Abstract
Anogenital distance (AGD) is regarded as a potential biomarker for endometriosis, and a measurement on MRI images has been found to be promising. This study aimed to evaluate the measurement of AGD on MRI to predict the surgical diagnosis of endometriosis. We included 127 patients who received an MRI for endometriosis between October 2018 and February 2023. AGD was measured on MRI by two readers (MRI-AGD-AC: clitoris to anus; MRI-AGD-AF: posterior fourchette to anus). The feasibility and interobserver reliability of AGD measurements were evaluated. Differences in AGD between patient groups were analyzed. The intraclass correlation coefficient estimates indicated a good to excellent reliability of MRI-AGD-AC (0.92; 95% CI: 0.83–0.95) and a poor to good reliability of MRI-AGD-AF (0.68; 95% CI: 0.27–0.83). No statistically significant differences in the mean MRI-AGD-AC and MRI-AGD-AF in patients with and without surgical diagnosis of DIE (p = 0.413; p = 0.110), peritoneal endometriosis with and without DIE (p = 0.641; p = 0.323), and ovarian endometriosis (p = 0.155; p = 0.150) were found. The AUC ranged from 0.475 (95% CI: 0.365–0.584) to 0.586 (95% CI: 0.454–0.718). Thus, AGD does not constitute a valuable biomarker for patients with clinically suspected endometriosis.
Similar content being viewed by others
Introduction
Anogenital distance (AGD) is the distance between the anus and the genital tubercle that is present during the development of the reproductive system. Animal and human studies have shown that AGD is dependent on the early prenatal hormonal environment1,2. A longer AGD is considered to be a consequence of androgen exposure, and a shorter AGD a consequence of estrogen exposure. The AGD is regarded as a potential biomarker for various urogenital diseases, including hypospadias, prostate cancer, polycystic ovary syndrome (PCOS), and endometriosis3. As there currently is no test that can definitively confirm or rule out endometriosis non-invasively (although imaging is considered valuable4,5,6), the identification of biomarkers for this common disease is of interest7, with recent advances in the utilization of a saliva-based micro-ribonucleic acid (miRNA) signature for endometriosis8. About 10–15% of women of childbearing age are affected by endometriosis9. Several studies have shown promising results in predicting endometriosis based on AGD10,11,12,13, utilizing two clinical measurements: From the upper verge of the anus to the anterior surface of the clitoris (AGD-AC) and to the posterior fourchette (AGD-AF). Crestani et al. found that measurement of the AGD on MRI is more accurate than clinical measurement, and they reported significantly different values for AGD in endometriosis (n = 67) and non-endometriosis (n = 31) groups14. Conversely, Buggio et al. found no significant association between clinical AGD measurements and the presence of endometriosis in a case-control study, including 45 patients with deep infiltrating endometriosis (DIE), 45 patients with ovarian endometriomas and 45 controls15. Although all patients in the study by Crestani et al. underwent surgery, the patients in the control group were operated on for various different reasons (e.g., myomectomies, sacrocolpopexies), resulting in heterogeneity between the endometriosis group and the non-endometriosis group14. In the study by Buggio et al., the control group also came from a different population than the disease groups (e.g., periodic well-woman visits, cervical cancer screening)15.
Since it is necessary to validate a supposed biomarker on a study population that reflects the target population in which it will be used, we conducted a study on a collective of patients who had not undergone prior abdominal surgery, had an MRI scan for endometriosis and had subsequently undergone surgery within a maximum of one year of the MRI examination. This study aimed to compare measurements of MRI-AGD-AC and MRI-AGD-AF between patients with and without a subsequent surgical diagnosis of DIE, peritoneal endometriosis with and without DIE, and ovarian endometriosis (endometriomas). The value of AGD for the prediction of endometriosis was to be evaluated.
Methods
This study was approved by the Ethics Committee of the Faculty of Medicine, Justus Liebig University Giessen (reference: 209/22). Informed consent was waived for this retrospective analysis by the Ethics Committee of the Faculty of Medicine, Justus-Liebig-University Giessen. All research was performed in accordance with relevant guidelines and regulations.
Study population and design
All patients aged at least 18 years who had received a pelvic MRI for endometriosis between October 2018 and February 2023 were consecutively included. Patients who had not undergone surgery within one year of the MRI examination (as the disease status might have changed over an extended period16), patients with prior abdominal surgeries, and patients with insufficient coverage of the sagittal T2 sequence were excluded.
MRI scans were conducted at two 1.5 Tesla scanners (n = 70; n = 56) and one 3 Tesla scanner (n = 1). The MRI protocol included T2-weighted FSE (fast spin echo) sequences of the pelvis (axial, sagittal, coronal) as recommended in current guidelines17,18. The further sequences acquired were not relevant for the evaluation presented here. Patient preparation included rectal opacification with water (n = 121) and vaginal opacification with ultrasound gel (n = 107). These measures are considered optional or recommended conditionally in current guidelines and are performed by many institutions17,18. A shift of the anatomical landmarks relevant for the measurement of AGD is not to be expected by vaginal gel or rectal water given the rigid fibrous skeleton constituted by the interposed perineal body. Nevertheless, significant differences between the measurements performed on examinations with and without vaginal opacification have been excluded, as described in the results section.
Image analysis
AGD measurements were performed independently by a reader with eight years of experience in pelvic MRI (reader 1, radiologist) and a reader not experienced in pelvic MRI (reader 2, advanced medical student). Reader 2 received a detailed tutorial on how to identify the relevant anatomical structures on T2-weighted images of the pelvis by reader 1 in advance. Measurements of AGD were performed on sagittal T2-weighted images from the anus to the clitoris (MRI-AGD-AC) and from the anus to the posterior fourchette (MRI-AGD-AF) as demonstrated in Fig. 1.
Two examples of anogenital distance (AGD) measurements in a 35-year-old woman without deep infiltrating endometriosis (DIE) (a, b) and a 31-year-old woman with DIE (c, d) on sagittal T2-weighted FSE (fast spin echo) sequences. a, c Anatomical landmarks for the measurements are the lower edge of the midline of the anal canal (dotted line), the posterior fourchette (curved arrow), and the posterior wall of the clitoris (straight arrow). b, d MRI-AGD-AC measurements from the posterior wall of the clitoris to the lower edge of the anal canal giving distances of 99.5 mm and 72.6 mm, respectively (white lines and numbers); MRI-AGD-AF measurements from the posterior fourchette to the lower edge of the anal canal giving distances of 35.9 mm and 34.2 mm, respectively (orange lines and numbers). Asterisk in c: Typical DIE with involvement of the rectum and the uterine cervix.
The center of the lower edge of the anal canal (i.e., the anus) served as posterior reference point for the measurements due to the favorable reproducibility, like Liu et al. described for clinical AGD measurements19. To record potential difficulties with the AGD measurements, readers noted measurability for each examination using a two-point scale.
Surgical procedures
Surgeries were performed by senior surgeons with at least 10 years’ experience in endometriosis surgery, certified for minimally invasive surgery by the German Gynecological Endoscopy Working Group (AGE). The attending Department of Gynecology and Obstetrics is a training center for minimally invasive surgery, certified by the German Gynecological Endoscopy Working Group and by EuroEndoCert on behalf of the German Endometriosis Research Foundation (level III endometriosis center). Detection of DIE, peritoneal endometriosis with and without DIE, and ovarian endometriosis was noted as recorded in the surgery reports (laparoscopy, n = 123; laparotomy, n = 4).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 29.0 (IBM), R 4.3.2 (The R Foundation for Statistical Computing), and RStudio 2023.12.1 + 402 (Posit Software). Continuous variables are presented as mean and standard deviation and categorical variables are presented as counts and percentages. The chi-square test and Fisher’s exact test were used to compare differences in the frequencies of categorical variables. Intraclass correlation coefficient (ICC) estimates were calculated for the AGD measurements of the readers, based on a mean-rating (k = 2), absolute-agreement, 2-way mixed-effects model and interpreted as suggested by Koo and Li20. Mean values of the measurements of MRI-AGD-AC and MRI-AGD-AF of the two readers were calculated for each patient. The Shapiro-Wilk test was used to test if the AGD measurements (MRI-AGD-AC, MRI-AGD-AF) were normally distributed in the group of patients with and without a surgical diagnosis of DIE. The Wilcoxon test (i.e., Mann-Whitney U test/ Wilcoxon rank-sum test) was then performed to assess differences in the mean AGD measurements between readers and patient groups. Binary logistic regression models were calculated to investigate the dependence between age, body mass index (BMI), MRI-AGD-AC, MRI-AGD-AF, and the variables “DIE”, “peritoneal endometriosis with and without DIE” and “ovarian endometriosis”. The sample size was based on the common requirement of a minimum number of events per variable of ten. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated as a measure of the ability to discriminate the presence of DIE, peritoneal endometriosis with and without DIE, and ovarian endometriosis. P-values ≤ 0.05 were considered statistically significant.
Results
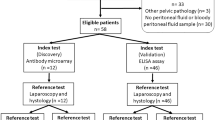
127 consecutive patients (mean age, 30.4 years ± 6.7 [standard deviation]) were evaluated. The flowchart of our study cohort is provided in Fig. 2.
Characteristics of the study population are summarized in Table 1.
Cases and controls of the primary categories of the examination (surgical detection of DIE, n = 67 / no surgical detection of DIE, n = 60) had similar demographic and clinical characteristics. Only the symptoms infertility and diarrhea/obstipation were significantly more frequent in patients with subsequent surgical diagnosis of DIE (p = 0.010 and p = 0.043).
Reader 1 noted good measurability of AGD in 93/127 (73.2%) cases and limited measurability in 34/127 (26.8%) cases. Reader 2 noted good measurability of AGD in 57/127 (44.9%) cases and limited measurability in 70/127 (55.1%) cases. These differences in the categorizations of the measurability of AGD by the two readers were statistically significant (p < 0.001, chi-square test).
The mean values of the MRI-AGD-AC were 74.7 mm ± 10.4 (reader 1) and 77.4 mm ± 10.1 (reader 2) (p = 0.034, Wilcoxon test). The mean values of the MRI-AGD-AF were 31.3 mm ± 5.6 (reader 1) and 34.9 mm ± 5.4 (reader 2) (p < 0.001, Wilcoxon test). The averaged mean values were 76.0 mm ± 9.9 (reader 1 + 2) for MRI-AGD-AC, and 33.1 mm ± 5.0 (reader 1 + 2) for MRI-AGD-AF. Given the statistically significantly different mean values of AGD-measurements of both readers, analyses were performed for the AGD measurements of each reader individually and for averaged measurements. Figure 3 shows the frequency distribution of the averaged values of the AGD measurements of readers 1 and 2.
The ICC estimate of MRI-AGD-AC was 0.92 (95% CI: 0.83–0.95), indicating good to excellent reliability. The ICC estimate of MRI-AGD-AF was 0.68 (95% CI: 0.27–0.83), indicating poor to good reliability. When considering only the measurements for which good measurability was documented by both readers (n = 53), the ICC estimate of MRI-AGD-AC was 0.96 (95% CI: 0.90–0.98), indicating excellent reliability, and the ICC estimate of MRI-AGD-AF was 0.73 (95% CI: 0.43–0.86), indicating poor to good reliability.
The respective mean MRI-AGD-AC and MRI-AGD-AF were 76.1 mm ± 9.8 and 33.1 mm ± 4.8 in examinations with vaginal opacification (n = 107), and 75.9 mm ± 10.9 and 33.6 mm ± 6.0 in examinations without vaginal opacification (n = 20). No statistically significant differences between the measurements performed on examinations with and without vaginal opacification could be found (p = 0.784 and p = 0.686, respectively, Wilcoxon test).
The Shapiro-Wilk test for MRI-AGD-AC and MRI-AGD-AF did show a significant deviation from normal distribution for patients without a surgical diagnosis of DIE (p = 0.013 and p = 0.009, respectively) and no significant deviation from normal distribution for patients with a surgical diagnosis of DIE (p = 0.589 and p = 0.172, respectively). The analysis of the differences of AGD measurements depending on surgical findings showed no statistically significant results, both when looking at the mean values formed from the measurements of the two readers and when looking at the individual measurements of readers 1 and 2 (Table 2).
A comparison of the AGDs only for the measurements with good measurability noted by both readers (n = 53) also revealed no statistically significant differences in the individual patient groups (Table 3).
The average AGD measurements and their distribution within patient groups are summarized in Fig. 4.
Distribution of the MRI-AGD-AC (a) and the MRI-AGD-AF (b) values depending on the surgical diagnosis of DIE, peritoneal endometriosis with and without DIE, and ovarian endometriosis. The boxes represent the values for the median and the 25th and 75th percentiles. The whisker plots represent the maximum and minimum values, excluding outliers.
Results of the binary logistic regression models for the variables “DIE”, “peritoneal endometriosis with and without DIE”, and “ovarian endometriosis” are shown in Table 4.
ROC curves are presented in Fig. 5.
The AUC of MRI-AGD-AC was 0.542 (95% CI: 0.441–0.644; p = 0.415) for DIE, 0.475 (95% CI: 0.365–0.584; p = 0.648) for peritoneal endometriosis with and without DIE, and 0.585 (95% CI: 0.472–0.698; p = 0.140) for ovarian endometriosis. The AUC of MRI-AGD-AF was 0.582 (95% CI: 0.482–0.683 p = 0.109) for DIE, 0.554 (95% CI: 0.450–0.658; p = 0.310) for peritoneal endometriosis with and without DIE, and 0.586 (95% CI: 0.454–0.718; p = 0.200) for ovarian endometriosis.
Discussion
This study aimed to evaluate measurements of MRI-AGD-AC and MRI-AGD-AF for the prediction of endometriosis in patients without prior abdominal surgeries, examined for endometriosis on MRI, and subsequently undergoing surgery within one year of the MRI examination. Our findings did not indicate a statistically significant difference in the averaged AGD measurements of two readers between patients with and without a surgical diagnosis of DIE (p = 0.110 to p = 0.413), between patients with and without a surgical diagnosis of peritoneal endometriosis with and without DIE (p = 0.323 to p = 0.641), and between patients with and without a surgical diagnosis of ovarian endometriosis (p = 0.150 to p = 0.155). Also, no statistically significant differences were found between these patient groups when only the measurements of the more experienced reader were considered (p = 0.161 to p = 0.731).
No data are available to date on the interobserver agreement of MRI-based AGD measurements. The applicability of AGD measurements on MRI in our study was good, with ICC analysis showing better agreement between the two readers in the measurement of MRI-AGD-AC (ICC 0.92; 95% CI: 0.83–0.95) than in the measurement of MRI-AGD-AF (ICC 0.68; 95% CI: 0.27–0.83). This is probably due to the less distinct definition of the posterior fourchette in the MRI images, whereas the anatomical landmarks of the MRI-AGD-AC are more often clearly identifiable. In the only available study in which AGD measurements were performed in MRI by Crestani et al., the measurements were performed by a single radiologist, and the reproducibility of the measurements was not investigated14. Our data favor the measurement of MRI-AGD-AC over MRI-AGD-AF as a more robust and easily reproducible parameter, even for inexperienced readers (e.g., medical students following a training measure, as in our study).
In general, and perhaps unsurprisingly, the more experienced reader rated the measurability more favorably than the inexperienced reader (good measurability in 73.2% and 44.9%, respectively, p < 0.001). For the AGD measurements for which good measurability was documented by both readers (n = 53), the agreement was slightly better than in the entire collective (increase in ICC for MRI-AGD-AC from 0.92 to 0.96 and for MRI-AGD-AF from 0.68 to 0.73). As these differences are only minor, this confirms that AGD measurements on MRI are possible for inexperienced readers despite the perceived difficulty in measurements compared to more experienced readers.
The challenging question arises as to why our results cannot confirm the results of several other studies on AGD3,10,11,12,13,14,19,21, which found some degree of association between AGD and endometriosis. Crestani et al. reported an AUC of 0.840 (95% CI: 0.782–0.898) for AGD-AF and an AUC of 0.756 (95% CI: 0.684–0.828) for AGD-AC for predicting the presence of endometriosis13. Sánchez-Ferrer et al. reported an AUC of 0.91 (95% CI: 0.84–0.97) for AGD-AF and an AUC of 0.64 (95% CI: 0.53–0.75) for AGD-AC for predicting the presence of DIE11. In contrast to our study, other groups examined different collectives with possible confounders. The control groups did not correspond to the collective for which AGD measurements to predict endometriosis would be applied. In the study by Crestani et al., surgeries in the control group were performed for various reasons (e.g., hysterectomy for leiomyomas, salpingo-oophorectomy for ovarian lesions, etc.), but not to diagnose and possibly treat endometriosis13. The specification of our study population, in contrast, is that it represents the population for which the use of a biomarker for endometriosis would be reasonable—i.e., patients with clinically suspected endometriosis who have not yet undergone surgery and in whom endometriosis is therefore not yet confirmed. In the study by Buggio et al., in which care was taken to avoid confounders through the selection criteria, no correlation was found between clinically measured AGD and the presence of endometriosis, as in our study15.
There is heterogeneity in the execution of AGD measurements between different groups. In the only study in which AGD measurements were performed on MRI by Crestani et al., it is stated that measurements were taken from the anterior edge of the anal canal to the posterior wall of the clitoris/vagina, but no MR images are presented14. Mean values of 40.4 mm ± 7.3 for MRI-AGD-AC and 13.3 mm ± 3.9 for MRI-AGD-AF were reported for the endometriosis group, i.e., substantially shorter distances than in our investigation (75.5 mm ± 9.3 and 33.4 mm ± 5.1, respectively). For clinical measurements of AGD, Buggio et al. reported mean values of 76.0 mm ± 12.1 for AGD-AC and 22.8 mm ± 5.0 for AGD-AF in a patient group affected by DIE15. Thus, the values for AGD-AC were similar to those found in our study, but the values for AGD-AF were substantially lower. We decided to perform the AGD measurements on MRI from the center of the lower edge of the anal canal (i.e., the anus) due to the favorable reproducibility, like Liu et al. described for clinical AGD measurements19. The good to excellent agreement between two readers for MRI-AGD-AC in our study documents the reliability of this approach, whereas, in the measurement of MRI-AGD-AF, a lower reliability was shown possibly due to the less clearly defined posterior fourchette.
It has been discussed that pathophysiologically, a short AGD in women with endometriosis indicates an early exposure to genetic and epigenetic factors and endocrine disruptors during intrauterine life13. Various other diseases have been identified that show a relationship to AGD, such as prostate cancer, PCOS, pelvic organ prolapse (POP), hypospadias, testicular germ cell tumors, testicular microlithiasis, and azoospermia3,22,23,24. Our results do not provide a favorable answer regarding the value of AGD measurements on MRI for predicting endometriosis. Nevertheless, MRI is a valuable non-invasive diagnostic tool for detecting and determining the extent of endometriosis4,5.
Numerous biomarkers for endometriosis are currently the subject of research, including glycoproteins, inflammatory cytokines, apoptosis markers, immunological markers, angiogenesis markers, hormone markers, miRNAs, neuropeptide markers, proteomics, metabolomics, genomics, and the microbiome7,25,26. A robust and reliable non-invasive marker for the diagnosis of endometriosis has not yet been identified27,28. A questionnaire-based approach has recently shown promising results with a sensitivity of 90.4% and a PPV of 87.4%29. Given the frequency and significance of the disease, which has a substantial negative impact on patients’ quality of life, further efforts to identify non-invasive diagnostic markers of endometriosis and to facilitate effective and early treatment are of great interest30.
There are limitations to our study. The study was conducted retrospectively at a single center. We have used surgery as the gold standard, although limitations of laparoscopy are known31, such as in the visual identification of lesions with the conventional white light technique32. Therefore, we cannot completely rule out cases in the control groups (no surgical detection of DIE, peritoneal endometriosis with and without DIE, or ovarian endometriosis). On the other hand, surgical exclusion of endometriosis should be regarded as a high-quality criterion, even if there is an increasing restraint in the indication of surgery for endometriosis in favor of non-invasive diagnostic and therapeutic approaches33.
In conclusion, our results do not indicate that anogenital distance, measured on MRI, is a valuable biomarker for patients with clinically suspected endometriosis. No association of MRI-AGD with deep infiltrating endometriosis, peritoneal endometriosis with and without DIE, or ovarian endometriosis was demonstrated.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Jain, V. G. et al. Anogenital distance is determined during early gestation in humans. Hum. Reprod. 33, 1619–1627 (2018).
Gandelman, R., Simon, N. G. & McDermott, N. J. Prenatal exposure to testosterone and its precursors influences morphology and later behavioral responsiveness to testosterone of female mice. Physiol. Behav. 23, 23–26 (1979).
Zamani, P. et al. Association between anogenital distance as a noninvasive index in the diagnosis and prognosis of reproductive disorder: a systematic review. Int. J. Reprod. Biomed. 21, 599–618 (2023).
Avery, J. C. et al. Noninvasive diagnostic imaging for endometriosis part 2: a systematic review of recent developments in magnetic resonance imaging, nuclear medicine and computed tomography. Fertil. Steril. 121, 189–211 (2024).
Harth, S., Roller, F. C., Zeppernick, F., Meinhold-Heerlein, I. & Krombach, G. A. Deep infiltrating endometriosis: diagnostic accuracy of Preoperative Magnetic Resonance Imaging with respect to morphological criteria. Diagnostics (Basel). 13, 1794 (2023).
Harth, S. et al. Application of the #Enzian classification for endometriosis on MRI: prospective evaluation of inter- and intraobserver agreement. Front. Med. (Lausanne). 10, 1303593 (2023).
Pant, A., Moar, K., Arora, T. K. & Maurya, P. K. Biomarkers of endometriosis. Clin. Chim. Acta. 549, 117563 (2023).
Bendifallah, S. et al. Validation of a salivary miRNA signature of endometriosis - interim data. NEJM Evid. 2, EVIDoa2200282 (2023).
Smolarz, B., Szyłło, K., Romanowicz, H. & Endometriosis Epidemiology, classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 22, 10554 (2021).
Mendiola, J. et al. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum. Reprod. 31, 2377–2383 (2016).
Sánchez-Ferrer, M. L. et al. Investigation of anogenital distance as a diagnostic tool in endometriosis. Reprod. Biomed. Online. 34, 375–382 (2017).
Sánchez-Ferrer, M. L. et al. Accuracy of anogenital distance and anti-Müllerian hormone in the diagnosis of endometriosis without surgery. Int. J. Gynaecol. Obstet. 144, 90–96 (2019).
Crestani, A. et al. Anogenital distance in adult women is a strong marker of endometriosis: results of a prospective study with laparoscopic and histological findings. Hum. Reprod. Open. 2020, hoaa023 (2020).
Crestani, A. et al. A short anogenital distance on MRI is a marker of endometriosis. Hum. Reprod. Open. 2021, hoab003 (2021).
Buggio, L. et al. Anogenital distance and endometriosis: results of a case-control study. Reprod. Sci. 29, 3508–3515 (2022).
Nisenblat, V., Bossuyt, P. M. M., Farquhar, C. & Johnson, N. Hull, M. L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2, CD009591 (2016).
Bazot, M. et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 27, 2765–2775 (2017).
Tong, A. et al. Recommendations for MRI technique in the evaluation of pelvic endometriosis: consensus statement from the Society of Abdominal Radiology endometriosis disease-focused panel. Abdom. Radiol. (NY). 45, 1569–1586 (2020).
Liu, X., Ding, D., Shen, M., Yan, D. & Guo, S. W. Shorter Anogenital Distance in women with ovarian endometriomas and adenomyosis, but not uterine Leiomyomas. Biomedicines 11, 2618 (2023).
Koo, T. K. & Li, M. Y. A Guideline of selecting and reporting Intraclass correlation coefficients for Reliability Research. J. Chiropr. Med. 15, 155–163 (2016).
García-Peñarrubia, P., Ruiz-Alcaraz, A. J., Martínez-Esparza, M., Marín, P. & Machado-Linde, F. Hypothetical roadmap towards endometriosis: prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Hum. Reprod. Update. 26, 214–246 (2020).
Schwartz, C. L. et al. Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch. Toxicol. 93, 253–272 (2019).
Moreno-Mendoza, D. et al. Short anogenital distance is associated with testicular germ cell tumour development. Andrology 8, 1770–1778 (2020).
Pedersen, M. R. V., Osther, P. J. & Rafaelsen, S. R. Shorter anogenital distance is observed in patients with testicular microlithiasis using magnetic resonance imaging. Insights Imaging. 12, 46 (2021).
Encalada Soto, D. et al. Endometriosis biomarkers of the disease: an update. Curr. Opin. Obstet. Gynecol. 34, 210–219 (2022).
Tummers, F. H. M. P. et al. K. Biomarker identification for endometriosis as a target for real-time intraoperative fluorescent imaging: a new approach using transcriptomic analysis to broaden the search for potential biomarkers. Eur. J. Obstet. Gynecol. Reprod. Biol. 288, 114–123 (2023).
Burghaus, S. et al. Multicenter evaluation of blood-based biomarkers for the detection of endometriosis and adenomyosis: a prospective non-interventional study. Int. J. Gynaecol. Obstet. 164, 305–314 (2024).
Pascoal, E. et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet. Gynecol. 60, 309–327 (2022).
Konrad, L. et al. Predictive model for the non-invasive diagnosis of Endometriosis based on clinical parameters. J. Clin. Med. 12, 4231 (2023).
Zondervan, K. T., Becker, C. M., Missmer, S. A. & Endometriosis N Engl. J. Med. 382:1244–1256 (2020).
Goncalves, M. O. et al. Systematic evaluation of endometriosis by transvaginal ultrasound can accurately replace diagnostic laparoscopy, mainly for deep and ovarian endometriosis. Hum. Reprod. 36, 1492–1500 (2021).
Vizzielli, G. et al. Real three-dimensional approach vs two-dimensional camera with and without real-time near-infrared imaging with indocyanine green for detection of endometriosis: a case-control study. Acta Obstet. Gynecol. Scand. 99, 1330–1338 (2020).
Chapron, C., Marcellin, L., Borghese, B. & Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 15, 666–682 (2019).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.H., L.M., D.L., F.C.R., F.Z., I.M.-H. and G.A.K. conceived and designed the study. S.H., L.M. and D.L. contributed to data curation. S.H., L.M., D.L., and A.B. carried out data analysis. S.H. and L.M. performed the investigation. S.H., L.M., D.L., F.C.R., A.B., F.Z., I.M.-H., and G.A.K. contributed to the methodology. S.H., L.M., F.C.R., F.Z., I.M.H., and G.A.K. contributed to project administration. S.H., F.C.R., F.Z., I.M.-H. and G.A.K. contributed to resources. D.L., F.C.R., F.Z., I.M.-H., and G.A.K. supervised the study. D.L., F.C.R., and A.B. contributed to study validation. S.H. and D.L. performed visualization. S.H. wrote the manuscript and interpretation of data. All authors reviewed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harth, S., Metze, L., Leufkens, D. et al. Anogenital distance on MRI does not correlate to surgical diagnosis of endometriosis in patients without prior abdominal surgery. Sci Rep 14, 30507 (2024). https://doi.org/10.1038/s41598-024-82407-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82407-6