Abstract
Heart transplantation remains the ultimate treatment strategy for neonates and children with medically refractory end-stage heart failure and utilization of donors after circulatory death (DCD) can expand th donor pool. We have previously shown that mitochondrial transplantation preserves myocardial function and viability in neonatal swine DCD hearts to levels similar to that observed in donation after brain death (DBD). Herein, we sought to investigate the transcriptomic and proteomic pathways implicated in these phenotypic changes using ex situ perfused swine hearts. Pathway analysis showed that ATP binding, voltage-gated K channel activity involved in cardiac cell muscle contraction and ribosomal RNA biogenesis were upregulated in the mitochondrial transplantation group, while mitochondria were the predicted source. Promotion of ribosome biogenesis and downregulation of apoptosis were the overlapping mechanisms between transcriptomic and proteomic alterations. Moreover, we showed that mitochondrial transplantation modulates ischemic transcriptomic and proteomic profiles to that of non-ischemia through the mitochondria. Replication of these findings in human in vivo experiments is warranted.
Similar content being viewed by others
Introduction
Heart transplantation remains the ultimate treatment strategy for neonates and children with medically refractory end-stage heart failure. However, the donor pool is limited given the scarcity of available donors after brain death (DBD) of young age and is further complicated by heart size mismatch. An acceptable way to increase the donor pool is to utilize hearts obtained from non-heart-beating donors, or donation after circulatory death (DCD) who have irreversible neurological injury but do not meet the criteria for brain death. The use of DCD hearts has not been widely accepted as an alternative because of the detrimental effects of warm ischemia to the myocardium inflicted by the withdrawal of life support1,2.
We have previously shown that mitochondrial transplantation, which entails replacing damaged myocardial mitochondria with viable respiratory-competent mitochondria, preserves myocardial function and viability in neonatal swine DCD hearts to levels similar to that observed in DBD hearts1,2. The mechanisms for this were unknown. Herein, we show that mitochondrial transplantation acts independently to modulate transcriptomic and proteomic pathways in neonatal and pediatric DCD hearts. Specifically, in neonates, mitochondrial transplantation was followed by changes in the transcriptome and proteome that did not differ from the “sham” non-ischemic group, suggesting a regulatory shift towards cellular normalcy. In pediatric DCD hearts treated with mitochondria, our findings are consistent with decreased apoptosis and inflammation and increased myocardial contractility and tissue regeneration.
Results
Neonatal hearts
Mitochondrial transplantation normalized transcriptomic changes associated with ischemia
We have previously showed that mitochondrial transplantation in neonatal hearts increased myocardial function as shown by left ventricular developed pressure (LVDP) and decreased infarct size (Fig. 1, upper panel)1. To investigate potential transcriptomic alterations and pathways involved in functional changes seen in neonatal hearts treated with mitochondria, heart tissue biopsies were obtained and further processed for gene expression and pathway enrichment analysis. Differentially expressed genes among DCD groups are summarized in Fig. 2A. A total of 826 genes were differentially expressed (log2fold change ≥ 1, padj ≤ 0.05 between DCD vehicle and mitochondria treated groups (474 upregulated and 352 downregulated). PCA plot shows a clear discrimination between DCD mitochondria and vehicle groups (Fig. 2B). When comparing DCD mitochondria hearts to sham (DBD) hearts that did not receive warm ischemia, only 9 genes (1 up, 8 down) were found to significantly differ between the two groups (Fig. 2C). This observation suggests that the transcriptome of the DCD mitochondria hearts resembles that of non-ischemic sham (DBD) hearts.
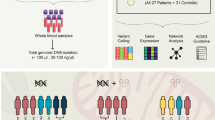
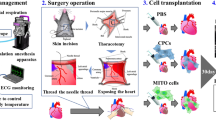
Graphic representation of the study outline. Neonatal (3–4.5 kg) or pediatric (10–15 kg) female Yorkshire pigs underwent cardiac death by induction of deep anesthesia and neuromuscular blockade and extubation. Hearts were harvested and received either vehicle or mitochondria and underwent ex situ heart perfusion. A group of sham hearts underwent ex situ heart perfusion but not the part of the cardiac death. Tissue was collected and snap frozen in the end of the perfusion and RNA seq and SOMAscan were performed. Left ventricle developed pressure (mmHg) and infarct size (% of total cardiac mass) following 2 h. of unloaded and 2 h. of loaded ex-situ heart blood perfusion for sham control hearts receiving no warm ischemia (SHAM, blue), DCD hearts receiving vehicle alone (VEH, black) and DCD hearts receiving vehicle containing 5 × 109 mitochondria (MITO, red). VEH and MITO were both delivered as a bolus antegrade into the aortic root over 5 s. (Figures retrieved from Alemany, et al.). *p < 0.05 for Mitochondria vs Vehicle groups; VEH: Vehicle group; MITO: Mitochondria group; SHAM: Sham group.
(A) Heatmap of transcriptomic regulation patterns in neonatal DCD Mitochondria (Mitochondria), DCD Vehicle (Vehicle) and Sham (DBD) control hearts. Τhe log fold change of gene expression is shown with pseudocolor scale (-2 to 2), with blue denoting downregulation and orange denoting upregulation when comparing hearts that received mitochondria to vehicle. Columns represent FC comparisons, and rows represent the genes. Neonatal experimental groups are indicated. (B) Principal component analysis (PCA) for neonatal hearts receiving mitochondria (red circles) or vehicle alone (green circles). Sham indicated in blue. (C) Volcano plot indicating the downregulated (blue) and upregulated (red) genes in the neonatal mitochondria group compared to vehicle. (D) Gene ontology (GO) pathway analysis indicating downregulated and upregulated biological process in the neonatal mitochondria group compared to vehicle. The size of the dot correlates with the number of genes and the color with the fold change. (E) Enrichment plot analysis indicating downregulated and upregulated pathways in the neonatal mitochondria group compared to vehicle. NES: Normalized enrichment plot; FDR: False discovery rate. (F) Gene ontology analysis indicating the most significant cellular compartment and molecular function in the neonatal mitochondria group compared to vehicle. (G) Gene ontology molecular function analysis indicating the top 200 genes in the neonatal mitochondria group compared to vehicle. (H) Network analysis indicating the top hub genes in significantly downregulated and upregulated pathways in the neonatal mitochondria group compared to vehicle. (I) Gene networks in upregulated and downregulated pathways in the neonatal mitochondria group compared to vehicle. All results are shown following 4 h perfusion, consisting of 2 h. unloaded and then 2 h of isovolumic loaded heart perfusion for each group.
Mitochondrial transplantation mitigates immune response to ischemia, while increases cardiomyocyte proliferation
When comparing DCD mitochondria to DCD vehicle, GO analysis showed that ribosome biogenesis was upregulated while fatty acid oxidation and phagosome maturation were downregulated (Fig. 2D). GSEA enrichment analysis suggests cardiomyocyte proliferation and p53 pathway to be significant hallmarks implicated (Fig. 2E). The mitochondria and Golgi to plasma membrane vesicle were the most prevalent cellular compartments associated with transcriptional changes while ATP and protein kinase binding were the most common GO molecular functions (Fig. 2F). Subsequent pathway enrichment analysis of the top 200 genes suggested increased “potassium channel activity” processes in the cardiac muscle of mitochondrial treated hearts. The top hub genes involved in those processes included potassium voltage-gated channel, Isk-related family, member 3 (KCNE3), potassium inwardly rectifying channel subfamily J member 14 (KCNJ14), KCNJ5 and potassium channel subfamily K member 5 (KCNK5) (Fig. 2G).
Regulation of ribosomal RNA metabolic processes and immune responses are primarily affected by mitochondrial transplantation in neonatal ischemic hearts
Investigation of gene regulatory networks using the PPI-Biogrid database showed that rRNA and ncRNA metabolic processes are significantly enriched in the DCD mitochondria hearts, compiling networks of hub genes that included the NIN1 binding protein 1 homolog (NOB1; apoptosis related gene), ribosome biogenesis regulator homolog (RRS1), ribosomal RNA processing 9 (RRP9), EBNA1 binding protein 2 (EBNA1BP2; cell proliferation related gene) and GTP binding protein 4 (GTPBP4) (Fig. 2H,I). Furthermore, mitochondrial transplantation decreased immune system processes and cellular response to stress following ischemia by downregulating the expression of various cytokines, cytokine receptors, collagen and proinflammatory genes such as Interleukin 1A (IL1A), C-X-C Motif Chemokine Ligand 8 (CXCL8), C-X-C Motif Chemokine Ligand 11 (CXCL11) and Heat Shock Protein Family A (Hsp70) Member 2 (HSPA2) among others (Fig. 2H,I).
Mitochondrial transplantation in neonatal hearts alters diminishes apoptosis
To investigate concomitant proteomic changes following mitochondrial transplantation in neonatal ischemic hearts, proteomics analysis of heart tissues using SOMAscan was performed. Differentially regulated proteins among groups are summarized in Fig. 3A. PCA plot shows the clear discrimination among groups (Fig. 3B). Α total of 142 proteins significantly differed between the DCD vehicle and mitochondria groups (Fig. 3C). GO analysis in two independent databases highlighted again the mitochondrion and its compartments as the primary cellular component regulating the myocardial proteome following autologous transplantation (Fig. 3D). The pathways significantly downregulated after mitochondrial transplantation included apoptosis, proteasome mediated catabolic processes and cell adhesion while significantly enriched pathways were cell proliferation, skeletal system development, glucose intake and adipose tissue browning (Fig. 3E). The molecular function of significantly regulated proteins suggested ATP, rRNA and Ca2+ binding (Fig. 3F). Disease-associated enrichment analysis revealed reperfusion injury, post myocardial infarction cardiac fibrosis and endothelial function mostly affected by mitochondrial treatment (Fig. 3G). Cardiac myocytes, endothelial cells and adipocytes were identified as the major cell types involved (Fig. 3H).
(A) Heatmap of proteomic regulation patterns in neonatal DCD Mitochondria (Mitochondria), DCD Vehicle (Vehicle) and Sham (DBD) control hearts. Τhe log fold change of gene expression is shown with pseudocolor scale (-2 to 2), with blue denoting downregulation and orange denoting upregulation. Columns represent FC comparisons, and rows represent the proteins. Neonatal experimental groups are found on the bottom. (B) Principal component analysis (PCA) for neonatal hearts receiving mitochondria (red circles) or vehicle alone (green circles). Sham indicated in blue. (C) Volcano plot indicating the downregulated (blue) and upregulated (red) proteins in the neonatal mitochondria group compared to vehicle. (D) Manhattan plot of two databases (Gene Ontology (GO) Cellular Component 2018 and Jensen Compartments) indicating the most significant GO cellular compartments when comparing neonatal mitochondria group to vehicle. (E) Barplots indicating upregulated and downregulated GO biological processes in the neonatal mitochondria group compared to vehicle. (F) Barplot indicating upregulated and downregulated GO molecular function in the neonatal mitochondria group compared to vehicle. (G) Dotplot indicating disease associated enrichment analysis when comparing neonatal mitochondria group to vehicle. (H) Dotplot indicating tissue/cell enrichment analysis when comparing neonatal mitochondria group to vehicle. (I) Venn diagram depicting the number and name of overlapping genes as well as the respective processes of the RNA seq and proteomics data when comparing neonatal mitochondria group to vehicle. (J) Common predicted upstream transcription factors (TF) in RNA seq and proteomics data when comparing neonatal mitochondria group to vehicle. Interaction network of ribosome biogenesis (orange) and immune response (blue) also shown. FDR: False discovery rate. All results are shown following 4 h perfusion, consisting of 2 h. unloaded and then 2 h of isovolumic loaded heart perfusion for each group.
Transcriptomic and proteomic crosslinks of cardioprotection afforded by mitochondrial transplantation
There were 8 common hubs between RNA seq and proteomics analysis that are mainly associated with cellular proliferation responses in DCD mitochondria hearts (Fig. 3I). Two of these genes, namely activating transcription factor 3 (ATF3) and marginal zone B and B1 cell specific protein (MZB1) exert cardioprotective properties following myocardial ischemia injury through modulation of mitochondrial function and alleviation of inflammation3,4. To identify regulatory mediators involved in transcriptomic and proteomic changes, upstream regulatory analysis of transcription factors (TF) was performed. The top common predicted transcription factors in both datasets were protein C-ets-1 (ETS1) and tumor suppressor protein 53 (TP53). Multi-omic 2-layer analysis of TFs and related RNA/protein changes revealed two major protein networks oppositely regulated by ETS1 and TP53, whereas GO analysis of related biological processes of those clusters revealed regulation of ribosome biogenesis and immune response (Fig. 3J). In addition to ETS1 and TP53, two other TFs, activating transcription factor 3 (ATF3) and CD8 subunit alpha (CD8A) were also characterized as significant regulatory elements of the clusters above (Fig. 3J).
Pediatric hearts
Heart regeneration and oxidative phosphorylation are upregulated following mitochondrial transplantation, while hypoxic response and apoptosis are suppressed
Αs previously shown, mitochondrial transplantation in pediatric hearts exerts a cardioprotective effect in the ischemic myocardium (Fig. 2, upper panel)1. Similarly to neonatal heart tissue, we also performed RNA sequencing and GO analysis in heart biopsies of pediatric sham and DCD hearts. Differentially expressed genes among groups are summarized in Fig. 4A. PCA plot suggests a visible transcriptional shift between the DCD mitochondria and DCD vehicle groups (Fig. 4B). A total of 334 genes were differentially expressed between the mitochondria and vehicle groups (Fig. 4C). GO analysis showed that tissue morphogenesis and development, cellular response to endogenous stimulus and organic substance were upregulated in the DCD mitochondria group compared to DCD vehicle, while regulation of immune system and cell migration were suppressed. (Fig. 4D). ABC transporters were found to be the most significant common domains in significant DEGs followed by homeobox genes. (Fig. 4E). KEGG pathway analysis revealed that ABC transporters and Wnt signaling pathway were upregulated in the DCD mitochondria group while leukocyte migration was downregulated compared to DCD vehicle (Fig. 4F). GSEA enrichment analysis indicated enrichment of cell cycle regulation at G2M checkpoint, oxidative phosphorylation and cardiac muscle contraction as well as downregulation of apoptosis, glycolysis and hypoxia related processes in the DCD mitochondria group (Fig. 4G).
(A) Heatmap of transcriptional regulation patterns at the end of the perfusion period. Τhe log fold change of gene expression is shown with pseudocolor scale (-2 to 2), with blue denoting downregulation and orange denoting upregulation. Columns represent FC comparisons, and rows represent the genes. Pediatric experimental groups are found on the bottom. (B) Principal component analysis (PCA) for pediatric hearts receiving mitochondria (red circles) or vehicle alone (green circles). Sham indicated in blue. (C) Volcano plot indicating the downregulated (blue) and upregulated (red) genes in the pediatric mitochondria group compared to vehicle. (D) Gene ontology (GO) pathway analysis indicating downregulated and upregulated biological process in the pediatric mitochondria group compared to vehicle. The size of the dot correlates with the number of genes and the color with the fold change. (E) Barplot indicating common domains of differentially expressed genes (DEGs) when comparing pediatric mitochondria group with vehicle. (F) Barplots indicating significant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways when comparing pediatric mitochondria group with vehicle. (G) Enrichment plot analysis indicating downregulated and upregulated pathways in the pediatric mitochondria group compared to vehicle. NES: Normalized enrichment plot; FDR: False discovery rate.
Inflammatory marker expression was dampened mitochondrial transplantation
To identify proteomic alterations and overlapping pathways, SOMAscan analysis of myocardial tissues of all groups was also performed. Differentially regulated proteins among groups are summarized in Fig. 5A. PCA plot shows the discrimination among groups (Fig. 5B). As seen in the heatmap in Fig. 5C, mitochondrial transplantation dampened the inflammatory response following ischemia in the myocardium. Collectively, a total of 40 proteins significantly differed between the DCD mitochondria and DCD vehicle groups (Fig. 5D). GO cellular component analysis of significant proteins suggested that those changes were attributed mostly to the mitochondrial respiratory chain complex I and the extracellular organelle, while mitochondrial ATP synthesis and negative regulation of apoptosis emerged as the top regulated biologic processes in the mitochondrial treated pediatric hearts (Fig. 5E,F). Further analysis highlighted 3 hub regulatory networks, whose nodes are involved in CCT/prefoldin complex, ATP metabolic process and immune response (Fig. 5G).
(A) Heatmap of proteomic regulation patterns at the end of the perfusion period. Τhe log fold change of gene expression is shown with pseudocolor, with blue denoting downregulation and orange denoting upregulation. Columns represent FC comparisons, and rows represent the proteins. Pediatric experimental groups are found on the bottom. (B) Principal component analysis (PCA) for pediatric hearts receiving mitochondria (red circles) or vehicle alone (green circles). Sham indicated in blue. (C) Heatmap of proteomic inflammatory alterations at the end of the perfusion period. Τhe log fold change of gene expression is shown with pseudocolor, with blue denoting downregulation and orange denoting upregulation. Columns represent FC comparisons, and rows represent the proteins. Pediatric experimental groups are found on the bottom. (D) Volcano plot indicating the downregulated (blue) and upregulated (red) proteins in the pediatric mitochondria group compared to vehicle. (E) Manhattan plot of two databases (Gene Ontology (GO) Cellular Component 2018 and Jensen Compartments) indicating the most significant GO cellular compartments when comparing pediatric mitochondria group to vehicle. (F) Dotplot indicating significant gene ontology (GO) biological processes when comparing pediatric mitochondria group to vehicle. (G) Protein networks in enrichment pathways when comparing neonatal mitochondria group to vehicle. FDR: False discovery rate. (H) Venn diagram depicting the number and name of overlapping genes as well as the respective processes of the RNA seq and proteomics data when comparing pediatric mitochondria group to vehicle. (I) Protein–protein interaction network for cell cycle, mitochondrion organization, heart development, muscle organ development and gluconeogenesis when comparing neonatal mitochondria group to vehicle. FDR: False discovery rate.
Overlapping transcriptomic and proteomic alterations associated with mitochondrial transplantation related changes
Common RNA seq and SOMAscan targets were shown to include MAGUK p55 subfamily member 5 (MPP5), mitogen activated protein kinase 1 (MAP4K1), tenascin C (TNC), fibrinogen C domain containing 1 (FIBCD1), C type lectin (CD209) and aldehyde dehydrogenase 1 family member A1 (ALDH1A1) (Fig. 5H). Protein–protein interaction networks of both datasets indicated cell cycle, mitochondrion organization, heart development, gluconeogenesis and muscle organ development among the significantly regulated processes in the pediatric hearts treated with mitochondria (Fig. 5I), suggesting a significant cardioprotective effect of mitochondrial transplantation in the ischemic myocardium.
Discussion
We have previously shown that mitochondrial transplantation increases myocardial function and decreases cardiac injury in neonatal DCD swine hearts1. Herein, we have extended these studies to elucidate the effect of mitochondrial transplantation on transcriptomic and proteomic profiles. Pathway analysis showed that ATP binding, voltage-gated K channel activity involved in cardiac cell muscle contraction and ribosomal RNA biogenesis were upregulated in the mitochondrial transplantation group, while mitochondria were the predicted source. Promotion of ribosome biogenesis and downregulation of apoptosis were the overlapping mechanisms between transcriptomic and proteomic alterations (Fig. 6).
We have previously shown the mitochondria are rapidly taken up by cardiac cells (cardiomyocytes and non-cardiomyocytes) and then fuse with existing mitochondria close to the nucleus. This would allow for rapid mitochondrial-nuclear signaling and message or second messenger signaling via EPR and/or Golgi. This is beyond this paper but worthy of speculation and would be supported by the findings5. We have previously reported that dead mitochondria, mitochondrial proteins, mitochondrial DNA and RNA or exogenous ATP do not offer the cardioprotective effects afforded by mitochondrial transplantation6.
These findings recapitulate our previous experiments in rabbits and swine showing decreased cardiac apoptosis and necrosis following mitochondrial transplantation7,8,9. In the study by Masuzawa et al., proteomic analysis showed upregulated immunomodulation and downregulation of apoptosis associated with mitochondrial transplantation 28 days following regional myocardial ischemia reperfusion injury5.
Of particular interest is the upregulation of voltage-gated K channel activity observed in our study which is in agreement with Shin et al.2019 showing mitochondria-induced coronary vasodilation modulated by KCN family genes regulating inwardly rectifying channels in swine hearts in vivo. This increase in coronary flow may merely contribute to the enhanced myocardial function observed following mitochondrial transplantation10,11,12.
The up regulation of proteomic and metabolomic pathways for energy production, mitochondrial function, cellular respiration and mitochondrial function and the metabolic pathway for mitochondrial function agree with our studies showing that mitochondrial transplantation increases total tissue ATP content, myocardial oxygen consumption and cellular respiration12,13.
An anti-apoptotic effect of mitochondrial transplantation was also observed through modulation of the p53 and ETS1 pathways. p53 is a thoroughly studied transcriptional factor involved in the regulation of various cellular processes including protein interactions, genome stability, cell division, apoptosis and cell energy metabolism. Growing evidence shows that it is increased in cardiovascular disease. It has also been shown that p53 is essential regulator of mitochondrial repair and biogenesis and its upregulation is associated with mitochondrial apoptosis and dysfunction14,15. ETS1 was found to be increased in the DCD mitochondria group, while its loss is associated has been showed to be associated with impaired coronary vascular development leading to ventricular noncompation16.
Increased ribosome synthesis was consistent in both neonatal and pediatric DCD hearts that were treated with mitochondria; a process that plays an essential role in cell proliferation, differentiation, and development in response to external stimuli17. Current data from studies in the skeletal muscle suggest a positive correlation between ribosome and mitochondrial biogenesis and provide increased aerobic capacity18. Moreover, bulk RNA seq cannot differentiate between cellular and mitochondrial ribosome; thus, it could be speculated that part of the observed outcomes are associated with upregulated mitoribosome synthesis. This process is substantial for mitochondrial function and structure as well as synthesis and maintenance of bioenergetic proteins, while its disturbance can lead to mitochondrial dysfunction19,20.
Our findings are consistent with existing literature suggesting the anti-inflammatory properties of mitochondrial transplantation. These are not limited to the myocardial ischemia reperfusion injury2,5,21 but include cases of acute kidney injury22,23, chemotherapy-induced brain injury24 and cardiomyopathy21, acute lung injury25, tendinopathy26 and acute limb ischemia27. The most pertinent inflammatory markers that were downregulated were members of the interleukin and CXC families which are consistent with the multiplex assay results observed in our previous studies investigating any potential autoimmune or autoinflammatory response to mitochondrial transplantation8,27,28.
Up-regulation of transcriptomic pathways for muscle contraction and muscle development and proteomic pathways for muscle function and metabolomic pathways for muscle function also agree with our measured contractile indices where we have shown that mitochondrial transplantation enhances post-ischemic myocardial contractile function that includes increased left ventricular developed pressure, maintenance of left ventricular end diastolic pressure, increased systolic shortening, increased ejection fraction1,2,5,7,10,29.
Novel genes that were upregulated in the neonatal hearts that received mitochondrial transplantation include NOB-1 and RRS1 which have both been reported to be associated with upregulation of apoptosis when silenced suggesting its anti-apoptotic effect30,31. RRP-9 is another gene that was found to be upregulated and associated with resistance to chemotherapeutic agents by inhibiting apoptosis32. EBNA1BP2 which was also highly expressed in the mitochondria group has been reported to be downregulated in subjects with myocardial infarction or heart failure and similarly GTPBP4 has been shown to diminish immune response33.
Overlapping transcriptomic and proteomic targets in the neonatal hearts showed that ATF3 and MZB1 were both upregulated. ATF3 is a transcription factor providing cardioprotection by downregulation of p5315. MZB1 confers its cardioprotective effects by improving mitochondrial membrane potential, ATP levels and mitochondrial oxygen consumption rate, while reducing apoptosis by suppressing the levels of Bax/Bcl-2 and cytochrome c3,4,34.
Combined RNA seq and proteomic analyses in pediatric hearts showed that mitochondrial transplantation led to upregulation of MPP5 a negative regulator of apoptosis via p55 modulation, as well as MAP4K1, another antiapoptotic gene that modulates DNA damage/repair system and MAPK pathway35. TNC, an extracellular matrix glycoprotein, was also upregulated and it is reported that it plays a dynamic role in cardiac regeneration by controlling fibrosis and inflammation35. FIBCD1, CD209 and ALDH1A1 were also upregulated in the mitochondria treated groups with the latter exhibiting an important role in cell proliferation, while the role of the other two in myocardial injury has yet not been fully elucidated36,37,38.
Intriguingly, comparison of the myocardial transcriptome of the DCD mitochondrial transplantation group to the group of the sham DBD hearts revealed only 9 differentially expressed genes. This observation is in accordance with that transplanted mitochondria replace mitochondria damaged by ischemia reperfusion injury which may explain its durable cardioprotective properties. This finding also justified the transplantation of healthy non-ischemic mitochondria in cases of ischemia reperfusion injury of mitochondria with impaired function at baseline due to chronic conditions such as diabetes mellitus or mitochondrial genetic disorders.
Despite the encouraging results of this study, there are certain limitations that should be taken into consideration. These findings are derived from a swine mode of ex situ heart perfusion; thus, an in vivo study of heart transplantation may be more representative of the physiology and immunology of a heart transplant in addition to allowing for a longer follow up period. These were also healthy animals, not allowing to assess the effect of different medical diseases on mitochondrial transplantation such as diabetes given its known relationship with impaired mitochondrial function. Moreover, despite the similarities of swine and human transcriptome and proteome, it should not be neglected that preclinical findings do not always translate to human data. Additionally, relevant clinical scenarios of our study include normothermic cardiac arrest or iatrogenic myocardial ischemia reperfusion injury which are common in these populations. It would be also interesting to assess the epigenetic alterations associated with the transplantation of mitochondria isolated from cells grown in vitro.
In conclusion, mitochondrial transplantation is associated with transcriptomic and proteomic alterations consistent with decreased apoptosis and inflammation and increased myocardial contractility and angiogenesis in neonatal and pediatric DCD hearts. These findings further unveil the underlaying mechanisms of action of mitochondrial transplantation, while our next steps are focused in translating these findings in human DCD hearts.
Methods
Animal care and biosafety
This study was conducted according to the National Institutes of Health’s guidelines and was approved by the Boston Children’s Hospital’s Animal Care and Use Committee (Protocol 00,001,364). All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (https://www.nap.edu/catalog/12910/guide-for-the-care-and-use-of-laboratory-animals-eighth).
Experimental model
The experimental model has been previously described in detail (Fig. 1)1. Briefly, female Yorkshire neonatal (3–4 kg) and pediatric pigs (10–15 kg) underwent a standardized protocol mimicking the conditions of DCD including 20 min of warm ischemic time (WIT) and 10 min of cold ischemic time (CIT). The WIT represents the average time required for cardiac death from the time of extubation to time of heart cannulation and cardioplegic arrest (achieved by del Nido cardioplegia when the mean arterial pressure equalizes with the central vein pressure), and the CIT, the time from the end of WIT to warm reperfusion of the heart. Another group of hearts representing the Sham group did not undergo any WIT but only the 10 min of CIT since they were directly cannulated and arrested with cardioplegia to avoid any warm ischemia injury. The hearts were reperfused with warm swine blood drawn from the same animal on a custom-made ex situ heart perfusion (ESHP) system at a constant aortic root pressure of 40mmHg39. Mean aortic pressure, calcium, PaO2, PaCO2 and pH were maintained at a fixed level for all experiments. Hearts were paced and inotropic support was provided by dobutamine and epinephrine. Vehicle alone (VEH, 5 ml sterile buffer containing 300 mM Sucrose, 10 mM K + -HEPES pH 7.2, 1 mM K + -EGTA, pH 8.0) or 5 mL of sterile buffer containing isolated mitochondria (MITO, 1 × 109) was delivered as a bolus injection into the aortic root after the first 15 min of ESHP perfusion. Hearts were perfused for a total of 4 h consisting of 2 h of unloaded and then 2 h of isovolumic loaded heart perfusion. The first part represents the transportation time of the organ to its donor and the second part was to assess myocardial function in conditions similar to those post transplantation. The hearts used in this study are a subset of hearts used in a previous study, in which the first 3- 4 hearts from each group were used for RNA and protein extraction1.
Mitochondrial isolation for autologous transplantation
Two pieces of skeletal muscle tissue (0.1 g) were obtained from the pectoralis major prior to heart harvesting. Mitochondria were isolated by filtration as previously described40. The isolated mitochondria were resuspended in sterile buffer containing 300 mM Sucrose, 10 mM K + -HEPES pH 7.2, 1 mM K + -EGTA, pH 8.0 and used for transplantation as previously described (Alemany et al., 2024).
Tissue isolation
At the end of the ESHP, myocardial tissue samples were obtained from preselected areas of the free wall of the left ventricle and were snap frozen and then stored in liquid nitrogen prior to RNA Seq and SOMAscan analysis.
Bulk RNA sequencing and analysis
RNA Seq analysis was performed following ribodepletion and construction of a standard library using Illumina HiSeq2500 V4 2 × 100 PE (Genewiz, South Plainfeld, NJ). All samples were processed using an RNAseq pipeline implemented in the bcbio-nextgen project33. FastQC34 was used to examine raw reads’ quality and ensure that the library generation and sequencing were suitable for subsequent analysis. Trimmed reads were aligned to UCSC build Sscrofa11.1 of the pig genome, augmented with transcript information from Ensembl release 79 using STAR84. FastQC and Qualimap were used to check alignments for evenness of coverage, rRNA content and genomic context. Counts of reads aligning to known genes were generated by featureCounts85. Genes with adjusted False discovery rate (FDR) < 0.05 and log2-fold change (> or < 1) were characterized as differentially expressed genes (DEGs) for each comparison. MetaCore (v20.2) was used for functional enrichment analysis. DEGs having at least one log2-fold change and FDR < 0.05 were used for pathway enrichment analysis. The DEGs were visualized using hierarchical clustering plot and subjected to gene ontology (GO) analysis for enriched biological processes, molecular pathways and cellular localization using GSEA, Metacore, DAVID functional annotation tools and Appyter software.
SOMAscan and proteomic analysis
Protein was extracted using T-Per tissue protein extraction agent (Termo Scientifc, USA) and total protein for sample normalization was determined using the Micro BCAProtein Assay Kit (Termo Scientifc, USA). Proteomic profiling was performed using the SomaScan single-stranded DNA aptamer-based platform using SOMAmer reagents. FDR correction was applied to calculate the adjusted p-value. Proteins having at least one log2-fold change and FDR < 0.05 were used for pathway enrichment analysis. GO enrichment analysis was identified using g:Profiler database and Metascape. Visualization of enriched protein–protein networks was performed in Metascape and String. Using Omics-net we conducted a network-based analysis with significant proteins/genes, including pathway enrichment analysis among the “hub” nodes. In addition, we performed a multi-omic 2-layer network analysis of significant nodes and common predicted upstream regulators as indicated in the Trust V2. Database.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Alemany, V. S. et al. Mitochondrial transplantation preserves myocardial function and viability in pediatric and neonatal pig hearts donated after circulatory death. J Thorac Cardiovasc Surg 167, e6–e21 (2024).
Guariento, A. et al. Mitochondrial transplantation for myocardial protection in ex-situ-perfused hearts donated after circulatory death. J Heart Lung Transplant 39, 1279–1288 (2020).
Soraya, A. S. et al. ATF3 expression in cardiomyocytes and myofibroblasts following transverse aortic constriction displays distinct phenotypes. Int J Cardiol Heart Vasc 32, 100706 (2021).
Nobori, K. et al. ATF3 inhibits doxorubicin-induced apoptosis in cardiac myocytes: A novel cardioprotective role of ATF3. J Mol Cell Cardiol 34, 1387–1397 (2002).
Masuzawa, A. et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 304, H966 (2013).
McCully, J. D. et al. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol 296, H94–H105 (2009).
Guariento, A. et al. Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J Thorac Cardiovasc Surg 160, e15–e29 (2020).
Doulamis, I. P. et al. Transcriptomic and proteomic pathways of diabetic and non-diabetic mitochondrial transplantation. Sci Rep 12, 22101 (2022).
Cowan, D. B. et al. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS ONE 11, e0160889 (2016).
Shin, B. et al. A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: Safety and efficacy. JACC Basic Transl Sci 4, 871–888 (2019).
Wang, J., Toan, S. & Zhou, H. New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis 23, 299–314 (2020).
Ishii, K., Norota, I. & Obara, Y. Endocytic regulation of voltage-dependent potassium channels in the heart. J Pharmacol Sci 120, 264–269 (2012).
Thomas, C. & Tampé, R. Structural and mechanistic principles of ABC transporters. Annu Rev Biochem 89, 605–636 (2020).
Wang, H. et al. p53 contributes to cardiovascular diseases via mitochondria dysfunction: A new paradigm. Free Radic Biol Med 208, 846–858 (2023).
Mak, T. W., Hauck, L., Grothe, D. & Billia, F. p53 regulates the cardiac transcriptome. Proc Natl Acad Sci USA 114, 2331–2336 (2017).
Wang, L., Lin, L., Qi, H., Chen, J. & Grossfeld, P. Endothelial loss of ETS1 impairs coronary vascular development and leads to ventricular non-compaction. Circ Res 131, 371–387 (2022).
Jiao, L. et al. Ribosome biogenesis in disease: New players and therapeutic targets. Signal Transduct Target Ther 8, 15 (2023).
Mesquita, P. H. C. et al. Skeletal muscle ribosome and mitochondrial biogenesis in response to different exercise training modalities. Front Physiol 12, 725866 (2021).
Itoh, Y. et al. Mechanism of mitoribosomal small subunit biogenesis and preinitiation. Nature 606, 603–608 (2022).
Khawaja, A., Cipullo, M., Krüger, A. & Rorbach, J. Insights into mitoribosomal biogenesis from recent structural studies. Trends Biochem Sci 48, 629–641 (2023).
Sun, X. et al. Alda-1 treatment promotes the therapeutic effect of mitochondrial transplantation for myocardial ischemia-reperfusion injury. Bioact Mater 6, 2058–2069 (2021).
Doulamis, I. P. et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am J Physiol Renal Physiol 319, F403–F413 (2020).
Rossi, A. et al. Mitochondria transplantation mitigates damage in an in vitro model of renal tubular injury and in an ex vivo model of DCD renal transplantation. Ann Surg 278, e1313 (2023).
Alexander, J. F. et al. Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits. Theranostics 11, 3109 (2021).
Moskowitzova, K. et al. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 318, L78–L88 (2020).
Lee, J. M. et al. Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants https://doi.org/10.3390/antiox10050696 (2021).
Orfany, A. et al. Mitochondrial transplantation ameliorates acute limb ischemia. J Vasc Surg 71, 1014–1026 (2020).
Ramirez-Barbieri, G. et al. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion 46, 103–115 (2019).
Blitzer, D. et al. Delayed transplantation of autologous mitochondria for cardioprotection in a porcine model. Ann Thorac Surg 109, 711–719 (2020).
Song, J. et al. Functional role of RRS1 in breast cancer cell proliferation. J Cell Mol Med 22, 6304–6313 (2018).
Ren, Z., Yao, L., Liu, J., Qi, Z. & Li, J. Silencing NOB1 Can affect cell proliferation and apoptosis via the C-Jun N-terminal kinase pathway in colorectal cancer. Journal of Investigative Surgery 34, 819–825 (2021).
Zhang, Z. et al. RRP9 promotes gemcitabine resistance in pancreatic cancer via activating AKT signaling pathway. Cell Commun Signal 20, 188 (2022).
Feng, L., Guo, M. & Jin, C. Identification of alternative splicing and RNA-binding proteins involved in myocardial ischemia-reperfusion injury. Genome 66, 261–268 (2023).
Xue, Q. et al. NOG1 downregulates type I interferon production by targeting phosphorylated interferon regulatory factor 3. PLoS Pathog 19, e1011511 (2023).
Imanaka-Yoshida, K., Tawara, I. & Yoshida, T. Tenascin-C in cardiac disease: a sophisticated controller of inflammation, repair, and fibrosis. Am J Physiol Cell Physiol 319, C781–C796 (2020).
Geijtenbeek, T. B. H. & Gringhuis, S. I. Signalling through C-type lectin receptors: Shaping immune responses. Nature Reviews Immunology 9, 465–479 (2009).
Fell, C. W. et al. FIBCD1 is an endocytic GAG receptor associated with a novel neurodevelopmental disorder. EMBO Mol Med 14, 15829 (2022).
Tomita, H., Tanaka, K., Tanaka, T. & Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 7, 11018 (2016).
Duignan, T. et al. A multi-mode system for myocardial functional and physiological assessment during ex situ heart perfusion. J Extra Corpor Technol 52, 303–313 (2020).
Preble, J. M. et al. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J Vis Exp https://doi.org/10.3791/51682 (2014).
Funding
This work was supported by the Richard A. and Susan F. Smith President’s Innovation Award, Michael B. Klein and Family, The Sidman Family Foundation, The Michael B. Rukin Charitable Foundation, The Kenneth C. Griffin Charitable Research Fund, and The Boston Investment Council.
Author information
Authors and Affiliations
Contributions
IPD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing-original draft, Writing-review & editing; AT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing-original draft, Writing-review & editing; VSA: Data curation, Investigation, Methodology, Writing-review & editing; RSN: Data curation, Investigation, Methodology, Writing-review & editing; AC: Data curation, Investigation, Methodology, Writing-review & editing; DRP: Data curation, Investigation, Methodology, Writing-review & editing; MYS: Data curation, Investigation, Methodology, Supervision, Writing-review & editing; AG: Data curation, Methodology, Supervision, Writing-review & editing; SME: Conceptualization, Funding acquisition, Methodology, Supervision, Writing-review & editing; JP: Conceptualization, Funding acquisition, Methodology, Supervision, Writing-review & editing; PDN: Conceptualization, Funding acquisition, Methodology, Supervision, Writing-review & editing; JDM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Doulamis, I.P., Tzani, A., Alemany, V.S. et al. Mitochondrial transplantation normalizes transcriptomic and proteomic shift associated with ischemia reperfusion injury in neonatal hearts donated after circulatory death. Sci Rep 14, 31236 (2024). https://doi.org/10.1038/s41598-024-82578-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82578-2
Keywords
This article is cited by
-
Exploring mitochondrial health and transplantation strategies in DCD heart transplantation: a systematic review
Journal of Translational Medicine (2025)
-
Uptake mechanisms and functions of isolated mitochondria in mesenchymal stromal cells
Scientific Reports (2025)
-
Biotechnological approaches and therapeutic potential of mitochondria transfer and transplantation
Nature Communications (2025)