Abstract
The Restriction Spectrum Imaging restriction score (RSIrs) has been shown to improve the accuracy for diagnosis of clinically significant prostate cancer (csPCa) compared to standard DWI. Both diffusion and T2 properties of prostate tissue contribute to the signal measured in DWI, and studies have demonstrated that each may be valuable for distinguishing csPCa from benign tissue. The purpose of this retrospective study was to (1) determine whether prostate T2 varies across RSI compartments and in the presence of csPCa, and (2) evaluate whether csPCa detection with RSIrs is improved by acquiring multiple scans at different TEs to measure compartmental T2 (cT2). Data includes two cohorts scanned for csPCa with 3T multi-b-value diffusion-weighted sequences acquired at multiple TEs. cT2 values were computed from multi-TE RSI data and compared by compartment. CsPCa detection was compared between RSIrs and a logistic regression model (LRM) to predict the probability of csPCa using cT2 in combination with RSI measurements. Two-sample t-tests (α = 0.05) and the area under the receiver operating characteristic curve (AUC) were used for the statistical analyses. In both cohorts, T2 was different (p < 0.05) across the four RSI compartments (C1, C2, C3, C4). Voxel-level, cohort 1: T2 was different in csPCa for C1, C2, C3 (p < 0.001). Patient-level, cohort 1: T2 was different in csPCa patients in C3 (p = 0.02); cohort 2: T2 differed in csPCa patients in C1 (p = 0.01), C3 (p = 0.01) and C4 (p < 0.01). Voxel-level csPCa detection: cT2 did not improve discrimination over RSIrs alone (p = 0.9). Patient-level: RSIrs and the LRM performed better than diffusion alone (p < 0.001), but the difference in AUCs between RSIrs and the LRM was not significantly different (p = 0.54). In conclusion, significant differences in cT2 were observed between normal and cancerous prostatic tissue. With our data, however, consideration of cT2 in addition to diffusion did not significantly improve cancer detection performance.
Similar content being viewed by others
Introduction
Multiparametric magnetic resonance imaging (mpMRI) has become an important tool for the diagnosis of prostate cancer (PCa)1. MpMRI has proven to reduce unnecessary biopsies, mitigate overdiagnosis of clinically insignificant prostate cancer (indolent PCa), and enhance detection of clinically significant prostate cancer (csPCa, Grade Group ≥ 2)2,3. In standard reporting of prostate MRI (Prostate Imaging Reporting and Data System, PI-RADS v2.14), diffusion-weighted imaging (DWI) and T2 -weighted imaging are the principal modalities used to detect csPCa. DWI measures the random movement of water molecules within tissues, aiding in the visualization of areas of restricted diffusion associated with hypercellular csPCa5. Meanwhile, T2 -weighted imaging provides detailed anatomical information and facilitates the visualization of abnormalities in prostate tissue. The combined analysis of these two sequences allows for a more in-depth evaluation of potential tumor lesions, thereby contributing to csPCa detection and characterization.
The challenge with the interpretation of conventional mpMRI lies in its inherent subjectivity and variability6. The interpretations of imaging data by different radiologists that rely on qualitative assessment alone leads to inconsistencies in the identification and characterization of csPCa lesions7. Interobserver variability significantly limits the accuracy and reliability of csPCa diagnosis8. To enhance diagnostic accuracy, there is a growing emphasis on the development and adoption of quantitative MRI approaches. Quantitative MRI aims to provide objective metrics of tissue properties associated with the probability of csPCa, offering the potential for more standardized and reproducible image assessment.
Restriction Spectrum Imaging (RSI) is a quantitative approach to DWI for csPCa detection and characterization. RSI scans are acquired at multiple b-values (diffusion weightings) to distinguish diffusion signal from tissue micro-compartments (intracellular water, extracellular hindered water, freely diffusing water, and flowing fluid)9,10,11,12. However, RSI models typically do not incorporate quantitative T2 measurements. On the other hand, studies using luminal water imaging (LWI) and hybrid multidimensional MRI have shown that tissue T2 can differ between prostate tissue compartments and provide diagnostic information that is complementary to diffusion13,14.
In this study, we acquired prostate RSI data at multiple echo times (TEs) to measure compartmental T2 in addition to diffusion. We aimed to determine whether compartmental T2 (i.e., T2 within each RSI micro-compartment) differs between cancerous and normal prostate tissue, and whether consideration of compartmental T2 in RSI yields improved detection of csPCa.
Methods
Study population
This study was approved by the University of California San Diego (UCSD) institutional review board (IRB). All research was performed in accordance with relevant guidelines and regulations. An FDA-cleared, commercial version of RSI is used routinely in our center as part of clinical routine. The first cohort included 46 patients scanned for suspected or known PCa between August and December of 2016 with multiple TEs as part of a quality improvement project to determine the best TE. The second cohort included patients who were scanned for suspected or known PCa between March 2021 and January of 2023 with a multi-TE RSI protocol per clinical routine (the need to obtain informed consent was waived by the UCSD IRB for secondary use of routine clinical data) or after informed written consent as part of a clinical trial on treatment response assessment (clinicaltrials.gov NCT04349501). Patients were excluded if they had received any treatment for PCa prior to the MRI acquisition or if a lesion with PI-RADS score ≥ 3 was detected on MRI but no biopsy information was available.
Routine clinical evaluation
Patients in both cohorts underwent prostate MRI as part of routine clinical care for PCa, except for 38 patients in cohort 2 who were scanned as part of a prospective research study without clinical evaluation by a radiologist. MpMRI was performed according to PI-RADS guidelines, and interpretation was made per clinical routine using PI-RADS v2.1. Several patients, all from cohort 2, had PCa diagnosed on systematic biopsy without MRI, and then had an MRI with RSI before any treatment as part of a prospective study; PI-RADS scores are not available for these subjects. PI-RADS interpretation was done as part of clinical practice, but the original lesion segmentations were not available for the present study. Those segmentations are made in routine clinical practice using a proprietary software for biopsy that does not permit exporting the lesions. The radiologists in the present study segmented all biopsy-confirmed lesions, relying on the images themselves and the written description of those biopsy targets provided in the routine clinical PI-RADS reports. The presence of clinically significant prostate cancer (csPCa, grade group ≥ 2) was determined from biopsy results, typically systematic 12-core biopsy with additional targeted cores for suspicious lesions on MRI. Patients with PI-RADS lesions of 1 or 2 with no biopsy were considered negative for csPCa, in accordance with European Association of Urology (EAU) guidelines15,16,17.
RSI data acquisition and processing
All patients were scanned with an expanded MRI protocol that included two multi-b-value RSI acquisitions performed with different TEs. MRI acquisition details are summarized in Table 1. All MR imaging was performed on a 3T clinical scanner (Discovery MR750; GE Healthcare, Waukesha, WI, USA), using a 32-channel phased-array coil over the pelvis. For each patient, two axial, multi-b-value DWI volumes were separately acquired using two different TEs: 80 ms and 100 ms for cohort 1, and 76 ms and 90 ms for cohort 2. All other parameters were the same between scans. In addition to the DWI volumes, a single T2-weighted volume was acquired for anatomical reference using the same scan coverage as the DWI volumes. MRI post-processing was performed using programs implemented in MATLAB R2022a (MathWorks, Natick, MA, USA18). DWI volumes were corrected to account for B0-inhomogeneities, gradient nonlinearities, eddy currents19, and image noise10. Samples at each b-value were averaged together. Image registration20 was applied to correct for patient motion between acquisitions.
For patients in cohort 1, regions of interest (ROIs) were manually defined on T2-weighted images over the whole prostate, peripheral zone, and transition zone (the central zone was included with the transition zone). The contouring of the prostate zones and tumor lesions was performed using MIM software (MIM software version 7.2.6, Inc; Cleveland, OH, USA21), by a radiation oncologist with 3 years of experience and two board-certified sub-specialist radiologists with 4 and 6 years of experience, using all available clinical imaging and pathologic information11. Radiologist-certified contours of the prostate zones and lesions were not obtainable for cohort 2. Instead, automated prostate contours, which are generally highly accurate22, were obtained using an FDA-cleared commercial product (OnQ Prostate version 1.4, CorTechs.ai, San Diego, CA, USA23).
RSI modeling
Prior studies established and validated a four-compartment RSI model of the diffusion signal10,11,24:
S(b) denotes the measured DWI signal intensity at a particular b-value, which is modeled as a linear combination of exponential decays representing four diffusion compartments. Ci describes the compartmental signal contributions to be determined via model-fitting. The diffusion coefficients, Di, are fixed for each of the four tissue compartments to empirically determined values10 that broadly represent restricted diffusion, hindered diffusion, free diffusion, and vascular flow: 1.1e−4, 1.8e−3, 3.6e−3, and 0.1220 mm2/s, respectively. Signal-contribution (Ci) maps were computed for both DWI volumes per patient by fitting this model to the signal-vs.-b-value curve from each voxel. A previously validated biomarker for PCa called the RSI restriction score (RSIrs) was computed by dividing the signal intensity of the restricted diffusion compartment, C1, at each voxel by the median signal intensity within the whole prostate on the b = 0 mm2/s DWI images (an index of apparent T2-weighting in the prostate)11,24,25,26,27.
Compartmental T 2 mapping and analysis by csPCa status
T2 maps were computed for each compartment of the RSI model by fitting the monoexponential T2 decay formula to the signal values from the two Ci maps with different TEs. The median T2 within the whole prostate was then computed. Two-sample t-tests (α = 0.05) were used to determine whether there were significant differences in median T2 between compartments.
For cohort 1, two-sample t-tests (α = 0.05) were used to compare median compartmental T2 values between benign or clinically insignificant PCa tissue and csPCa lesions. For both cohorts, we used two-sample t-tests (α = 0.05) to compare each patient’s median T2 by compartment in the whole prostate, and whether compartmental T2 was significantly different between patients with and without csPCa. Any compartments with a significant difference in T2 between normal and cancerous tissue were noted for inclusion in subsequent multivariable modeling.
Logistic regression model fitting and evaluation of cancer-detection performance
A logistic regression model (LRM) was developed to estimate the probability that a given voxel of tissue contains csPCa given measurements of diffusion and compartmental T2. RSIrs11 was included as the diffusion parameter of the model. Compartmental T2 was included in the LRM for each compartment that showed a significant difference in T2 between normal and cancerous tissue. Cohort 1 had radiologist-certified lesion contours available and was therefore used to train the LRM. In patients with csPCa, voxels inside the lesion contours were labeled as csPCa-positive, while prostate voxels outside the lesion contours were labeled as csPCa-negative. In these patients, voxels labeled as csPCa-positive were included to train the LRM and all non-csPCa voxels were excluded. In patients without csPCa, diffusion and compartmental T2 measurements from all voxels within the entire prostate were used to train the LRM and labeled as csPCa-negative.
Ten-fold cross-validation was performed to evaluate voxel-level csPCa-detection performance of the model within cohort 1. We assessed csPCa-detection performance using the area under the receiver operating characteristic curve (AUC) and calculated 95% confidence intervals (CI) from 10,000 bootstrap samples.
Both cohorts were used to test the patient-level csPCa-detection performance of the model. For patient-level analysis of the LRM, the highest probability value observed within the whole prostate was used as the predictor variable. Similarly, the maximum RSIrs value within the whole prostate was used as the patient-level predictor for RSIrs. We also computed maximum C1 to obtain the patient-level performance of diffusion only, as RSIrs incorporates global prostate T2 signal in addition to diffusion signal. AUC values were computed for maximum C1, maximum RSIrs, and the LRM, and compared using two-sample t-tests (α = 0.05). The 95% confidence intervals were estimated through random sampling with replacement from 10,000 bootstrap patient samples.
Results
Study population
Cohort 1 comprised 46 patients (age: 70 ± 10 years; PSA: 10.6 ± 16.9 ng/mL). Cohort 2 comprised 195 patients (age: 69 ± 8 years; PSA: 8.2 ± 8.5 ng/mL). In cohort 1, 22 of 46 patients (47.8%) had csPCa, while the remaining 24 had either low-grade (grade group 1) disease or no cancer. In cohort 2, 96 of 195 (49.2%) patients had csPCa. 38 participants from cohort 2 had no PI-RADS scores available because csPCa was diagnosed on systematic biopsy without MRI and then had an MRI with RSI acquisition as part of a separate, prospective study. Table 2 summarizes the patient characteristics of both cohorts.
Compartmental T 2 mapping
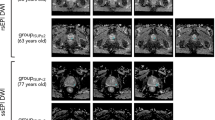
Figure 1 shows compartmental T2 maps for two patients with csPCa, one from each cohort.
RSI signal contribution (Ci) and compartmental T2 (cT2) maps for two patients with csPCa. (A) Patient from cohort 1. (B) Patient from cohort 2. Compartmental T2 maps were computed for each RSI model compartment by measuring the T2 -weighted signal decay of the signal-contribution map for different TEs. The signal-contribution maps shown here were computed from the DWI acquisition with shorter TE. Whole-mount (WM) histopathology results were available for the patient from cohort 2 and illustrate the lesion contour in the prostate. For cohort 1, the contouring of the prostate zones and tumor lesions was performed using MIM software (MIM software version 7.2.6, Inc; Cleveland, OH, USA21). For cohort 2, automated prostate contours were obtained using an FDA-cleared commercial product (OnQ Prostate version 1.4, CorTechs.ai, San Diego, CA, USA23).
Figure 2 shows violin plots of median T2 within the whole prostate for each RSI model compartment. In both cohorts, the highest median T2 values were observed in C3, followed by C2, C4, and finally C1. Compartmental T2 was significantly different between any two compartments (p < 0.05).
Violin plots showing the distribution of median T2 values in the whole prostate for each of the four RSI model compartments. Within each cohort, compartmental T2 was significantly different between any two compartments (p < 0.05). (A) cohort 1 (n = 46). (B) cohort 2 (n = 195). Plots were created using MATLAB R2022a (MathWorks, Natick, MA, USA18). For cohort 1, the contouring of the prostate zones and tumor lesions was performed using MIM software (MIM software version 7.2.6, Inc; Cleveland, OH, USA21). For cohort 2, automated prostate contours were obtained using an FDA-cleared commercial product (OnQ Prostate version 1.4, CorTechs.ai, San Diego, CA, USA23).
Voxel-level analysis of compartmental T 2 values by csPCa status
For each compartment of the RSI model, the comparison of T2 values between csPCa and prostate tissue outside of csPCa lesions is illustrated in Fig. 3. This figure corresponds to cohort 1, which has radiologist-certified csPCa lesion contours. csPCa lesions showed significantly higher compartmental T2 values in compartment 1 (p << 0.001) and compartment 2 (p < < 0.001) than normal tissue. In addition, the compartmental T2 values of compartment C3 were significantly lower in csPCa lesions (p << 0.001). The compartmental T2 values for compartment C4 were not significantly different between csPCa and normal tissues (p = 0.17).
Violin plots showing the distribution of median compartmental T2 values from cohort 1 in both csPCa lesions and the surrounding prostate tissue. Each panel corresponds to one of the RSI diffusion compartments. csPCa lesions showed significantly higher compartmental T2 values in C1 (p-value < < 0.001) and C2 (p-value < < 0.001) than tissues outside of csPCa lesions. Compartmental T2 values of C3 were also significantly lower in csPCa lesions than outside csPCa lesions (p-value < < 0.001). A red star indicates a significant difference (p-value < 0.05) in compartmental T2 between csPCa lesions and the prostate tissue outside the lesions. WP: whole prostate. Plots were created using MATLAB R2022a (MathWorks, Natick, MA, USA18). For cohort 1, the contouring of the prostate zones and tumor lesions was performed using MIM software (MIM software version 7.2.6, Inc; Cleveland, OH, USA21).
Patient-level analysis of compartmental T 2 values by csPCa status
The comparison of compartmental T2 values between patients with and without csPCa is shown in Fig. 4. In both cohorts, patients with csPCa had higher compartmental T2 values in compartment C1 than patients with no csPCa. In cohort 2, median C1 compartmental T2 was significantly higher (p = 0.07 for cohort 1; p = 0.01 for cohort 2). Median C3 compartmental T2 was significantly different between csPCa and patients without csPCa in both cohorts (p = 0.02 for cohort 1; p = 0.01 for cohort 2). Median C4 compartmental T2 was also significantly different in cohort 2 (p < < 0.01). Compartmental T2 values for the other compartments were not significantly different between patients with and without csPCa.
Violin plots comparing compartmental T2 within the whole prostate between patients with csPCa and those without. A red star indicates a significant difference (p-value < 0.05) in whole-prostate compartmental T2 between csPCa and non-csPCa patients. (A) cohort 1 (n = 46). (B) cohort 2 (n = 195). Plots were created using MATLAB R2022a (MathWorks, Natick, MA, USA18). For cohort 1, the contouring of the prostate zones and tumor lesions was performed using MIM software (MIM software version 7.2.6, Inc; Cleveland, OH, USA21). For cohort 2, automated prostate contours were obtained using an FDA-cleared commercial product (OnQ Prostate version 1.4, CorTechs.ai, San Diego, CA, USA23).
Logistic regression model fitting and evaluation of csPCa-detection performance
T2 measurements from RSI compartments 1, 2, and 3 (C1, C2, C3) were included as the T2 parameters of the LRM. These three compartments showed significantly different median T2 signal between csPCa lesions vs csPCa-negative voxels. The LRM predictors were RSIrs, C1-T2, C2-T2, and C3-T2. The model coefficients with 95% confidence interval for the y-intercept and RSIrs were 6.367 (6.323, 6.416) and -51.621 (-52.354, -50.888), respectively. The weights for C1-T2, C2-T2, and C3 T2 were < 0.005. Example probability maps computed from the model are shown in Fig. 5 for two patients with csPCa, alongside maps of RSIrs.
RSI restriction score and multivariable model probability maps of the prostate for patients with csPCa. Top panel: Patient from cohort 1 with a lesion in the left peripheral zone. Bottom panel: Patient from cohort 2 with a lesion in the transition zone. The multivariable model uses compartmental T2 measurements from each voxel in addition to the RSI restriction score to determine the probability that it contains csPCa. For the patient from cohort 1, the contouring of the prostate zones and tumor lesions was performed using MIM software (MIM software version 7.2.6, Inc; Cleveland, OH, USA21). For patient from cohort 2, automated prostate contours were obtained using an FDA-cleared commercial product (OnQ Prostate version 1.4, CorTechs.ai, San Diego, CA, USA23).
Voxel-level cancer detection
For voxel-level cancer detection, the tenfold cross-validation mean AUC of the LRM was 0.98 [95% CI: 0.957–0.985], versus 0.98 [95% CI: 0.958–0.986] for maximum RSIrs, indicating that incorporating compartmental T2 did not improve discrimination over RSIrs alone (p = 0.9).
Patient-level cancer detection
For cohort 1, the AUC of the LRM was 0.804 [0.648–0.930], versus 0.805 [0.648–0.931] for RSIrs. The mean AUC for maximum C1 was 0.695 [0.530–0.851]. The difference in AUCs between RSIrs and the multivariable model was not significantly different (p = 0.26). Both RSIrs and the LRM performed significantly better than diffusion (maximum C1) alone (both p << 0.001).
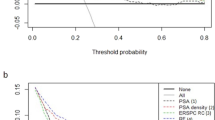
For cohort 2, the mean LRM AUC for 10,000 bootstrapped samples was 0.724 [0.650–0.793]. The mean AUC of RSIrs for 10,000 bootstrapped samples was 0.725 [0.652–0.794]. For maximum C1 the mean AUC was 0.654 [0.573, 0.730]. The difference in AUCs between RSIrs and the multivariable model was not significantly different (p = 0.54). Both RSIrs and the LRM performed significantly better than diffusion (maximum C1) alone (both p << 0.001). ROC curves for both cohorts are shown in Fig. 6.
Patient-level diagnostic performance of maximum RSIrs, the LRM and maximum C1 (max C1) for cohorts 1 and 2. (A) For cohort 1, mean AUC values were 0.695 for maximum C1, 0.805 for RSIrs and 0.804 for the multivariable model. (B) For cohort 2, mean AUC values were 0.654 for maximum C1, 0.725 for RSIrs and 0.724 for the multivariable model. Plots were created using MATLAB R2022a (MathWorks, Natick, MA, USA18). For cohort 1, the contouring of the prostate zones and tumor lesions was performed using MIM software (MIM software version 7.2.6, Inc; Cleveland, OH, USA21). For cohort 2, automated prostate contours were obtained using an FDA-cleared commercial product (OnQ Prostate version 1.4, CorTechs.ai, San Diego, CA, USA23).
Discussion
We found that compartmental T2 values were significantly different across RSI diffusion compartments. Moreover, compartmental T2 values differed between csPCa lesions and benign tissue or low-grade PCa in RSI compartments C1, C2, and C3. At the patient level, there were also differences in whole-prostate T2 between patients with no csPCa and those with biopsy-proven csPCa. Quantitative differences in compartmental T2 may provide insight into the microstructural changes associated with PCa. For example, extracellular matrix remodeling may contribute to the increased compartmental T2 observed in C212. Lower compartmental T2 values in C3 may reflect hyperplasia-induced reductions in luminal space13. While the elevated compartmental T2 observed in C1 of csPCa patients may seem opposed to the known overall hypointense appearance of csPCa on T2-weighted MRI, it is consistent with an increase in nuclear volume fraction that is typical of cancer cells28.
We have previously shown that RSIrs is a useful quantitative DWI biomarker for csPCa at the voxel- and patient-level11,24,26. Here, we evaluated whether incorporating compartmental T2 values would improve csPCa discrimination over RSIrs alone. We demonstrated that T2 effects have csPCa discriminatory value, as they showed higher AUC values compared to diffusion alone in both cohorts. However, consideration of compartmental T2 did not significantly improve csPCa-detection performance over maximum RSIrs at the voxel- or patient-level. This finding may suggest an overlap of microstructural information that is captured by diffusion and T2-weighted imaging. A number of studies have demonstrated an interdependence between apparent diffusion coefficient (ADC) measurements and T2 values29,30. Diffusion measurements from RSI and T2 measurements from techniques like Luminal Water Imaging (LWI) are both also strongly correlated with microstructural tissue features, including cellularity and luminal water space, that are indicative of cancer31. It may be that the RSI and T2 measurements in this study both reflect similar aspects of tissue microstructure, and their combination therefore does not yield a substantial increase of diagnostically useful information32.
As with any conventional DWI acquisition, signal from the individual RSI compartments is also partially T2-weighted. RSIrs further incorporates a measure of global T2-weighted signal in the prostate, namely the median signal within the prostate on the b = 0 s/mm2 volume. These T2-weighting factors intrinsic to RSIrs are correlated with the values obtained from quantitative T2-mapping, so the addition of T2-mapping may not have provided sufficiently complementary information to improve csPCa discrimination performance.
Other advanced imaging techniques have also been used to measure T2 in the prostate. LWI utilizes the unique T2 relaxation rates associated with various components of prostate tissue to quantify the fractional volume of glandular lumen, denoted as luminal water fraction (LWF)33. This method takes advantage of the observed alterations in the composition of prostatic tissue in the presence of cancer and the Gleason grade of the cancer for PCa diagnosis31. Hybrid multidimensional MRI (HM-MRI) exploits the interdependence of T2 and ADC values to measure prostate volume fractions of the lumen, epithelium, and stroma34. Studies using LWI and HM-MRI demonstrate lower T2 values in cancer lesions compared to normal prostate tissue with increasing Gleason Grade. The decrease in T2 results from a decrease in luminal volume due to cellular hyperplasia31,35. This trend agrees with the decrease observed in this study of T2 in compartment C3 of patients with csPCa. This RSI compartment reflects signal from freely diffusing water in the prostate, which we expect to find predominantly in luminal tissue and to be impacted by a reduction in luminal space. Prior work with HM-MRI showed that the decrease in luminal space is largely the result of epithelial tissue proliferation, indicated by an increase in the measured epithelial volume fraction34. Since RSI does not explicitly assign signal contributions to a particular tissue type, this aspect of HM-MRI is harder to align with the present study. However, we can be sure that the changes observed in the T2 of compartment C1 reflect, at least in part, an increase in the overall tissue cellularity36. Signal contributions in this compartment are also dependent upon the nuclear volume fraction of cells in the tissue28, and the increase in the T2 observed in C1 of csPCa patients suggests an increase in nuclear volume fraction.
While quantitative T2 did not yield improved patient-level csPCa discrimination compared to RSI alone, the ROI-based analysis of this study suggests that it may be helpful for lesion-level detection and characterization of tumors at a microscopic level. Whole-mount histopathology (WMHP) data are currently being collected as part of an ongoing study to map changes in compartmental RSI signal to the histological restructuring of prostate tissue due to csPCa. This mapping aims to correlate RSI and T2 signal with specific microscopic alterations observed in tumors. This approach could also aid in identifying variations in T2 across different tumor grades, providing a non-invasive means to predict grade group and assess the aggressiveness of cancer13, a crucial prognostic indicator. Another ongoing, prospective trial (ART-Pro; NCT06579417) is evaluating the impact of RSIrs and compartmental T2 on csPCa diagnosis in a heterogeneous multi-center and multi-reader dataset37.
Future work that pairs RSI with a more advanced, multicompartmental approach to T2 measurement, such as LWI, would allow for deeper insight into the relationship between compartmental diffusion and T2 than was achievable here. Our acquisition protocol involved only a sparse sampling of two TEs compared to 5 b-values, while LWI would provide many more TE measurements to enable a more granular assessment of the T2 microenvironment. This would help determine whether diffusion and T2 measurements truly provide complementary information about the prostate and serve to enhance csPCa detection accuracy when used together.
Limitations
The use of separate acquisitions and only two TEs per patient may have limited the accuracy of voxel-wise compartmental T2 measurements. However, the inclusion of additional TEs was restricted to avoid excessive protocol length on active clinical scanners.
While other quantitative MRI approaches use lower TEs (< 30 ms) for T2 mapping14,31, the high b-values required by RSI to optimally estimate C1 are generally incompatible with very low TEs on clinical scanners. Separate acquisitions would therefore be required to evaluate the combination of optimal RSIrs and T2 estimated with very low TE. Another limitation of this study is that our voxel-level data included only high-confidence csPCa and control categories, leaving little room for improvement over RSIrs for the voxel-level analysis11.
Conclusion
T2 mapping affords insights into characteristics of benign and cancerous prostate tissue, but we did not find compelling evidence that acquisitions with multiple TE is necessary for patient-level csPCa detection with RSI.
Data availability
Data are available for bona fide researchers who request it from the authors. Please contact Dr. Tyler Seibert (tseibert@health.ucsd.edu) if you would like to request the data from this study.
References
Leung, D.K.-W., Chiu, P.K.-F., Ng, C.-F. & Teoh, J.Y.-C. Role of pre-biopsy multiparametric MRI in prostate cancer diagnosis: Evidence from the literature. Turk. J. Urol. 47(Suppl 1), S65–S70. https://doi.org/10.5152/tud.2020.20360 (2021).
de Rooij, M., Hamoen, E. H. J., Fütterer, J. J., Barentsz, J. O. & Rovers, M. M. Accuracy of multiparametric MRI for prostate cancer detection: A meta-analysis. AJR Am. J. Roentgenol. 202(2), 343–351. https://doi.org/10.2214/AJR.13.11046 (2014).
Schoots, I. G. et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: A systematic review and meta-analysis. Eur. Urol. 68(3), 438–450. https://doi.org/10.1016/j.eururo.2014.11.037 (2015).
Scott, R., Misser, S. K., Cioni, D. & Neri, E. PI-RADS v2.1: What has changed and how to report. SA J. Radiol. 25(1), 2062. https://doi.org/10.4102/sajr.v25i1.2062 (2021).
Demirel, H. C. & Davis, J. W. Multiparametric magnetic resonance imaging: Overview of the technique, clinical applications in prostate biopsy and future directions. Turk. J. Urol. 44(2), 93–102. https://doi.org/10.5152/tud.2018.56056 (2018).
Pickersgill, N. A. et al. Accuracy and variability of prostate multiparametric magnetic resonance imaging interpretation using the prostate imaging reporting and data system: A blinded comparison of radiologists. Eur. Urol. Focus 6(2), 267–272. https://doi.org/10.1016/j.euf.2018.10.008 (2020).
Midiri, F., Vernuccio, F., Purpura, P., Alongi, P. & Bartolotta, T. V. Multiparametric MRI and radiomics in prostate cancer: A review of the current literature. Diagn. Basel Switz. 11(10), 1829. https://doi.org/10.3390/diagnostics11101829 (2021).
Stabile, A. et al. Factors influencing variability in the performance of multiparametric magnetic resonance imaging in detecting clinically significant prostate cancer: A systematic literature review. Eur. Urol. Oncol. 3(2), 145. https://doi.org/10.1016/j.euo.2020.02.005 (2020).
Brunsing, R. L. et al. Restriction spectrum imaging: An evolving imaging biomarker in prostate MRI: Prostate MRI with restriction spectrum imaging: A review. J. Magn. Reson. Imaging 45(2), 323–336. https://doi.org/10.1002/jmri.25419 (2017).
Conlin, C. C. et al. Improved characterization of diffusion in normal and cancerous prostate tissue through optimization of the restriction spectrum imaging signal model. Radiol. Imaging https://doi.org/10.1101/2020.03.27.20042069 (2020).
Feng, C. H. et al. Voxel-level classification of prostate cancer on magnetic resonance imaging: Improving accuracy using four-compartment restriction spectrum imaging. J. Magn. Reson. Imaging 54(3), 975–984. https://doi.org/10.1002/jmri.27623 (2021).
White, N. S. et al. Diffusion-weighted imaging in cancer: Physical foundations and applications of restriction spectrum imaging. Cancer Res. 74(17), 4638–4652. https://doi.org/10.1158/0008-5472.CAN-13-3534 (2014).
Hectors, S. J., Said, D., Gnerre, J., Tewari, A. & Taouli, B. Luminal water imaging: Comparison With diffusion-weighted imaging (DWI) and PI-RADS for characterization of prostate cancer aggressiveness. J. Magn. Reson. Imaging 52(1), 271–279. https://doi.org/10.1002/jmri.27050 (2020).
Chatterjee, A. et al. Diagnosis of prostate cancer with noninvasive estimation of prostate tissue composition by using hybrid multidimensional MR imaging: A feasibility study. Radiology 287(3), 864–873. https://doi.org/10.1148/radiol.2018171130 (2018).
EAU Guidelines on Prostate Cancer—Uroweb. Uroweb - European Association of Urology. Accessed: Oct. 16, 2023. [Online]. https://uroweb.org/guidelines/prostate-cancer
Abdul Raheem, R. et al. Can a prostate biopsy be safely deferred on PI-RADS 1,2 or 3 lesions seen on pre-biopsy mp-MRI?. Arab. J. Urol. 21(1), 10–17. https://doi.org/10.1080/2090598X.2022.2119711 (2023).
Reijnen, J. S. et al. Results from a PI-RADS-based MRI-directed diagnostic pathway for biopsy-naive patients in a non-university hospital. Abdom. Radiol. N. Y. 46(12), 5639–5646. https://doi.org/10.1007/s00261-021-03249-8 (2021).
“MATLAB.” Accessed: Nov. 19, 2024. [Online]. https://www.mathworks.com/products/matlab.html
Holland, D., Kuperman, J. M. & Dale, A. M. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. NeuroImage 50(1), 175–183. https://doi.org/10.1016/j.neuroimage.2009.11.044 (2010).
Paquin, D., Levy, D., Schreibmann, E. & Xing, L. Multiscale image registration. Math. Biosci. Eng. 3(2), 389–418 (2006).
MIM Software Inc. | Precision Care Simplified. Accessed: Nov. 17, 2024. [Online]. http://mimsoftware-5300642.hs-sites.com
Y. Song et al. Precise prostate contours: Setting the bar and meticulously evaluating AI performance. https://doi.org/10.1101/2024.10.21.24315771 (2024).
“OnQTM Prostate - Cortechs.ai.” Accessed: Nov. 14, 2024. [Online]. https://www.cortechs.ai/solution/onq-prostate/
Zhong, A. Y. et al. Automated patient-level prostate cancer detection with quantitative diffusion magnetic resonance imaging. Eur. Urol. Open Sci. 47, 20–28. https://doi.org/10.1016/j.euros.2022.11.009 (2023).
Conlin, C. C. et al. Background prostate tissue is quantitatively abnormal on MRI in patients with clinically significant prostate cancer. Radiol. Imaging https://doi.org/10.1101/2022.10.12.22280855 (2022).
Lui, A. J. et al. ReIGNITE RT boost: An international study testing the accuracy and feasibility of using restriction spectrum imaging (RSI) MRI to guide intraprostatic tumor target volume for radiotherapy boost. Oncology https://doi.org/10.1101/2022.12.13.22283420 (2022).
Kallis, K. et al. Comparison of synthesized and acquired high b-value diffusion-weighted MRI for detection of prostate cancer. Radiol. Imaging https://doi.org/10.1101/2023.02.17.23286100 (2023).
White, N. S. & Dale, A. M. Distinct effects of nuclear volume fraction and cell diameter on high b-value diffusion MRI contrast in tumors: Diffusion in Tumor Cells. Magn. Reson. Med. 72(5), 1435–1443. https://doi.org/10.1002/mrm.25039 (2014).
Sadinski, M. et al. Pilot study of the use of hybrid multidimensional T2-weighted imaging–DWI for the diagnosis of prostate cancer and evaluation of Gleason score. Am. J. Roentgenol. 207(3), 592–598. https://doi.org/10.2214/AJR.15.15626 (2016).
Wang, S. et al. Hybrid multidimensional T2 and diffusion-weighted MRI for prostate cancer detection. J. Magn. Reson. Imaging JMRI 39(4), 781. https://doi.org/10.1002/jmri.24212 (2013).
Sabouri, S. et al. Luminal water imaging: A new MR imaging T2 mapping technique for prostate cancer diagnosis. Radiology 284(2), 451–459. https://doi.org/10.1148/radiol.2017161687 (2017).
Hepp, T. et al. T2 mapping for the characterization of prostate lesions. World J. Urol. 40(6), 1455. https://doi.org/10.1007/s00345-022-03991-8 (2022).
Chatterjee, A., Harmath, C. & Oto, A. New prostate MRI techniques and sequences. Abdom. Radiol. 45(12), 4052–4062. https://doi.org/10.1007/s00261-020-02504-8 (2020).
Lee, G. H. et al. Comparing radiologist performance in diagnosing clinically significant prostate cancer with multiparametric versus hybrid multidimensional MRI. Radiology 305(2), 399–407. https://doi.org/10.1148/radiol.211895 (2022).
Chatterjee, A. et al. Changes in epithelium, stroma, and lumen space correlate more strongly with Gleason pattern and are stronger predictors of prostate ADC changes than cellularity metrics. Radiology 277(3), 751–762. https://doi.org/10.1148/radiol.2015142414 (2015).
Liss, M. A. et al. MRI-derived restriction spectrum imaging cellularity index is associated with high grade prostate cancer on radical prostatectomy specimens. Front. Oncol. https://doi.org/10.3389/fonc.2015.00030 (2015).
M. T. Baxter et al. 2024 Advanced Restriction imaging and reconstruction Technology for Prostate MRI (ART-Pro): Study protocol for a multicenter, multinational trial evaluating biparametric MRI and advanced, quantitative diffusion MRI for detection of prostate cancer. medRxiv https://doi.org/10.1101/2024.08.29.24311575 (2024).
Acknowledgements
This work was supported, in part, by the National Institutes of Health (NIH/NIBIB K08EB026503, NIH UL1TR000100), the American Society for Radiation Oncology, the Prostate Cancer Foundation (PCF20YOUN01), and the U.S. Department of Defense (DOD/CDMRP PC220278).
Author information
Authors and Affiliations
Contributions
M.R.D. and C.C.C. contributed equally to the work. Drafting of the manuscript: M.R.D., C.C.C., T.M.S. Statistical Analyses, Conception and Design: M.R.D., C.C.C., R.K., T.M.S. Data acquisition: C.O., M.T.B., D.D.D., M.R.D., C.C.C., T.M.S. Manuscript revision: all authors. Corresponding Author: T.M.S.
Corresponding author
Ethics declarations
Competing interests
M.E.H. reports honoraria from Multimodal Imaging Services Corporation and research funding from GE Healthcare. R.R.P. has an equity interest in CorTechs Labs and Curemetrix, serves on the scientific advisory board of Imagine Scientific, and has received consulting fees from Bayer and research funding from GE Healthcare. A.M.D. is a founder of and holds equity in CorTechs Labs, Inc, and serves on its scientific advisory board; he also is a member of the scientific advisory board of Human Longevity, Inc, and receives funding through research agreements with GE Healthcare. T.M.S. reports honoraria from CorTechs Labs, Varian Medical Systems, WebMD, GE Healthcare, and Janssen; has an equity interest in CorTechs Labs, Inc, and serves on its scientific advisory board; and has received in-kind research support from GE Healthcare via a research agreement with the University of California, San Diego. These companies might potentially benefit from the research results. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. All the remaining authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rojo Domingo, M., Conlin, C.C., Karunamuni, R. et al. Utility of quantitative measurement of T2 using restriction spectrum imaging for detection of clinically significant prostate cancer. Sci Rep 14, 31318 (2024). https://doi.org/10.1038/s41598-024-82742-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82742-8