 mm and 17.36 mm with advanced glaucoma, as evidenced by cup-disc ratios (CD) of 0.85 and 0.9. The eye with a CD of 0.9 underwent a second trabeculectomy and experienced an intraoperative malignant glaucoma event during glaucoma surgery. The second patient, a 60-year-old woman, had axial lengths of 19.29 mm and 19.10 mm and presented with early glaucoma, characterized by CD of 0.6 in both eyes, which was well-controlled with a single topical glaucoma medication. She underwent uncomplicated cataract surgery in one eye. The third patient, a 58-year-old man without glaucoma, had axial lengths of 16.97 mm and 17.16 mm and underwent uneventful piggyback intraocular lens implantation in both eyes. The prevalence of angle-closure disease in this microphthalmos cohort was 66.67%, significantly higher than in the RAM cohort, where it was 17.39%. These findings are consistent with the study by N.Relhan, which reported a similar prevalence of angle-closure disease in 69.23% of microphthalmic eyes31.
Cataract surgery-related complication
In Table 5, we compared surgical complications between our study and the previous one by Nihalani et al.6 While the prior study reported a higher incidence of Descemet’s detachment and uveal inflammation, none of these complications were observed in our study. This apparent anomaly could be due to potential under-reporting in our dataset of minor, self-resolving complications, such as non-visual axis or planar Descemet’s detachment, and uveal inflammation.
This study highlights a significantly higher likelihood of complications in RAM patients compared to general cataract surgery patients, where complication rates are typically under 10%32,33. In the present study, RAM patients experienced transient corneal edema surpassing 30%.
Furthermore, patients with RAM may face elevated risks, particularly when compounded by additional risk factors such as the involvement of less experienced surgeons, ACD ≤ 2.2 mm, and LAF ≥ 2.3. These findings are consistent with those of previous research, which has shown an association between surgical difficulty, increased endothelium damage resulting in post-operative corneal edema, challenges posed by limited working space, and a higher incidence of corneal guttata5,6.
Study strengths and limitations
Current study had a strength of large dataset from a real-world practice. The optical biometer demonstrated a convenient tool to assess RAM. However, the study had some inherent limitations. Firstly, its retrospective nature may have introduced biases and could have resulted in incomplete data, potentially leading to under-reporting of surgical complications and impacting observed prevalence rates and associations. Inconsistencies in non-Goldmann applanation intraocular pressure data could also have further complicated matters. Additionally, as the study was conducted at a single center, the generalizability of our findings to broader populations may be restricted. The involvement of multiple surgeons using varied techniques also complicates the interpretation of surgical outcomes. Despite employing advanced technology like the IOL Master 700, variations in measurement techniques and device calibration may have affected the accuracy and reliability of our measurements. However, these limitations may simply mirror the complexities encountered in real-world scenarios.
Conclusion
This study investigated the prevalence of RAM and its correlation with glaucoma using real-world clinical data and advanced technology in the form of the optical biometer. While the prevalence of RAM was lower than previously thought, we found a higher incidence of glaucoma among RAM patients, especially in the closed-angle subtype. This underscores the importance of tailored management strategies for treatment of RAM patients with concurrent glaucoma in order to optimize outcomes and minimize complications. Additionally, early detection and preventive interventions, such as laser peripheral iridotomy, are crucial to mitigate the risk of acute angle-closure crises. These findings offer insights into the clinical importance of RAM, with the IOL Master serving as a valuable diagnostic tool, informing future interventions into these complex conditions.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the exceptionally large data size but are available from the corresponding author on reasonable request.
References
Hoffman, R. S. et al. Cataract surgery in the small eye. J. Cataract Refract. Surg. 41 (11), 2565–2575 (2015).
Bateman, J. B. & Microphthalmos Int. Ophthalmol. Clin. ;24(1):87–107. (1984) Spring.
Carricondo, P. C., Andrade, T., Prasov, L., Ayres, B. M. & Moroi, S. E. Nanophthalmos: a review of the Clinical Spectrum and Genetics. J. Ophthalmol. 2018, 2735465 (2018).
Naumann, G. O. H. Pathologie Des Auges 2nd edn p.1264–1265 (Springer, 1997).
Auffarth, G. U., Blum, M., Tetz, M. R. & Völcker, H. E. Relative anterior microphthalmos morphometric analysis and its implications for cataract surgery. Ophthalmology 107 (8), 1555–1560 (2000).
Nihalani, B. R., Jani, U. D., Vasavada, A. R. & Auffarth, G. U. Cataract surgery in relative anterior microphthalmos. Ophthalmology 112 (8), 1360–1367 (2005).
Wanichwecharungruang, B. et al. Clinical evaluation of ocular biometry of dual Scheimpflug analyzer, GALILEI G6 and swept source optical coherence tomography. ANTERION Sci. Rep. ;12(1). (2022).
Wanichwecharungruang, B., Amornpetchsathaporn, A., Wongwijitsook, W., Kongsomboon, K. & Chantra, S. Evaluation of ocular biometry in primary angle-closure disease with two swept source optical coherence tomography devices. PLoS One. 17 (3), e0265844 (2022).
Hirzenberger, C. K. & Hitzenberger, C. K. Optical measurement of the Axial Eye length by laser Doppler Interferometry. Invest. Ophthalmol. Vis. Sci. 32 (3), 616–624 (1991).
Findl, O., Drexler, W., Menapace, R., Hitzenberger, C. K. & Fercher, A. F. High precision biometry of pseudophakic eyes using partial coherence interferometry. J. Cataract Refract. Surg. 24 (8), 1087–1093 (1998).
Rajan, M. S., Keilhorn, I. & Bell, J. A. Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculations. Eye 16 (5), 552–556 (2002).
Jung, K. I., Yang, J. W., Lee, Y. C. & Kim, S. Y. Cataract surgery in eyes with Nanophthalmos and relative anterior microphthalmos. Am. J. Ophthalmol. 153 (6), 1161–1168e1 (2012).
Foster, P. J., Buhrmann, R., Quigley, H. A. & Johnson, G. J. The definition and classification of glaucoma in prevalence surveys. Br. J. Ophthalmol. 86 (2), 238–242 (2002).
Stein, J. D. Serious adverse events after cataract surgery. Curr. Opin. Ophthalmol. 23 (3), 219–225 (2012).
Lin, I. H. et al. Predisposing factors for severe complications after cataract surgery: a Nationwide Population-based study. J. Clin. Med. 10 (15), 3336 (2021).
Markowitz, S. N. & Donald Morin, J. The ratio of Lens thickness to axial length for biometric standardization in Angle-Closure Glaucoma. Am. J. Ophthalmol. 99 (4), 400–402 (1985).
Ang, R. E. T., Reyes, E. K. F., Ayuyao, F. A. J., Umali, M. I. N. & Cruz, E. M. Comparison of white-to-white measurements using four devices and their determination of ICL sizing. Eye Vis. 9 (1), 36 (2022).
Baumeister, M., Terzi, E., Ekici, Y. & Kohnen, T. Comparison of manual and automated methods to determine horizontal corneal diameter. J. Cataract Refract. Surg. 30 (2), 374–380 (2004).
Dong, J. et al. Comparison of axial length, anterior chamber depth and intraocular lens power between IOL Master and ultrasound in normal, long and short eyes. PLoS One. 13 (3), e0194273 (2018).
Bai, Q. H., Wang, J. L., Wang, Q., Yan, Q. C. & Zhang, J. S. The measurement of anterior chamber depth andaxial length with the IOL Master compared with contact ultrasonic axial scan. Int. J. Ophthalmol. 1, 151–154 (2007).
Gaballa, S. H., Allam, R. S., Abouhussein, N. B. & Raafat, K. A. IOL Master and A-scan biometry in axial length and intraocular lens power measurements. Delta J. Ophthalmol. 18 (1), 13–19 (2017).
Rathod, S. et al. A study on comparison of axial length and IOL Power in A-Scan Biometry versus IOL Master. J. Evid. Based Med. Healthc. 7 (12), 587–590 (2020).
Gopi, R. & Sathyan, S. Comparison of ocular biometry parameters between IOL Master and applanation A-scan in eyes with short, medium, long, and very long axial lengths. Kerala J. Ophthalmol. 29 (1), 35–40 (2017).
Rose, L. T. & Moshegov, C. N. Comparison of the Zeiss IOL Master and applanation A-scan ultrasound: biometry for intraocular lens calculation. Clin. Exp. Ophthalmol. 31 (2), 121–124 (2003).
Blake, C. R., Lai, W. W. & Edward, D. P. Racial and ethnic differences in ocular anatomy. Int. Ophthalmol. Clin. 43 (4), 9–25 (2003).
Fahnehjelm, C. et al. Anophthalmia and microphthalmia in children: associated ocular, somatic and genetic morbidities and quality of life. Ophthalmic Genet. 43 (2), 172–183 (2022).
Tham, Y. C. et al. Global prevalence of Glaucoma and projections of Glaucoma burden through 2040. Ophthalmology 121 (11), 2081–2090 (2014).
Allison, K., Patel, D. & Alabi, O. Epidemiology of Glaucoma: the past, Present, and predictions for the future. Cureus 12 (11), e11686 (2020).
Park, S. J., Park, K. H., Kim, T. W. & Park, B. J. Nationwide incidence of Acute Angle Closure Glaucoma in Korea from 2011 to 2015. J. Korean Med. Sci. 34 (48), e306 (2019).
Seah, S. K. et al. Incidence of Acute Primary Angle-closure Glaucoma in Singapore. Arch. Ophthalmol. 115 (11), 1436–1440 (1997).
Relhan, N. et al. High-hyperopia database, part I: clinical characterisation including morphometric (biometric) differentiation of posterior microphthalmos from nanophthalmos. Eye (Lond). 30 (1), 120–126 (2016).
Tufail, A., Foss, A. J. & Hamilton, A. M. Is the first day postoperative review necessary after cataract extraction? Br. J. Ophthalmol. 79 (7), 646–648 (1995).
Tan, J. H., Newman, D. K., Klunker, C., Watts, S. E. & Burton, R. L. Phacoemulsification cataract surgery: is routine review necessary on the first post-operative day? Eye 14 (1), 53–55 (2000).
Acknowledgements
We gratefully acknowledge the funding provided by Rajavithi Hospital in support of this research endeavor.
Funding
Rajavithi Hospital.
Author information
Authors and Affiliations
Contributions
KA was responsible for the overall direction and planning of the study and performed the analysis. WS contributed to interpreting the results and wrote the manuscript with support from KS. MC and NA processed the experimental data. KK provided statistical expertise. BW conceived the initial idea for the study and proofread the manuscript outline. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Annopawong, K., Sriyuttagrai, W., Chainakul, M. et al. Prevalence and clinical associations of relative anterior microphthalmos assessed with an optical biometer. Sci Rep 14, 31026 (2024). https://doi.org/10.1038/s41598-024-82246-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82246-5
This article is cited by
-
Risk factors for glaucoma in nanophthalmos – a systematic review and meta-analysis
BMC Ophthalmology (2025)