Abstract
This study used ultra-widefield swept-source optical coherence tomography angiography (UWF SS-OCTA) to analyze and compare choroidal blood flow and anatomical changes in eyes affected by central serous chorioretinopathy (CSC), pachychoroid neovasculopathy (PNV), and uncomplicated pachychoroid (UCP). The findings revealed distribution patterns of vortex veins across the three patient groups and provided initial findings insights into the origin of choroidal neovascularization (CNV) in PNV. A total of 44 patients with CSC, 38 with PNV, and 46 with UCP were included in the analysis. Three-dimensional data were obtained from scans measuring 20 mm vertically by 24 mm horizontally with a scan depth of 6 mm, covering nine subfields. The results showed that CSC groups exhibited significantly higher choroidal vessel volume per unit area (mCVV/a), choroidal vascularity index (CVI), and choroidal thickness (CT) compared to the PNV and UCP groups. While the PNV and UCP groups shared similar choroidal vascular characteristics, the PNV group exhibited slightly higher choroidal vessel volume and notably the lowest foveal choriocapillaris density. These findings suggest that PNV and CSC may represent a continuous pathological spectrum, while UCP might be considered a precursor to CSC or a stage following its resolution. Additionally, further analysis indicated that CNV in PNV could be attributed to choriocapillaris occlusion and ischemia resulting from mechanical compression by hypertrophic choroidal vessels. These findings enhance our understanding of choroidal structure and hemodynamics within pachychoroid spectrum diseases (PSD), shedding light on shared etiological mechanisms and advancing the characterization of their pathophysiology.
Similar content being viewed by others
Introduction
Pachychoroid spectrum disease (PSD) is associated with structural and functional changes in the choroid, primarily characterized by diffuse choroidal thickening and the presence of “pachyvessels”1. The term was introduced by Warrow et al.2 in 2013 to describe ocular diseases with similar features, including central serous chorioretinopathy (CSC) and polypoidal choroidal vasculopathy (PCV), and has since become an important classification standard in ophthalmic imaging. However, classifying PSD has become increasingly complex due to overlapping imaging and clinical features among subtypes. Additionally, variations in treatment response further complicate clinical decision-making. To refine the classification of PSD, Siedlecki et al.3 proposed a system that categorizes pachychoroid spectrum diseases into five types: Type 0/UCP (uncomplicated pachychoroid), Type I/PPE (pachychoroid pigment epitheliopathy), Type II/CSC, Type III/PNV (pachychoroid neovasculopathy), and Type IV/AT1, now termed pachychoroid aneurysmal type 1 choroidal neovascularization, formerly referred to as PCV.
Choroidal thickening and vortex vein dilation are key contributors to abnormal choroidal blood flow in PSD4,5. Studies indicate that vortex vein remodeling, including connections between superior and inferior vortex veins, is commonly observed in PSD and may be closely linked to CSC development and progression4,5,6,7. However, the relationship between vortex vein dilation and PSD subtypes is still underexplored. Based on Siedlecki’s classification, this study systematically analyzes the characteristics of vortex vein dilation across different PSD subtypes. We hypothesize that vortex vein dilation exhibits distinct pathological features in each subtype, reflecting unique pathophysiological mechanisms. Through investigating these dilation characteristics, we aim to clarify the role of vortex veins in PSD progression and assess their potential as biomarkers for personalized treatment.
To achieve this, we employ ultra-widefield swept-source optical coherence tomography angiography (UWF SS-OCTA)—an advanced imaging technology that provides comprehensive data and structural insights critical for quantitative PSD analysis. Compared to traditional OCT, UWF SS-OCTA enhances visualization of choroidal blood flow, making it a valuable tool for studying vascular changes in UCP, CSC, and PNV patients8. This study leverages UWF SS-OCTA to evaluate and compare choroidal vascular alterations, with a specific focus on the relationship between choroidal neovascularization (CNV) and vortex vein dilation in PNV to reveal potential pathophysiological mechanisms.
Methodology
Participants
This cross-sectional research was conducted at the Ophthalmology Department of the General Hospital under the Central Theater Command from April 2023 to February 2024. A total of 44 CSC patients, 38 PNV patients, and 46 UCP patients were enrolled in the study. Ethical approval was obtained from the Ethics Review Board of the General Hospital under the Central Theater Command, in accordance with the Declaration of Helsinki. All participants provided written informed consent before participating in the study.
The CSC diagnosis is based on specific criteria: (1) the presence of subretinal fluid (SRF) and/or pigment epithelial detachment (PED) visible on UWF SS-OCTA, (2) retinal pigment epithelium (RPE) dye leakage on fundus fluorescein angiography (FFA), and (3) enhanced choroidal vascular permeability detectable in the late phase of indocyanine green angiography (ICGA). PNV is diagnosed when CNV associated with a thick choroid is detected by FFA, ICGA, UWF SS-OCTA, or a combination of these imaging techniques. Notably, CNV associated with polypoidal lesions, termed PCV, is not classified within the PNV category. UCP9 is identified by increased subfoveal choroidal thickness (SFCT) > 300 µm or an extrafoveal region at least 50 µm thicker), dilated choroidal vessels, inner choroidal thinning, and the absence of abnormalities such as PED, CSC, PNV, or PCV on fundus angiography (FA), ICGA, or UWF SS-OCTA. Exclusion criteria included: (1) patients with macular neuroepithelial detachment and PED resulting from age-related macular degeneration (AMD), along with other underlying diseases; (2) high myopia, refractive stromal opacity, or inability to cooperate during examination; (3) patients undergoing intraocular surgery other than cataract surgery; (4) poor image quality (OCT or OCTA score below 8); and (5) severe systemic diseases.
Ultra-widefield swept-source optical coherence tomography angiography
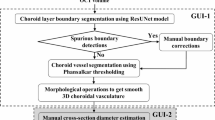
In this study, a 400 kHz UWF SS-OCTA device (BMizar, TowardPi Medical Technology, Beijing, China) was used for high-resolution imaging. This device employs a 1060 nm vertical-cavity surface-emitting laser (VCSEL) with a scan range of 24 × 20 mm, a depth of 6 mm, an axial resolution of 3.8 µm, and a lateral resolution of 10 µm, enabling detailed visualization of retinal and choroidal structures. During image acquisition, a higher-order moment amplitude decorrelation angiography (HMADA) algorithm was applied to minimize motion artifacts and enhance vessel clarity. Artificial intelligence-assisted segmentation software was used to precisely identify ocular structural layers, including Bruch’s membrane (BM) and the choroid-scleral interface (CSI). Initial segmentation results were generated by the automated software, which were then reviewed for accuracy by two experienced ophthalmologists (SYP and YM). When automated segmentation was inadequate, trained technical staff manually adjusted the segmentation boundaries to ensure precision, thereby enhancing the reliability of the image quality.
The choroid was classified into two main layers based on vessel diameter: the choriocapillaris layer (CC layer), defined as the region from Bruch’s membrane to 29 µm below it, and the large and medium choroidal vessel layer (LMCV layer), extending from 29 µm below Bruch’s membrane to the CSI. Data were collected from nine regions: superotemporal, superior, superonasal, temporal, central, nasal, inferotemporal, inferior, and inferonasal. The measured parameters included choroidal thickness (CT), choroidal vascularity index (CVI), choroidal vessel volume per unit area (mCVV/a), and choriocapillaris density (VD-cc). CT was defined as the vertical distance from Bruch’s membrane to CSI. CVI was defined as the ratio of choroidal vessel volume (CVV) to total choroidal volume, reflecting the vascular density of the choroid. VD-cc was automatically calculated as the ratio of the vessel pixel area to the total pixel area within each designated region. mCVV/a represented the choroidal vessel volume per unit area, assessing vascular distribution within the choroid. All parameters were automatically calculated by the software, with manual adjustments performed by technical staff when necessary to ensure data accuracy (Fig. 1).
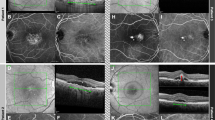
Representative ultra-widefield swept-source optical coherence tomography angiography (UWF SS-OCTA) images. The maps include choroidal vessel volume per unit area (a–c), choroidal vessel volume index (d–f), choriocapillaris density (g–i), and choroidal thickness (j–l) for central serous chorioretinopathy (CSC) (a,d,g,j), pachychoroidal neovasculopathy (PNV) (b,e,h,k), and uncomplicated pachychoroid (UCP) (c,f,i,l) eyes.
To correct for magnification errors associated with axial length (AL) in the quantification of vascular parameters, a standardized measurement method was applied. Specifically, the modified Littmann formula was used, with adjustments based on the Bennett procedure, to standardize the measurement range across all participants. This approach ensured consistent and reliable comparisons of choroidal vascular parameters across individuals in the study population.
Image quality control
To minimize the influence of diurnal variation on choroidal thickness, all UWF SS-OCT/OCTA images were acquired between 8:00 a.m. and 11:30 a.m. To ensure image quality, all patients underwent imaging post-dilation, which improved field clarity and ensured imaging consistency. Only images with a quality score of 8 or higher were included, and each patient underwent two scans, from which the highest quality and most structurally clear image was selected for further analysis. To maintain stable eye positioning during imaging, patients were instructed to focus on a fixed point.
VVDP classification
In this study, the VVPD classification criteria were based on the line connecting the optic disc and the fovea, which was used to divide the upper and lower regions to observe vortex vein dilation patterns. The VVPD classification includes the following three patterns: Upper-Dominant: In en face UWF SS-OCTA images, with the line connecting the optic disc and fovea as the boundary, primary dilation is concentrated above the line. The upper vortex veins exhibit significantly greater dilation and stronger blood flow signals compared to the lower region. Lower-Dominant: Dilation is mainly concentrated below the line connecting the optic disc and fovea, with the lower vortex veins showing more pronounced dilation and stronger blood flow signals than the upper region. Symmetric: The dilation of the upper and lower vortex veins is approximately equal, with relatively uniform blood flow distribution along the line connecting the optic disc and fovea (Fig. 2).
To ensure classification consistency, VVDP was independently assessed by two experienced retinal specialists (SYP and YM) based on en face UWF SS-OCTA images. In cases of disagreement, a third retinal imaging specialist was consulted to resolve inconsistencies. Inter-rater consistency was quantified using Cohen’s Kappa coefficient.
Statistical analysis
Data analysis was performed using IBM SPSS software (version 27.0). Normally distributed data were reported as the mean ± standard deviation (SD) and evaluated for normality using the Kolmogorov–Smirnov test. Patient characteristics were summarized using descriptive statistics, including mean (SD), median, interquartile range (IQR), and proportions. Comparisons between CSC, PNV, and UCP eyes were conducted using ANOVA for normally distributed variables and the Kruskal–Wallis test for non-normally distributed variables. A P-value of < 0.05 was considered statistically significant.
Results
The demographic and clinical profiles of patients with PSD are presented in Table 1 and Fig. 1. Our cohort included 44 eyes with CSC, 38 eyes with PNV, and 46 eyes with UCP. Among the eyes with CSC, 34 were male (77.3%) and 10 were female (22.7%), with a mean age of 45.45 ± 6.94 years. The BCVA (logMAR) was 0.29 ± 0.26 and SFCT was 432.11 ± 103.29 µm. In the PNV eyes, 25 patients were male (73.3%) and 13 were female (26.7%), with a mean age of 51.55 ± 10.19 years. The BCVA (logMAR) was 0.34 ± 0.20, and the SFCT was 374.37 ± 102.59 µm. The UCP eyes included 30 males (65.5%) and 16 females (34.5%), with a mean age of 48.87 ± 7.52 years. The BCVA (logMAR) was 0.04 ± 0.05, and the SFCT was 416.96 ± 61.72 µm.
Significant differences in ages were observed between patients with CSC and PNV, as well as between CSC and UCP (P < 0.05) (Fig. 3a). However, no significant age differences were found between PNV and UCP. Patients with CSC and PNV eyes showed significantly worse BCVA compared to UCP eyes (P < 0.0001). Furthermore, BCVA was statistically worse in PNV eyes compared to CSC eyes (P < 0.05) (Fig. 3b). SFCT measurements also revealed significant differences between CSC and PNV (P < 0.05) (Fig. 3c), while no significant differences were observed between PNV and UCP or between CSC and UCP.
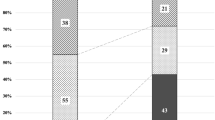
Significant differences were observed in the proportion of eyes exhibiting anastomosis between CSC and UCP eyes (P < 0.01). However, no significant differences were found in the proportion of eyes showing anastomosis between the CSC and PNV eyes, nor between PNV and CSC. Among the 44 CSC eyes, 26 eyes (59.1%) showed a symmetrical distribution of superior and inferior vortex veins, while 12 eyes (27.3%) exhibited an upper-dominant pattern and 6 eyes (13.6%) exhibited a lower-dominant pattern. Among the 38 PNV eyes, 11 eyes (28.9%) showed a symmetrical distribution of the superior and inferior vortex veins. Eighteen eyes (47.4%) exhibited an upper-dominant pattern, while 6 eyes (15.8%) showed a lower-dominant pattern. Additionally, a horizontal watershed was observed in 3 eyes (7.9%). In the group of 46 UCP eyes, a symmetrical distribution of the superior and inferior vortex veins was found in 14 eyes (30.4%). Among these, 10 eyes (21.7%) displayed an upper-dominant pattern, and 13 eyes (28.3%) exhibited a lower-dominant pattern. Furthermore, a horizontal watershed was found in 9 eyes (19.6%) (Table 1).
Table 2 summarizes the differences in choroidal features across various PSD conditions. We evaluated choroidal parameters in nine subfields for CSC, PNV, and UCP eyes. The mCVV/a in CSC eyes was elevated compared to PNV eyes in most regions, except for the superotemporal, nasal, and inferonasal regions (P < 0.05). Similarly, mCVV/a in CSC eyes was higher than in UCP eyes in most regions, except the nasal and inferonasal areas (P < 0.05). The VD-cc in PNV eyes was lower compared to CSC and UCP eyes in the central regions (P < 0.05), while VD-cc in UCP eyes was higher than in PNV and CSC eyes in nasal regions. Compared to PNV eyes, significant differences in CVI were observed in CSC eyes for nasal, inferotemporal, lower, and inferonasal subfields, as well as the average value. Additionally, we examined vortex vein distribution patterns. CVI in CSC eyes exceeded values in UCP eyes in most regions, except for the temporal, nasal, and central areas. The CT in CSC eyes was significantly higher than in PNV eyes in most regions, except the nasal and superonasal areas (P < 0.05). Similarly, CT in CSC eyes was significantly elevated compared to UCP eyes in most regions, except the superotemporal, upper, temporal, central, and inferotemporal areas (P < 0.05). However, no significant differences in mCVV/a, CVI, or CT were observed across all subfields when comparing PNV and UCP eyes (P > 0.05) (Fig. 4).
Using UWF SS-OCTA, we detected CNV network vessels situated between the detached RPE and Bruch’s membrane in 35 PNV eyes. These vessels predominantly originated from the temporal side of the optic disc or adjacent areas. Image analysis showed that CNV network vessels were consistently located either at sites of symmetric vortex vein dilation anastomosis or at the endpoints of dominant vortex vein dilation (Figs. 5, 6).
Imaging of a 34-year-old male with pachychoroid neovasculopathy (PNV). (a) Optical coherence tomography (OCT) scans through the fovea show medium to high reflectivity beneath the retinal pigment epithelium (RPE). (b–d) OCT angiography (6 mm × 6 mm) reveals choroidal neovascularization (CNV) network vessels located between the detached RPE and Bruch’s membrane (highlighted by orange circles). (e,f) En face UWF SS-OCTA (24 mm × 20 mm) images display dilated vortex veins in the deep choroidal layer. The orange arrows in (e,f) mark the endpoints of dominant vortex vein dilation where CNV network vessels are observed. En face UWF SS-OCTA images correspond to the OCT angiography, showing CNV network vessels at the endpoint of the dominant vortex vein dilation.
Imaging of a 48-year-old male with pachychoroid neovasculopathy (PNV). (a) Optical coherence tomography (OCT) scans through the fovea reveal medium- and high-reflectivity beneath the retinal pigment epithelium (RPE). (b–d) OCT angiography (6 mm × 6 mm) shows choroidal neovascularization (CNV) network vessels situated between the detached RPE and Bruch’s membrane (highlighted by orange circles). (e,f) En face UWF SS-OCTA (24 mm × 20 mm) images depict dilated vortex veins in the deep choroidal layer. The orange arrows in (e,f) mark the sites of symmetric vortex vein dilation and anastomosis, where CNV network vessels are observed. En face UWF SS-OCTA images align with the OCT angiography, indicating CNV network vessels at the site of symmetric vortex vein dilation and anastomosis.
In this study, we performed an inter-rater reliability analysis for VVDP classification. The Cohen’s Kappa coefficient for VVDP classification was 0.88, indicating a high level of inter-rater reliability.
Discussion
This study utilized UWF SS-OCTA technology to analyze changes in choroidal blood flow and anatomical structure in relation to PSD. The results indicate that the vortex vein drainage system plays a key role in the pathogenesis of PSD. The choroidal thickness in CSC patients was significantly greater than that in PNV patients, while the central choroidal vascular density was lowest in PNV patients. Furthermore, the incidence of vortex vein anastomosis was higher in CSC and PNV eyes, whereas it was lower in UCP eyes. This finding suggests that different types of PSD may exhibit distinct choroidal blood flow and structural characteristics, providing important clues for further pathological studies.
We observed that the high incidence of vortex vein anastomosis is consistent with the findings of Matsumoto et al.4, who reported that the majority of PSD patients, particularly those with CSC (90.2%) and PNV (95.1%), exhibit vortex vein anastomosis. However, in our study, the incidence of vortex vein anastomosis was higher in CSC patients than in PNV patients (100% vs. 92.1%), which may be attributed to differences in race and inclusion criteria. Additionally, we found that the incidence of vortex vein anastomosis in UCP eyes was 80.4%, significantly higher than the 38–50% rate reported by Mori et al.10 in healthy eyes, further suggesting that choroidal thickening may be associated with the occurrence of vortex vein anastomosis.
Vortex vein expansion pattern analysis revealed that 59.1% of CSC eyes exhibited symmetrical expansion, 47.4% of PNV eyes exhibited superior-dominant expansion, and UCP eyes showed relatively uniform expansion. This finding suggests that the superior-dominant vortex vein drainage system may serve as the primary drainage pathway for the choroid in PNV patients. A comparison with CVI further supports this idea: the CVI thickening in CSC eyes was multidirectional, whereas in PNV eyes, it was predominantly concentrated in the superior, superonasal, and central regions. Despite recent literature suggesting that the vortex vein drainage pattern in CSC eyes is asymmetrical, particularly concentrated in the temporal quadrant6,11,12, our study found that vortex vein expansion in CSC eyes was relatively symmetrical. However, the main area of choroidal thickening still concentrated in the temporal region, which is consistent with the literature describing the temporal region as the primary drainage zone. Matsumoto et al.12 noted that the diameter of the vortex vein in CSC eyes was significantly larger than in PCV and PNV eyes, suggesting that congested vortex veins may lead to vascular connections and anastomosis. This is consistent with our findings and further indicates that abnormal vortex veins in CSC eyes, as reflected by mCVV/a and increased CVI, result in increased choroidal vascular volume and density.
We observed that choroidal blood flow and thickness in CSC patients were predominantly concentrated in the temporal side, particularly in the drainage area of the temporal segment. This phenomenon may be related to the watershed vascular region of the optic disc and the anatomical structure of the optic nerve13. As CSC progresses or spontaneously resolves, compensatory expansion of the vortex vein drainage area in the superior region may occur to alleviate venous congestion. In contrast, vortex vein expansion in PNV patients, particularly the superior-dominant type, may lead to localized hypoperfusion and ischemia, creating conditions favorable for CNV formation. CNV in PNV has not shown a significant correlation with Vascular Endothelial Growth Factor (VEGF) levels14,15, However, Studies suggest that mechanical pressure induced by vortex vein dilation may play a crucial role in the formation of CNV. The expansion of vortex veins is believed to compress the choroidal capillaries, leading to ischemia, which subsequently triggers angiogenesis. Additionally, prolonged elevation of venous pressure, particularly in the superior-expanded or anastomotic regions, is thought to exert chronic stress on the choroidal vascular system. This continuous strain may result in structural changes to the vascular walls, thereby increasing the susceptibility of the affected areas to neovascularization16,17,18. It was observed that CNV networks in PNV patients were often located at sites of symmetrical vortex vein expansion anastomosis or at the terminal sites of superior-dominant expansion (Figs. 5, 6), which is consistent with the findings of Matsumoto et al.19, who also noted that CNV originated from vortex vein anastomotic vessels. Based on these findings, we hypothesize that the formation of CNV in PNV may not be directly driven by VEGF, but rather induced by mechanical pressure from choroidal vascular proliferation, leading to localized ischemia and vascular remodeling, which subsequently promotes CNV formation. Overall, vortex vein expansion in PNV patients, particularly the superior–dominant or symmetrical expansion pattern, may serve as an important predictor for CNV development. This mechanism suggests that in PNV patients, vortex vein expansion and hemodynamic changes play a more crucial role in CNV formation than VEGF, and these changes may act through chronic pressure and localized ischemia to drive pathological CNV formation.
In UCP patients, although the incidence of vortex vein anastomosis was lower than in PNV, their choroidal blood flow anatomical features resembled those of PNV, suggesting that UCP may have a potential structural basis for developing CSC or PNV. Additionally, the higher VD-cc value in UCP patients indicates that their choroidal blood flow may be close to normal, which provides a relatively independent mechanism for its pathological progression. Our study also found that CSC patients were generally younger than UCP and PNV patients, and this age difference suggests that CSC and PNV may represent different stages of a continuum in PSD, with UCP potentially being a precursor or remission state of CSC.
While the precise pathogenic mechanism of choroidal changes remains unclear, existing studies allow us to propose reasonable hypotheses. In the early stages of CSC, multiple factors may trigger excessive choroidal perfusion, leading to changes in UCP. To cope with congestion, CSC patients may improve choroidal drainage by creating vascular connections between the superior and inferior vortex veins to alleviate venous pressure6,7,19,20,21. However, when this drainage system becomes ineffective, subretinal fluid accumulation may trigger the onset of CSC. The asymmetry of the drainage system may lead to uneven venous blood flow distribution, further exacerbating choroidal thickening and eventually causing CSC. The different areas of vortex vein anastomosis may serve as a compensatory mechanism to restore blood flow. Importantly, the main drainage pathway in the superior and temporal regions may help facilitate more blood from the sclera into the choroid22, thus alleviating local venous congestion. This mechanism may explain the clinical differences observed in CSC patients: in some, compensatory anastomosis can assist blood flow and promote self-healing, while in others, abnormal arteriovenous anastomoses may lead to CSC progression, accompanied by venous overload, chronic congestion, and choroidal vascular thickening, ultimately compressing choroidal capillaries and triggering CNV.
In PNV patients, the superior-dominant expansion pattern of the vortex veins may serve as the main choroidal drainage pathway, further highlighting the anatomical susceptibility of PNV. Additionally, the asymmetrical distribution of vortex vein drainage pathways in PNV eyes, particularly the developmental abnormalities in the superior and inferior vortex veins, suggests congenital anatomical variations7,23.
Meanwhile, these results further suggest that choroidal vascular characteristics in PNV patients are closely associated with the development of CNV. In particular, PNV patients exhibited significantly lower VD-cc in the central foveal region compared to CSC and UCP patients, which may serve as an early indicator of CNV development. Our study found that CNV typically occurs in areas with significant choroidal structural changes. The reduction in CVI and the decrease in VD-cc in PNV patients indicate an elevated risk for CNV development. Therefore, quantitative analysis of choroidal vascular changes may serve as a valuable prognostic factor for CNV. Furthermore, our study also revealed that CSC patients generally exhibited greater CT, while PNV patients showed notably lower VD-cc. These changes provide insights into disease progression and prognosis. Quantifying choroidal vasculature enables clinicians to assess disease progression and make personalized treatment decisions. Regular monitoring of CVI, VD-cc, and CT can aid in the early identification of CNV risk. Patients exhibiting elevated CVI and pronounced vortex vein dilation may indicate that the disease is in its active or chronic stages, thereby offering clinical guidance for early intervention. These findings underscore the importance of early monitoring and open the door for future research into the potential use of choroidal vascular parameters, such as vortex vein expansion and CVI, as predictive markers for CNV development in larger, longitudinal cohorts. Further investigation into how these biomarkers interact with other clinical factors will enhance our understanding of the underlying pathophysiology of PSD and contribute to the development of targeted therapeutic strategies.
The cross-sectional nature and limited sample size of this study impose restrictions on establishing a definitive causal relationship between vortex vein drainage and PSD. Future studies should expand the sample size and use longitudinal designs to further investigate the role of vortex vein drainage in PSD, particularly the progression from CSC to PNV, to determine whether changes in vortex vein distribution are triggering factors for PSD development. Furthermore, future research could include subgroup analyses, especially focusing on the different types of vortex vein expansion patterns and their impact on clinical manifestations of PSD, thus providing more insights for early diagnosis and personalized treatment.
This study highlights the significant role of vortex vein structure and hemodynamics in the clinical presentation and pathophysiology of PSD. The findings underscore the importance of the vortex vein drainage system in CSC and PNV, suggesting that choroidal structural and hemodynamic changes could serve as biomarkers for clinical diagnosis and treatment. Additionally, this study offers valuable insights into the potential common etiologies of PSD, providing a foundation for future longitudinal studies and enhancing our understanding of choroidal pathology mechanisms.
Data availability
Data are available upon reasonable request. Deidentified participant data that underline the results reported in this article (text, tables and figures) could be shared upon reasonable request sent to the corresponding author.
References
Borooah, S. et al. Pachychoroid spectrum disease. Acta Ophthalmol. 99, e806–e822. https://doi.org/10.1111/aos.14683 (2021).
Warrow, D. J., Hoang, Q. V. & Freund, K. B. Pachychoroid pigment epitheliopathy. Retina 33, 1659–1672. https://doi.org/10.1097/IAE.0b013e3182953df4 (2013).
Siedlecki, J., Schworm, B. & Priglinger, S. G. The pachychoroid disease spectrum-and the need for a uniform classification system. Ophthalmol. Retina 3, 1013–1015. https://doi.org/10.1016/j.oret.2019.08.002 (2019).
Matsumoto, H. et al. Vortex vein anastomosis at the watershed in pachychoroid spectrum diseases. Ophthalmol. Retina 4, 938–945. https://doi.org/10.1016/j.oret.2020.03.024 (2020).
Matsumoto, H., Kishi, S., Mukai, R. & Akiyama, H. Remodeling of macular vortex veins in pachychoroid neovasculopathy. Sci. Rep. 9, 14689. https://doi.org/10.1038/s41598-019-51268-9 (2019).
Bacci, T., Oh, D. J., Singer, M., Sadda, S. & Freund, K. B. Ultra-widefield indocyanine green angiography reveals patterns of choroidal venous insufficiency influencing pachychoroid disease. Investig. Ophthalmol. Vis. Sci. 63, 17. https://doi.org/10.1167/iovs.63.1.17 (2022).
Hiroe, T. & Kishi, S. Dilatation of asymmetric vortex vein in central serous chorioretinopathy. Ophthalmol. Retina 2, 152–161. https://doi.org/10.1016/j.oret.2017.05.013 (2018).
Moussa, M., Leila, M. & Khalid, H. Imaging choroidal neovascular membrane using en face swept-source optical coherence tomography angiography. Clin. Ophthalmol. 11, 1859–1869. https://doi.org/10.2147/opth.S143018 (2017).
Kim, M. S. et al. Wide-field swept-source OCT analysis of interocular symmetry of choroidal thickness in subjects with uncomplicated pachychoroid. J. Clin. Med. 10, 253. https://doi.org/10.3390/jcm10184253 (2021).
Mori, K., Gehlbach, P. L., Yoneya, S. & Shimizu, K. Asymmetry of choroidal venous vascular patterns in the human eye. Ophthalmology 111, 507–512. https://doi.org/10.1016/j.ophtha.2003.06.009 (2004).
Luo, Z. et al. Choroidal vortex vein drainage system in central serous chorioretinopathy using ultra-widefield optical coherence tomography angiography. Transl. Vis. Sci. Technol. 12, 17. https://doi.org/10.1167/tvst.12.9.17 (2023).
Matsumoto, H. et al. Quantitative measures of vortex veins in the posterior pole in eyes with pachychoroid spectrum diseases. Sci. Rep. 10, 19505. https://doi.org/10.1038/s41598-020-75789-w (2020).
Yazdani, N. et al. Wide-field choroidal thickness and vascularity index in myopes and emmetropes. Ophthal. Physiol. Opt. 41, 1308–1319. https://doi.org/10.1111/opo.12875 (2021).
Chrząszcz, M. et al. Changes in plasma VEGF and PEDF levels in patients with central serous chorioretinopathy. Medicina 57, 63. https://doi.org/10.3390/medicina57101063 (2021).
Terao, N. et al. Distinct aqueous humour cytokine profiles of patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration. Sci. Rep. 8, 10520. https://doi.org/10.1038/s41598-018-28484-w (2018).
Yusef, Y. N., Kurguzova, A. G. & Budzinskaya, M. V. Choroidal neovascularization in central serous chorioretinopathy. Vestn. Oftalmol. 139, 152–157. https://doi.org/10.17116/oftalma2023139061152 (2023).
Spaide, R. F. Choroidal neovascularization. Retina 37, 609–610. https://doi.org/10.1097/iae.0000000000001575 (2017).
Grossniklaus, H. E. & Green, W. R. Choroidal neovascularization. Am. J. Ophthalmol. 137, 496–503. https://doi.org/10.1016/j.ajo.2003.09.042 (2004).
Kishi, S. & Matsumoto, H. A new insight into pachychoroid diseases: Remodeling of choroidal vasculature. Graefes Arch. Clin. Exp. Ophthalmol. 260, 3405–3417. https://doi.org/10.1007/s00417-022-05687-6 (2022).
Chung, S. E., Kang, S. W., Kim, J. H., Kim, Y. T. & Park, D. Y. Engorgement of vortex vein and polypoidal choroidal vasculopathy. Retina 33, 834–840. https://doi.org/10.1097/IAE.0b013e31826af540 (2013).
Spaide, R. F. et al. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog. Retin. Eye Res. 86, 100973. https://doi.org/10.1016/j.preteyeres.2021.100973 (2022).
Koizumi, H., Imanaga, N. & Terao, N. Central serous chorioretinopathy and the sclera: What we have learned so far. Jpn. J. Ophthalmol. 68, 419–428. https://doi.org/10.1007/s10384-024-01101-2 (2024).
Mihara, N. et al. Sex- and age-dependent wide-field choroidal thickness differences in healthy eyes. J. Clin. Med. 12, 505. https://doi.org/10.3390/jcm12041505 (2023).
Funding
The key project of the National Key R&D Program “Research on the Prevention and Treatment of Common Frequent Diseases” (2022YFC2502800).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: Bei Xiao; Acquisition of data: Ya Ye, Zhen Huang; Analysis and interpretation of the data: Ya Ye, Zhen Huang; Statistical analysis: Bei Xiao, Ming Yan; Obtaining financing: Yan-Ping Song; Writing of the manuscript: Bei Xiao; Critical revision of the manuscript for intellectual content: Yan-Ping Song, Bei Xiao.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of General Hospital of Central Theater Commandand with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, B., Song, Y., Yan, M. et al. Quantitative analysis of choroidal vascular structures and anatomical changes in pachychoroid spectrum diseases using ultra-widefield SS-OCTA. Sci Rep 15, 344 (2025). https://doi.org/10.1038/s41598-024-82745-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82745-5
Keywords
This article is cited by
-
Structural and microvascular changes in anisometropic amblyopia: a comparative analysis of swept-source OCTA
BMC Ophthalmology (2026)
-
Investigation of 3D choroidal components in myopic populations using ultra-widefield OCTA
Eye (2026)
-
Longitudinal quantitative assessment of macular retinal and choroidal remodeling after trabeculectomy in chronic primary angle-closure glaucoma using ultra-widefield SS-OCTA
Scientific Reports (2025)