Abstract
Colonoscopy is a valuable tool for colorectal cancer screening and health checkups, with increasing utilization annually. Assisted entry is a standard procedure during electronic colonoscopy. In China, most clinically assisted colonoscopy procedures involve a nurse directly applying abdominal pressure to the patient’s abdomen. This maneuver provides a fulcrum for the physician performing the procedure, facilitating smoother access to the colon. To reduce labor, optimize resource utilization, and enhance efficiency, this preliminary study aimed to develop and evaluate an adjunctive pressurized removable lap band for colonoscopy. This prospective randomized controlled trial randomized participants into control and experimental groups for observational comparison during follow-up, mitigating retrospective bias. Data were collected from 150 participants in a tertiary hospital endoscopy department between March and September 2023. Participants were evenly divided into groups using a randomized number table. Demographic data, including gender, age, height, weight, and abdominal circumference, were collected to ensure group representativeness and comparability. No significant pre-test differences were found between the groups. The experimental group demonstrated a significant reduction in examination-only insertion time compared to the control group (p < 0.001), median respectively 2.5, 3. Additionally, compared with control group (41.3%), the experimental group (24%) required fewer nurse assistance (p = 0.024). No significant differences were observed in systolic and diastolic blood pressure changes between the groups (p = 0.07, p = 0.43). However, compared with control group (0.89 ± 1.17), the experimental group (2.19 ± 0.94) reported lower pain scores (p < 0.001). Overall, this preliminary study validates the adjunctive pressurized removable lap band as an effective tool for improving colonoscopy efficiency, reducing patient pain, and conserving medical resources.
Trial registration: Registration Authority: Chinese Clinical Trial Registry (ChiCTR). Number: ChiCTR2400082664.
Similar content being viewed by others
Introduction
The latest data released by the International Agency for Research on Cancer indicates that in 2022, the global incidence of malignant tumors was projected to reach 18.74 million cases, resulting in 9.67 million deaths. Among these, colorectal cancer (CRC) was estimated to account for 1.92 million new cases, representing approximately 10.2% of all malignant tumors. Furthermore, CRC was responsible for 904,000 deaths, constituting approximately 9.3% of all malignant tumor-related deaths1. This places CRC as the third most commonly diagnosed cancer globally, second only to lung and breast cancer, and the second leading cause of cancer-related mortality worldwide (after lung cancer). The 2022 Annual Cancer Report from the National Cancer Center of China revealed that CRC was the second most frequently diagnosed cancer and the fourth leading cause of cancer-related death in China2. Overall, the morbidity and mortality rates of CRC rank among the top five both globally and domestically3, posing a significant threat to public health and imposing a substantial disease burden. As the third most prevalent cancer worldwide, CRC is a serious global health concern, causing approximately 700,000 deaths annually4,5,6,7. Studies have shown a general correlation between CRC incidence and age8,9,10, with most cases occurring after the age of 5011,12. However, due to modern lifestyles, dietary factors, and environmental influences, the age of CRC onset is gradually decreasing13. The accelerating pace of life has led to significant lifestyle changes, including the adoption of unhealthy dietary habits characterized by high-salt, high-fat, high-sugar, fried, and irritating foods. To alleviate work-related and life pressures, many individuals resort to smoking, excessive alcohol consumption, and late-night activities. Additionally, sedentary lifestyles, overweight, and obesity are common factors contributing to CRC risk. These factors have prompted increased awareness of the need to lower the screening age for CRC9. Historical data from the United States indicates a decline in the overall incidence of CRC since the widespread adoption of CRC screening modalities in the 1980s14,15,16,17,18. This evidence underscores the effectiveness of early detection and prevention strategies for CRC15,16,19,20,21. Colonoscopy is a widely used technique for screening and treating CRC and related conditions22,23,24,25,26. It is considered the gold standard for diagnosing mucosal lesions in the lower gastrointestinal tract, including the colon and ileum. A camera lens mounted on the colonoscope transmits images of the colonic mucosa to a computer processing center, which then displays them on a monitor, allowing for the visualization of subtle mucosal changes. While water immersion colonoscopy is limited by bowel preparation quality, colonoscopy with air insufflation is currently the primary modality for colonoscopy in China. Our study was conducted within the context of colonoscopy with air insufflation. The device we developed focuses on narrowing the abdominal cavity and manipulating specific positions to facilitate colonoscope insertion. This device enables patients to change positions during the procedure without significant restrictions, potentially reducing procedure time and improving patient comfort. In the Gastrointestinal Endoscopy Laboratory of a tertiary-A hospital in Harbin, a typical colonoscopy procedure requires the assistance of at least one nurse. This assistance is necessary because, even when the patient is positioned correctly and the anus is relaxed, the colonoscope may encounter difficulties during insertion, such as loop formation. To overcome these challenges, nurses often apply direct manual pressure to the patient’s abdomen to provide a fulcrum for the operator, facilitating smooth colonoscope insertion. However, this technique requires significant force, particularly in cases involving obese patients, which may necessitate the assistance of multiple nurses.
Nowadays, assisted compression devices have emerged as a superior option for colonoscopy, offering improved accuracy in compression positioning and enhanced patient comfort. Compared to manual compression performed by nurses, these devices allow for adjustable compression force, accommodating diverse body types, and enabling precise, safe, and effective compression. While abdominal compression devices are beneficial during colonoscopy insertion, there remains a need for auxiliary compression devices that provide more powerful assistance and precise compression.
Therefore, we designed and developed a novel auxiliary compression device. Unlike previous assisted compression devices, this device features removable modules for precise compression, a wider range of pressure adjustments, a three-stage compression selection mechanism, and real-time patient respiratory monitoring. This study aimed to (1) design and develop an assisted compression device for colonoscopy and (2) evaluate its efficacy.
Method
We designed and introduced an auxiliary pressurized removable compression device for colonoscopy. To inform the design, we observed and documented the techniques and positions employed by nurses during manual pressurization to assist with colonoscopy in the gastrointestinal endoscopy room of a tertiary-A hospital in Harbin. Based on these observations, we developed a preliminary design for the compression device. To ensure the safety and adaptability of the device, we consulted with two experienced nurses who had worked in the endoscopy unit for over a decade.
To facilitate colonoscopy, a compression device was designed and developed in this study. This device consists of a lap belt, a length-adjustable belt, and an inflatable component. The device offers three levels of pressurization: (1) Lap-belt pressurization: By adjusting the elasticity of the lap belt, abdominal circumference is narrowed, intestinal lumen is fixed, and free space is reduced, enabling smoother passage of the colonoscope. (2) Module-fixed pressurization: A spherical module allows for precise pressurization, aiding in the navigation of common obstruction points like the hepatic flexure, transverse colon, and sigmoid colon. (3) Pneumatic device pressurization: A combination of abdominal band and pneumatic device creates a double-pressure system, further assisting colonoscope insertion. This three-stage pressurization system accommodates patients of various sizes, enhancing the effectiveness of the auxiliary device. A visual representation of the auxiliary pressurized compression device for colonoscopy is provided in Fig. 1.
Demonstration of an adjunctive pressurized removable compression device for colonoscopy. (A) Abdominal belt. (B) Ring retaining strap. (C) Inflation device (manual and automatic duplex option). (D) Pressurized modules (magnet). (E) Length adjustment strap. (F) Disposable length adjustment with protective pouch. (G) Disposable lap band with protective pouch.
An inflatable pressurized lap belt was developed, utilizing a pump to control inflation and deflation via a switch button. Precise pressurization was achieved through the lap belt, an airbag, and a module. Before clinical implementation, the device underwent rigorous pre-experimentation and safety testing on group members. By observing nurse-assisted e-colonoscopy procedures from August to November 2022, the researchers estimated the necessary pressurization force and determined appropriate lap belt length and safety pressure ranges. During pre-experimentation, participants underwent a three-step pressurization process. Initially, primary pressurization (lap belt contraction) was applied, followed by secondary pressurization (automatic pump inflation) within a 60–100 mmHg range. The pump pressure was adjusted based on colonoscope insertion ease, with a maximum setting of 100 mmHg. For challenging insertions, tertiary pressurization (modular precision pressurization) was employed to target specific areas. From December 2022 to 2023, volunteers were recruited to test the device and ensure that the inflation pressure remained within safe physiological limits and caused no discomfort. The device’s materials were continuously refined to optimize comfort. The final product, the colonoscopy-assisted pressurized removable lap band, is a non-invasive external device proven safe for clinical use.
We developed a simple-to-operate compression device that did not require complex training. Nurses could learn to use the compression device correctly by watching a short video, eliminating the need for extensive training and delaying their implementation in clinical care. We ensured that clear and concise operating videos, instructions, and step-by-step sketches were provided to facilitate rapid learning and application in clinical practice.
Study
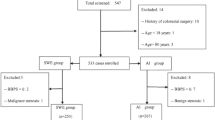
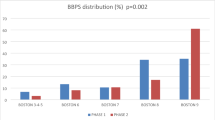
Preliminary validation of the effectiveness of the auxiliary pressurized removable compression device for colonoscopy: Participant Selection and Grouping Methods. Participants were recruited from individuals requiring a colonoscopy. Inclusion criteria were as follows: (1) Age between 18 and 80 years old, conscious, and able to cooperate with wearing the pressurized compression device during colonoscopy; no psychiatric disease that would prevent cooperation with the examination, and no contraindications to colonoscopy. Notably, not all psychiatric or psychological conditions are excluded, but only those that significantly impair a patient’s ability to cooperate with the healthcare provider. (2) Voluntary signing of the informed consent form. Exclusion criteria were as follows: (1) Inadequate bowel preparation (defined as a Boston scale score of < 6), severe inflammatory bowel disease, or intestinal obstruction; (2) Failure to sign the informed consent form; (3) Serious complications from other diseases, such as abdominal wound dehiscence or infection; (4) Inability to cooperate with the examination. Investigator recruitment for the experiment commenced after the establishment of the inclusion and exclusion criteria. All research subjects in this study were under the close supervision of qualified medical professionals. The decision to proceed with colonoscopy was made only after a comprehensive evaluation of each patient’s individual health condition and potential risks. This rigorous assessment ensured that colonoscopy was performed only on suitable candidates. Therefore, factors such as a history of pelvic or abdominal surgery or diverticulosis or diverticulitis were carefully considered. These conditions, however, were not expected to interfere with the study’s conduct or pose any harm to participants or investigators. Diverticular disease, often diagnosed through colonoscopy, is not directly linked to the pressurized removable abdominal band used in the study. This external device, designed to facilitate colonoscopy, is considered safe and poses no risk to patients with simple colonic diverticular disease. Years of experience with manual nurse-assisted pressure and the more recent use of the pressurized abdominal compression device in colonoscopy have not shown any adverse effects on patients with diverticulosis. This may be attributed to the protective layers of skin, fat, and peritoneal fluid that shield the abdominal wall.
To ensure the safety and well-being of all participants, the study required individuals to be fully conscious and free from any diagnosed mental illness. A total of 150 participants were ultimately selected for the study.
Before participation, all individuals underwent a series of tests to assess their suitability. Participants who were scheduled for colonoscopy but could not undergo the procedure due to inadequate bowel preparation were excluded from the study. Additionally, to gather information about potential surgical history, the nurse conducted a pre-colonoscopy interview with each patient, inquiring about any past surgeries or significant medical conditions that might impact their eligibility. While a routine CT scan is not typically required before a colonoscopy, it may be necessary in specific cases where there is a suspicion of intestinal space-occupying lesions or other concerning conditions.
The details of the test
All study participants who met the predetermined eligibility criteria were enrolled in the study following the acquisition of their informed consent. Subjects were assigned to experimental groups through a randomized process based on a table of random numbers. To ensure participant safety and monitor any potential changes in their condition, each subject was accompanied by a dedicated nurse throughout the study. To facilitate a smooth examination, all participants were required to undergo bowel cleansing the day prior to the colonoscopy procedure. To guarantee the proficiency and expertise of the medical team, all endoscopists and nurses involved in the study were selected based on their extensive experience of more than five years in the field. While a blind study design was not feasible due to the inherent nature of the intervention, additional measures were implemented to mitigate bias. These measures included randomization, standardized assessment protocols, and blinding of data analysts to group information, thereby enhancing the objectivity and reliability of the study results 6).
To maintain fairness and minimize potential interference, all tests were conducted under direct supervision, and participants were instructed to maintain a certain distance from one another. Additionally, to optimize the assessment of the safety and efficacy of the pressurized removable compression device, no sedation was administered during the colonoscopy procedures.
Implementation method
We positioned all subjects on their left side on the examination bed, instructing them to maintain a relaxed posture. The nurse then explained the examination process and associated precautions to the participants. While the pressurized removable abdominal compression device enables patients to change positions, it is designed to minimize positional changes and enhance patient comfort.
The control group received traditional manual pressurization assistance from one or more nurse to the abdomen to immobilize the intestinal lumen, as illustrated in Fig. 2. In contrast, the experimental group utilized a pressurized removable compression device to facilitate colonoscopy insertion, as depicted in Fig. 3. Patients were positioned on their left side on the examination table. The nursing staff secured the abdominal belt around the patient’s abdomen, adjusting its length and securing it with ring-shaped fixation belts to compress the abdominal circumference, immobilize the intestinal lumen, and reduce intestinal movement, achieving the first level of pressurization. During the colonoscopy procedure, if the scope encountered resistance, nursing staff inflated the inflatable balloon using the loading device, applying pressure to the entire abdomen to achieve the second level of pressurization. The inflatable balloon, equipped with a one-way valve, allowed for controlled deflation. The automatic inflation and pressurization pump enabled one-touch pressurization and pausing, providing flexibility for patient comfort and rapid inflation/deflation as needed. Removable and movable compression modules were integrated into the device. These modules, housed in magnetically secured bags, utilized magnetic attraction to position spherical compression modules over the sigmoid colon, transverse colon, and hepatic flexure, critical areas prone to resistance. This automated pressure application replaced manual pressure, streamlining the scope insertion process and freeing the nurse’s hands, thus achieving the third level of pressurization.
The feasibility and safety of this application were verified by assessing electronic colonoscopy time (from the start of the examination to reaching the ileocecal valve), blood pressure, the need for nurse-assisted insertion, and patient comfort, as measured by pain scores and healthcare satisfaction.
Blood pressure was selected as an indicator due to its relative stability and ability to objectively reflect patient response during colonoscopy. Our analysis revealed no significant difference in blood pressure between the groups with and without compression device use, indicating that the compression device does not compromise patient safety. While oxygen saturation and heart rate are common physiological indicators, their significant fluctuations during colonoscopy, influenced by procedural factors and other variables, can hinder accurate assessment of corset safety and introduce bias. Consequently, we consider blood pressure to be a more suitable evaluation metric.
At the end of the examination, patient pain and discomfort were observed and evaluated. In this study, facial expressions were assessed using the Facial Visual Analogue Scale (F-VAS). Scores of one to three were categorized as mild pain, four to seven as moderate pain, and eight to ten as severe pain. To assess patient satisfaction, an indirect approach was employed. Patient satisfaction was inferred from their pain scores and their response to pain management interventions. A decrease in pain scores indicated increased patient satisfaction.
To enhance medical staff satisfaction, we conducted in-depth interviews with department personnel to identify the key characteristics they deemed essential in optimal medical equipment. The collected insights were utilized to develop a satisfaction evaluation form, incorporating both the Likert scoring method and the physical characteristics method. Following the questionnaire’s initial draft, a pilot study was conducted to refine the instrument. Subsequently, the finalized questionnaire was administered to collect valuable feedback.
Ethics declarations
Our study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (YLXJ2021-107). The approval date was November 2021 and the study was completed within the validity period. Prior to participation, informed consent was obtained from all study participants. All procedures involving human subjects were performed in compliance with the Declaration of Helsinki.
Statistical analysis
Statistical analyses were conducted using SPSS 25.0. Continuous variables were assessed for normality through histogram examination. For normally distributed data, t-tests were employed to compare means. For non-normally distributed data, rank-sum tests were utilized. Categorical variables were analyzed using the chi-square test. Statistical significance was determined at the α < 0.05 level.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
Study population
One hundred fifty patients, seventy-five in each group, were enrolled in an electronic colonoscopy study conducted between March and September 2023 at the Department of Gastroenterology, Second Affiliated Hospital of Harbin Medical University. The experimental group comprised forty-two males and thirty-three females, aged thirty-five to seventy-seven years. The control group consisted of forty males and thirty-five females, aged forty-one to seventy-four years. A comparison of gender, age, height, weight, and abdominal circumference between the two groups revealed no significant differences, with p-values of 0.743, 0.792, 0.500, 0.196, and 0.729, respectively (Table 1).
Results of study 1
A comparison of enrollment times between the two groups of participants who underwent the examination-only procedure was conducted. The experimental group, utilizing the compression device, exhibited a significantly reduced examination insertion time (reverse eyepatch) compared to the control group (p < 0.001) (Table 2).
Results of study 2
A comparison of the number of examinations requiring nurse-assisted insertion was conducted between the two groups. The experimental group, utilizing the lap belt, demonstrated a significant reduction in the need for nurse-assisted insertion compared to the control group. Specifically, 24% of patients in the experimental group required nurse assistance, while 76% did not, representing a 17.3% improvement over the control group. This difference was statistically significant (p < 0.05, p = 0.024) (Table 3).
Results of study 3
The safety of the lap band was verified for both groups of patients. A comparison of systolic and diastolic blood pressure measurements before and after colonoscopy between the experimental and control groups revealed no significant differences (systolic blood pressure: p > 0.05, p = 0.07; diastolic blood pressure: p > 0.05, p = 0.43). These findings indicate that using the lap band had no major impact on blood pressure changes, suggesting its safe application (Table 4).
Results of study 4
A comparison of pain scores between the two groups revealed a reduction in pain scores following the use of the compression device in the experimental group. The experimental group exhibited a mean pain score of 0.89 ± 1.17 points, while the control group demonstrated a mean pain score of 2.19 ± 0.94 points (p < 0.001). These findings suggest that the compression device effectively reduces patient pain and provides a more comfortable experience compared to auxiliary manual compression. The statistically significant difference in pain scores between the two groups further supports this conclusion (Table 5).
Results of study 5
A comparison of satisfaction scores between the two groups of healthcare professionals revealed that the compression device group experienced shorter operative times, smoother scope insertion and withdrawal, and mean satisfaction scores exceeding 4.5 for both groups. Notably, the compression device group demonstrated greater satisfaction with the clinical application. The results, rated on a scale of 1 to 5, are presented at the conclusion of the article (Table 6).
No adverse events attributable to the intervention were observed during this study. The equipment, incorporating three-stage pressure, precise module pressure, and airbag pressure, was designed with integrated technology to enhance safety and scientific rigor. All participants remained in good health throughout the trial, and were monitored and protected in accordance with strict ethical guidelines. While procedures were in place to promptly record and address any potential adverse events, none were encountered during this trial.
Discussion
Principal findings
The first major outcome of this study was the development of an adjunctive compression device for use in colonoscopy. As the number of individuals undergoing colonoscopy increases annually, and approximately 79% experience colonic loops during the procedure, nurse-led external abdominal compression has become a widely adopted clinical practice to address this issue. However, the rising demand for endoscopies has led to increased nurse fatigue and potential musculoskeletal injuries due to prolonged external abdominal compression. Furthermore, the technique can be challenging to master, potentially causing patient discomfort. To address these limitations, external abdominal pressure devices have emerged as a potential solution. While foreign research and development efforts have produced innovative designs, these devices often face challenges such as high costs, complex operations, and limited clinical adoption. Domestically, the application of such devices remains relatively limited. This project investigated an abdominal compression device designed to assist with colonoscopy. The device aims to overcome the drawbacks of existing solutions, offering a cost-effective, user-friendly, and three-stage compression system. By replacing manual compression, the device effectively reduces intestinal movement, immobilizes the intestinal lumen, improves the success rate of endoscopic examinations, frees up healthcare personnel, alleviates patient discomfort, and significantly enhances both efficiency and patient satisfaction.
The second primary outcome of this study was to evaluate the effectiveness of the lap band in colonoscopy and compare the differences between the two groups. The results demonstrated that the compression device significantly reduced the time required for colonoscopy insertion. By comparing the insertion time from the anus to the ileum between the two groups, it was evident that the group utilizing the pressurized compression device achieved a significantly shorter insertion time. This finding suggests that the pressurized compression device can effectively enhance the efficiency of the physician’s examination. Colonic nodal loops commonly occur in the sigmoid region, followed by the splenic and hepatic flexures of the transverse colon. The pressurized compression device designed in this study is scientifically sound and effective, offering three levels of pressurization. The modular design allows for disassembly and repositioning, enabling precise, targeted compression and further reducing insertion time and improving workflow efficiency.
The third primary outcome assessed the impact of the compression device on healthcare worker satisfaction. Results indicated that the use of the pressurized compression device reduced the number of nursing assistants required, decreased nurse workload, and increased overall job satisfaction. Additionally, physician satisfaction scores improved. During colonoscopy or treatment procedures, challenges often arise when inserting the colonoscope. Clinicians typically rely on nurses to manually compress the patient’s abdomen to provide a fulcrum for the operator, facilitating smooth insertion. The three-stage pressurization system, utilizing the module to mimic manual compression, effectively replaces this manual intervention, freeing the nurse’s hands. Furthermore, some physicians observed that the abdominal compression device reduced the amplitude of the patient’s abdominal respiration, enabling more precise treatment maneuvers. This precision may help avoid potential side effects such as bleeding and perforation during electrocautery procedures, ultimately leading to shorter procedure times and increased patient safety. In conclusion, the use of the pressurized abdominal compression device in this study not only accelerated insertion time and improved workflow efficiency but also enhanced patient safety and increased healthcare worker satisfaction.
Cost analysis
In the era of economic globalization, competition has intensified, making cost control a crucial factor for maintaining competitive advantage and sustainable development. The compression device can be adapted to various resource constraints. In resource-limited settings, a manual air pump can be employed, reducing production costs, while also offering simplicity in operation. Maintenance costs primarily involve disposable protective clothing, which may be necessary in regions with advanced medical facilities but can be omitted in resource-poor areas without compromising the compression device’s functionality or patient safety. Given the moderate-income level of hospital nurses, manual compression techniques typically require the cooperation of at least two nurses alongside the physician. In contrast, the removable abdominal compression device necessitates only one nurse, freeing up another for alternative tasks. This labor efficiency, coupled with the device’s low production cost, makes it a financially viable and scalable solution for widespread implementation.
Limitation
Our study has certain limitations that should be acknowledged. The pressurized removable compression device, as an adjunct to electronic colonoscopy, is still in its early stages of development, necessitating a larger and more diverse population to assess its generalizability. Additionally, the relatively small sample size of our study may require a larger and more varied population to validate and confirm its effectiveness. While our initial validation provides a foundation for future improvements, we plan to enhance the assisted pressurized removable compression device to version 2.0, incorporating insights from our findings. Lastly, although we have not yet employed multivariate analysis to compare patient satisfaction with common risk factors, we acknowledge the value of this approach and intend to incorporate it into future analyses as we collect a larger dataset.
Conclusion
The results of this study demonstrate that the auxiliary pressurized removable compression device for electronic colonoscopy significantly enhances patient comfort, improves the success rate of cecal intubation, reduces intubation time, and ensures patient safety during the examination process. By reducing the need for nurse-assisted manual pressurization, the device decreases the strain on healthcare workers and minimizes the risk of occupational injuries. The auxiliary pressurized removable compression device can be used as a valuable adjunct to electronic colonoscopy, alleviating patient discomfort and facilitating the smooth progression of clinical procedures. The device’s ease of use and focus on patient safety make it a promising tool for healthcare providers. Classified as a Class II medical device and recognized as a clinical job invention, the auxiliary pressurized removable compression device has been approved for application in Heilongjiang Province, China. Additionally, it has been granted a national utility model patent, confirming its safety and scientific basis for clinical use. To further optimize the device, we will continue to upgrade the device to provide more precise positional pressurization and advanced patient safety monitoring during colonoscopy.
Data availability
All data generated or analyzed during this study are included in this article. The datasets used and analyzed, as well as the complete trial protocol, are available upon reasonable request from the corresponding author.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263. https://doi.org/10.3322/caac.21834 (2024).
Zheng, R. S. et al. Analysis on the prevalence of malignant tumors in China in 2022. Chin. J. Cancer 46(3), 221–231 (2024).
Cao, W. et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin. Med. J. 134(7), 783–791. https://doi.org/10.1097/CM9.0000000000001474 (2021).
Bretthauer, M. et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N. Engl. J. Med. 387(17), 1547–1556. https://doi.org/10.1056/NEJMoa2208375 (2022).
Ladabaum, U., Dominitz, J. A., Kahi, C. & Schoen, R. E. Strategies for colorectal cancer screening. Gastroenterology 158(2), 418–432. https://doi.org/10.1053/j.gastro.2019.06.043 (2020).
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A. & Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 73(3), 233–254. https://doi.org/10.3322/caac.21772 (2023).
Maida, M. et al. Screening of colorectal cancer: present and future. Expert Rev. Anticancer Ther. 17(12), 1131–1146. https://doi.org/10.1080/14737140.2017.1392243 (2017).
Jacobsson, M., Wagner, V. & Kanneganti, S. Screening for colorectal cancer. Surg. Clin. North Am. 104(3), 595–607. https://doi.org/10.1016/j.suc.2023.11.009 (2024).
Fernández-Landa, M. J. et al. Quality indicators and patient satisfaction in colonoscopy. Gastroenterol. Hepatol. 42(2), 73–81. https://doi.org/10.1016/j.gastrohep.2018.07.006 (2019).
Jackson, C. S. & Kahi, C. Colorectal cancer screening: it does matter if you are black or white. Gastrointest. Endosc. 82(5), 884–886. https://doi.org/10.1016/j.gie.2015.04.033 (2015).
Imperiale, T. F., Kahi, C. J. & Rex, D. K. Lowering the starting age for colorectal cancer screening to 45 years: who will come...and should they? Clin. Gastroenterol. Hepatol. 16(10), 1541–1544. https://doi.org/10.1016/j.cgh.2018.08.023 (2018).
Mannucci, A. et al. Colorectal cancer screening from 45 years of age: thesis, antithesis and synthesis. World J. Gastroenterol. 25(21), 2565–2580. https://doi.org/10.3748/wjg.v25.i21.2565 (2019).
Sauer, A. G., Liu, B., Siegel, R. L., Jemal, A. & Fedewa, S. A. Comparing cancer screening estimates: behavioral risk factor surveillance system and national health interview survey. Prev. Med. 106, 94–100. https://doi.org/10.1016/j.ypmed.2017.10.019 (2018).
Ladabaum, U., Mannalithara, A., Meester, R. G. S., Gupta, S. & Schoen, R. E. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology 157(1), 137–148. https://doi.org/10.1053/j.gastro.2019.03.023 (2019).
Sullivan, J. F. & Dumot, J. A. Maximizing the effectiveness of colonoscopy in the prevention of colorectal cancer. Surg. Oncol. Clin. N. Am. 27(2), 367–376. https://doi.org/10.1016/j.soc.2017.11.009 (2018).
Salimzadeh, H., Khabiri, R., Khazaee-Pool, M., Salimzadeh, S. & Delavari, A. Motivational interviewing and screening colonoscopy in high-risk individuals. A randomized controlled trial. Patient Educ. Couns. 101(6), 1082–1087. https://doi.org/10.1016/j.pec.2018.01.015 (2018).
Swartz, A. W., Eberth, J. M. & Strayer, S. M. Preventing colorectal cancer or early diagnosis: which is best? A re-analysis of the U.S. preventive services task force evidence report. Prev. Med. 118, 104–112. https://doi.org/10.1016/j.ypmed.2018.10.014 (2019).
Rex, D. K. et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Gastroenterology 153(1), 307–323. https://doi.org/10.1053/j.gastro.2017.05.013 (2017).
Levin, T. R. et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology 155(5), 1383–1391. e5. https://doi.org/10.1053/j.gastro.2018.07.017 (2018).
Emile, H., Barsom, H. & Wexner, S. D. An updated review of the methods, guidelines of, and controversies on screening for colorectal cancer. Am. J. Surg. 224(1 Pt B), 339–347. https://doi.org/10.1016/j.amjsurg.2022.03.034 (2022).
Miller Wilson, L. A. et al. Opportunities and challenges in screening for colorectal cancer. Popul. Health Manag. 26(4), 246–253. https://doi.org/10.1089/pop.2023.0013 (2023).
Hadjipetrou, A., Anyfantakis, D., Galanakis, C. G., Kastanakis, M. & Kastanakis, S. Colorectal cancer, screening and primary care: a mini literature review. World J. Gastroenterol. 23(33), 6049–6058. https://doi.org/10.3748/wjg.v23.i33.6049 (2017).
Allison, J. E. Colorectal-cancer screening. N. Engl. J. Med. 366(22), 1230–1231. https://doi.org/10.1056/NEJMc1203544 (2012).
Fitzpatrick-Lewis, D. et al. Screening for colorectal cancer: a systematic review and meta-analysi. Clin. Colorectal Cancer 15(4), 298–313. https://doi.org/10.1016/j.clcc.2016.03.003 (2016).
Kaminski, M. F., Robertson, D. J., Senore, C. & Rex, D. K. Optimizing the quality of colorectal cancer screening worldwide. Gastroenterology 158(2), 404–417. https://doi.org/10.1053/j.gastro.2019.11.026 (2020).
Zhang, C. et al. Effect of flexible sigmoidoscopy-based screening on colorectal cancer incidence and mortality: an updated systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anticancer Ther. 23(11), 1217–1227. https://doi.org/10.1080/14737140.2023.2245564 (2023).
Acknowledgements
The authors are grateful to the teachers of HMU for their guidance and the who participants volunteered to in the test.
Author information
Authors and Affiliations
Contributions
L.S.Y., Z.Y.H. and L.X.D. determined the research direction and drew up the research plan. Z.Y.H., L.X.D, L.S.Y., W.J.N. and F.X.X. designed and developed the equipment. L.S.Y., Z.Y.H. and L.X.D. ensured the accuracy of the information provided. L.S.Y., Z.Y.H., L.X.D. and L.Y. carried out the test. L.S.Y., Z.Y.H. and L.X.D completed the statistics and analysis of the data, writing and modification of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary Material 2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Lu, X., Li, S. et al. A preliminary study of further attempt at the development, testing and application of an auxiliary equipment for electronic colonoscopy. Sci Rep 14, 31128 (2024). https://doi.org/10.1038/s41598-024-82415-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82415-6