Abstract
Hydrocephalus commonly occurs after subarachnoid hemorrhage (SAH) and is associated with increased morbidity and disability in patients with SAH. Choroid plexus cerebrospinal fluid (CSF) hypersecretion, obliterative arachnoiditis occluding the arachnoid villi, lymphatic obstruction, subarachnoid fibrosis, and glymphatic system injury are considered the main pathological mechanisms of hydrocephalus after SAH. Although the mechanisms of hydrocephalus after SAH are increasingly being revealed, the clinical prognosis of SAH still has not improved significantly. Further research on SAH is needed to reveal the underlying mechanisms of hydrocephalus and develop translatable therapies. A model that can stably mimic the histopathological and neuroethological features of hydrocephalus is critical for animal experiments. There have been fewer animal studies on hydrocephalus after SAH than on other stroke subtypes. The development of a reproducible and effective model of hydrocephalus after SAH is essential. In this study, we establish a mouse model of SAH that stably mimics brain injury and hydrocephalus after SAH through injections of autologous blood into the cisterna magna via different methods and characterize the model in terms of neurological behavior, histology, imaging, neuronal damage, and white matter damage.
Similar content being viewed by others
Introduction
Spontaneous subarachnoid hemorrhage (SAH), which accounts for 2–7% of all stroke cases, is a subtype of stroke in which intracranial arteries rupture and blood enter the subarachnoid space, causing a series of clinical symptoms1,2. The leading cause of SAH, accounting for 85% of all cases, is aneurysm rupture3,4. SAH remains a devastating disease characterized by a high mortality rate and significant morbidity rate. Patients who survive SAH often develop different complications that adversely impact the ability to work and quality of life5. Hydrocephalus is a severe complication that may develop days to weeks after SAH. Approximately 6 to 67% of patients exhibit ventricular dilatation, which is characterized by an acute increase in intracranial pressure, brain stem compression, cerebral hernia, neuronal impairment, white matter damage, consciousness disorders, language and cognitive dysfunction, impairment of urination and defecation, and even death in seriously ill patients, following SAH6,7,8,9. Several clinical studies have shown that hydrocephalus independently predicts poor outcomes8,10,11. Acute hydrocephalus necessitates the placement of an external ventricular drain (EVD) to reduce deleterious secondary effects after SAH, and up to 48% of EVD recipients require ventriculoperitoneal (VP) shunt insertion. However, the prolonged use of an EVD may complicate the treatment of SAH and increase the risk of meningitis or ventriculitis, requiring lengthy hospital stays for the administration of intravenous antibiotics, temporary EVD placement, and the placement of a new shunt, which severely compromises long-term prognosis and the quality of life of patients12,13,14,15. Therefore, further exploration of the mechanism underlying hydrocephalus after SAH and the development of effective preventive and treatment measures are highly important for improving clinical prognosis after SAH.
The development of hydrocephalus after SAH is multifaceted pathological process involving a cascade of molecular and cellular alterations. The impact of altered expression of TGF-β1 and AQP4 in the development of hydrocephalus after SAH has been extensively documented. TGF-β, secreted by astrocytes, neurons, and microglia, regulates extracellular matrix remodeling and promotes fibroblast-to-myofibroblast transition, thereby exacerbating fibrosis and contributing to hydrocephalus in SAH16,17,18,19. Compelling evidence reveals intricate crosstalk between the TGF-β/samds/CTGF signal pathway, TGF-β1/p38 signal pathway and hydrocephalus after SAH, leading to post-traumatic fibrotic scarring and angiogenesis and resultant chronic communicating hydrocephalus20,21. AQP4 predominantly expressed in the subpial astrocyte processes forming the glial-limiting membrane, perivascular astrocyte endfeet is significantly expressed in cerebral cortical of hydrocephalus rats22. In recent years, the role of AQP4 in the glymphatic system has been gradually elucidated. Alterations in the glymphatic system associated with the reduction or redistribution of AQP4 expression appear to participate in the etiology of idiopathic normal pressure hydrocephalus. However, whether a direct mechanistic association exists between the glymphatic system, AQP4, and hydrocephalus remains an area of ongoing investigation23.
Currently, preventive measures and effective drug treatment options for hydrocephalus after SAH are not available, and most relevant studies have been conducted in animal models24,25. Animal experiments are vital for elucidating pathophysiological changes after SAH, identifying relevant therapeutic measures, and performing preclinical studies. However, there have been few animal studies on chronic hydrocephalus after SAH, and these studies have not provided detailed information about the progression of, histological and behavioral features of, or molecular biological changes associated with hydrocephalus. Moreover, the methods used to model hydrocephalus after SAH vary widely, hindering the comparison of the study results and limiting their reference value. Therefore, developing a model that reliably develops hydrocephalus and exhibits the typical pathophysiological and behavioral manifestations of SAH is crucial for research on the mechanism of and prevention strategies for hydrocephalus. Previous studies have reported that single and double autologous blood injections into the cisterna occipitalis are commonly used to establish SAH models. However, the characteristics of hydrocephalus of these models are not completely clear26,27. While attempting to model SAH in mice, we found that mice that received a single injection of 90µL of blood into the cisterna occipitalis presented an increased incidence of hydrocephalus and histological and behavioral changes typical of this condition. In this study, we provide a detailed characterization of this model and compare it with two established mouse models. Based on the volume and frequency of blood injections, we designated the novel model as Single 90 group, and the other two models as Single 60 group and Double group, respectively. A sham group was also included as a control.
Result
SAH mortality rate and severity
A total of 251 mice were used in the experiment. The mortality rate of the sham group was 0%, the mortality rate of the Single 60 group was 0%, the mortality rate of the Single 90 group was 7.04%, and the mortality rate of the Double group was 15.38%. Two hours after modeling, visible blood clots were observed around the major arteries in the cerebral arterial circle and subarachnoid space in the Single 60 group and the Single 90 group but not in the sham group (Fig. 1c). Nevertheless, the blood clots appeared to be partially eliminated in the Double group (Fig. 1c). The SAH score of the SAH group was significantly different from that of the sham group (P < 0.01, Fig. 1d); the SAH score of the Single 90 group was significantly different from that of the Single 60 group and Double group after SAH (P < 0.01, Fig. 1d).
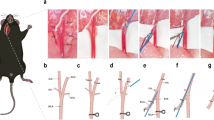
Establishment of the SAH mouse model. (a) Experimental design. (b) Schematic showing the location of cisterna magna autologous blood injection. (c) Representative image of blood distribution on the ventral surface of the brain; scale bar = 1 mm. (d) SAH score at 2 h after SAH was induced by cisterna magna autologous blood injection(n = 6). The values are presented as the means ± SDs. *: p < 0.05 versus the sham group; @: p < 0.05 versus the Single 90 group; one-way ANOVA followed by the least significant difference test.
SAH model mice presented varying degrees of acute ventricular dilatation and injury
T2-weighted magnetic resonance (MR) images and images of HE-stained samples from day 3 post-SAH are presented in Fig. 2. Compared with the sham group, the SAH groups showed different degrees of ventricular enlargement (Fig. 2a). the Evans ratios of the Single 90 and Double groups were significantly greater than those of the sham group and the Single 60 group, respectively, on day 3 post-SAH (P < 0.01; Fig. 2b). Additionally, compared with that of the sham group, the ventricular volumes of the SAH groups were significantly greater on day 3 post-SAH, and the ventricular volumes of the Single 90 and Double groups were significantly greater than that of the Single 60 group (P < 0.01; Fig. 2c). Moreover, there was no significant difference in ventricular volume between the Single 90 group and the Double group (P = 0.314; Fig. 2c). Microscopic observation of HE-stained sections showed ventricular enlargement with blunted contours. Compared with sham group, there was no significant difference in the ventricular wall structure, aside from some abnormal cellular arrangement (Fig. 2d). We used the diagnostic criteria for hydrocephalus to identify experimental mice with the condition and found that the incidence of hydrocephalus was 0%, 38.21%, 72.36%, and 61.23% in the sham, Single 60, Single 90, and Double groups, respectively. The incidence of hydrocephalus in the Single 90 and Double groups was significantly greater than that in the Single 60 group (P < 0.01).
Acute ventricular dilation and ventricular injury in mice after SAH. (a) Representative T2-weighted MR images from each group on day 3 after SAH. (b) Evans ratio in each group (n = 12). (c) Ventricular volume in each group (n = 12). (d) Representative images of HE-stained brain tissue sections showing the increase in the size of the lateral ventricle (Black arrows show disordered cellular arrangement); scale bar = 1 mm. The values are presented as the means ± SDs. *: p < 0.05 versus the sham group; #: p < 0.05 versus the Single 60 group; one-way ANOVA followed by the least significant difference test. MRI: magnetic resonance imaging;.
Mice in the SAH groups exhibited progressive ventricular dilatation and long-term ventricular damage
The MR images take on day 28 revealed that compared with those of the sham mice, the ventricles of SAH model mice were significantly dilated (Fig. 3a). Figure 3b shows that the Evans ratios of the Single 90 and Double groups were significantly greater than those of the sham group and the Single 60 group (P < 0.01), respectively.
Progressive ventricular dilatation and ventricular injury in mice on day 28 after SAH. (a) Representative T2-weighted MR images from each group. (b) Evans ratio in each group (n = 12). (c) Ventricular volume in each group (n = 12). (d) Comparison of the Evans ratio on days 3 and 28 post-SAH (n = 12). (e) Comparison of the ventricular volume on days 3 and 28 post-SAH (n = 12). (f) Representative images of HE-stained samples; scale bar = 1 mm (Black arrows show disordered cellular arrangement). The values are presented as the means ± SDs. *: p < 0.05 versus the sham group; #: p < 0.05 versus the SAH 60 group; ‡: p < 0.05 versus day 3 after SAH; one-way ANOVA followed by the least significant difference test.
Furthermore, our findings indicated that on the 28th day following SAH, the ventricular volume was markedly greater in the SAH groups than in the sham group. Specifically, the Single 90 group and Double 90 group presented significantly greater ventricular volumes than did the Single 60 group (P < 0.01; Fig. 3c). The Evans ratios (P = 0.008 and P = 0.0013; Fig. 3e) and ventricular volumes (P < 0.01 and P = 0.01; Fig. 3f) of the Single 90 and Double groups on day 28 post-SAH were significantly greater than those on day 3 post-SAH. However, no significant differences in the Evans ratio (P = 0.694 and P = 0.118; Fig. 3e) or ventricular volume (P = 0.806 and P = 0.061; Fig. 3f) between different time points were observed in the sham group or the Single 60 group. The ventricles of the Single 90 group and the Double group were further enlarged under microscope. Moreover, localized destruction or even fracture of the ventricular wall, loss of ependymal cells and inflammatory cell infiltration were observed. The damage of ventricle wall in Single 60 group was slighter than the Single 90 group and the Double group. (Fig. 3d). The incidence of hydrocephalus was 0%, 57.84%, 89.65%, and 72.53% in the sham, Single 60, Single 90, and Double groups, respectively. The incidence in the Single 90 and Double groups was greater than that in the sham group on day 28 post-SAH (P < 0.01). In addition, there was a significant increase in the incidence of hydrocephalus in the Single 90 and Double groups on day 28 post-SAH compared with day 3 post-SAH (p < 0.01).
Mice in the SAH groups exhibited different degrees of necroptosis and neuron shrinkage
TUNEL staining revealed that necroptosis on the surface of the cerebral cortex was increased on days 3 and 28 post-SAH (Fig. 4a). The density of TUNEL-positive cells in the Single 90 group was significantly different from that in the Single 60 and Double groups (P < 0.01, Fig. 4b), but the density of necroptosis cells was not significantly different between the Single 60 group and Double group (P = 0.084). The shrinkage of cortical neurons was observed in the SAH group through Nissl staining on days 3 and 28 post-SAH (Fig. 4d); the results revealed that the number of shrunken neurons in the SAH groups was significantly greater than that in the sham group (P < 0.01; Fig. 4e) and that the number of shrunken neurons in the Single 90 group was significantly greater than that in the Single 60 group and Double group (P < 0.01; Fig. 4e).
Acute and long-term nerve damage in mice after SAH. (a) Representative images of TUNEL staining of cortical tissues from each group on days 3 and 28 post-SAH showing necroptosis; scale bar = 50 µm. (b) TUNEL-positive cell density on day 3 post-SAH(n = 6). (c) TUNEL-positive cell density on day 28 post-SAH(n = 6). (d) Representative images of Nissl staining of the cortex on day 3 post-SAH showing changes in neuronal morphology; scale bar = 50 µm. (e) Comparison of the number of shrunken neurons among groups on day 3 post-SAH(n = 6). (f) Comparison of the number of shrunken neurons among groups on day 28 post-SAH(n = 6). The values are presented as the means ± SDs. *: p < 0.05 versus the sham group; @: p < 0.05 versus the Single 90 group; one-way ANOVA followed by the least significant difference test. DAPI: 4’,6-diamidino-2’-phenylindole; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
On day 28, the density of TUNEL-positive cells in the Single 90 group was significantly greater than that in the Single 60 and Double groups (P < 0.01; Fig. 4c); the density of necroptosis cells was not significantly different between the Single 60 group and Double group (P = 0.073; Fig. 4c). Furthermore, Nissl staining revealed that the number of shrunken neurons in the SAH group was significantly greater than that in the sham group and that the number of shrunken neurons in the Single 90 group was significantly greater than that in the Single 60 group and Double group (P < 0.01; Fig. 4f); however, there were no significant differences in the number of shrunken neurons between the Single 60 group and Double group (P = 0.293; Fig. 4f).
Mice in the SAH groups presented varying degrees of white matter damage
Immunofluorescence staining for MBP revealed that mice in the SAH group presented different degrees of thinning of the corpus callosum and different distributions of MBP fluorescence on days 3 and 28 after model establishment (Fig. 5a and b). A quantitative comparison of the fluorescence intensity in each region among the groups revealed that the fluorescence intensity in the Single 60 group was significantly greater than that in the Single 90 and Double groups on days 3 after modeling (P = 0.036 and P = 0.048; Fig. 5c), and the same result was observed on day 28 (P = 0.01 and P < 0.01; Fig. 5d), which indicated severe myelin sheath damage in the mice in the Single 90 and Double groups. We also assessed white matter damage through silver staining. As shown in Fig. 5e, disruption of nerve fiber arrangement and neuronal axons within the corpus callosum of mice in the SAH group was observed on days 3 and 28 after modeling, and the damage was most severe in the Single 90 group. On day 28, further aggravation of corpus callosum damage was observed in the Single 90 and Double groups, with thinning and fracture of the corpus callosum.
Acute and progressive white matter damage in mice after SAH. (a, b) Representative images of immunofluorescence staining for MBP in the corpus callosum in each group on days 3 and 28 post-SAH; scale bar = 50 µm. (c): Comparison of the MBP fluorescence intensity in the corpus callosum of the different group on days 3 post-SAH(n = 6). (d): Comparison of the MBP fluorescence intensity in the corpus callosum of the different group on days 28 post-SAH(n=6). (e) Representative images of silver staining of the corpus callosum of each group on days 3 and 28 post-SAH; scale bar = 50 µm. (f) TEM of neuronal and myelin ultrastructural alterations in the corpus callosum on days 3 and 28 post-SAH; scale bar = 1.0µm. The values are presented as the means ± SDs. *: p < 0.05 versus the sham group; #<0.05 versus the Single 60 group; one-way ANOVA followed by the least significant difference test. DAPI: 4’,6-diamidino-2’-phenylindole; MBP: myelin basic protein.
TEM was conducted to observe ultrastructural changes in axons and myelin sheaths in the corpus callosum (Fig. 5f). Typical myelin structure was observed in the corpus callosum region of the sham group, while the shrinkage and uneven thickness of myelin in the Single 60 group could be observed at both 3d and 28d, suggesting that white matter damage occurred in the Single 60 group at 3d and 28d after SAH. In the Single 90 group and the Double group, in addition to more obvious myelin destruction, different degrees of myelin disintegration, axonal degeneration, and subaxonal edema were also observed in the corpus callosum region. The results of these ultrastructural observations showed that the white matter damage was more pronounced in the Single 90 group and the Double group, which was consistent with the results of MBP quantitative comparison.
Acute and long-term neurological deficits were observed in SAH model mice
The weights of the mice in each group were measured and recorded at different time points (as shown in Fig. 6a). In the sham group, the body weight of the mice slightly decreased in the early stages after SAH, followed by an increasing trend. The body weight of mice in the Single 60 group showed a trend similar to that of mice in the sham group but was consistently lower. The body weight trends observed in the Single 90 group and the Double group were identical, with a significant decrease in the early stages following SAH. Additionally, there was a noticeable decrease in body weight from day 21 to 28 after SAH. The supplemental two-way repeated measures ANOVA showed that group (P = 0.000), time (P = 0.000), and interaction (group × time) (P = 0.000) were significant for changes in weight.
Body weight changes and neurological dysfunction in mice after SAH. (a) Changes in body weight in each group at 1, 3, 7, 14, 21 and 28 days. (b) Garcia scores showing the changes in neurological function at 1, 3, 7, 14, 21 and 28 days. (c) Typical trajectories in the Morris water maze test on day 28 after SAH. (d, e) Escape latency and swimming distance in the Morris water maze test on days 23 to 28 after hydrocephalus modeling(n = 6). (f, g) Number of platform crossings and time spent in the target quadrant in the Morris water maze test on day 28 after hydrocephalus modeling(n = 6). The values are presented as the means ± SDs. *: p < 0.05 versus the sham group; #: p < 0.05 versus the Single 60 group; @: p < 0.05 versus the Single 90 group; one-way ANOVA followed by the least significant difference test.
In the sham group, the Garcia score was slightly lower on day 1 after modeling but recovered and reached a normal level on days 3, 7, 14, 21, and 28 (Fig. 6b). Compared with that of the sham group, the Garcia score of the Single 60 group clearly decreased on day 1 after modeling, and recovered more slowly. Compared with those of the sham group and the Single 60 group, the Garcia score of the Single 90 and Double groups was significantly lower and recovered more slowly in the early stage after SAH (P < 0.01; Fig. 6b). Additionally, the Garcia scores of the Single 90 and Double groups markedly decreased from day 21 to 28 after SAH (P < 0.01; Fig. 6b). There was no significant difference in Garcia score between the Single 90 and Double groups. Moreover, there was a significant difference between the 4 groups in terms of Garcia scores at each time point (P = 0.000), and in addition the interaction analysis showed a difference in the trend of Garcia scores between the groups at different time points (P = 0.000).
The Morris water maze test was conducted to evaluate cognitive function among the groups. Representative trajectories of the mice in the acquisition phase are shown in Fig. 6C. The latency in the Single 90 group and Double group was greater than that in the Single 60 group and sham group, respectively, while there was no significant difference between the Single 90 and Double 90 groups (P < 0.01; Fig. 6d). In the spatial exploration phase, the swimming distance, number of platform crossings, and percentage of time spent in the target quadrant were lower in the Single 90 and Double groups than in the sham group and the Single 60 group (P < 0.01; Fig. 6e). Moreover, the number of crossing and percentage of time spent in the target quadrant showed the same trend as swimming distance among the groups (Fig. 6f and g), which suggests significant impairment of spatial memory and learning in the Single 90 and Double groups.
Levels of hydrocephalus-related proteins in on days 3 and 28 after SAH
Western blotting was performed to assess the protein levels of TGF-β1, smad2/3, p-smad2/3, p38, CTGF, and AQP4 in the periventricular parenchyma on days 3 and 28 after SAH (Fig. 7a). The expression of TGF-β1, CTGF, and P38 showed similar increasing trends on days 3 and 28 post-SAH (Fig. 7c, e, and f). The expression of these proteins was greater in the Single 90 group than in the other groups. Moreover, according to the p-smad/smad ratio, the phosphorylation level of smad in the Single 90 group was greater than that in the other groups on days 3 and 28 post-SAH (Fig. 7d). The expression of AQP4 increased, peaking on day 3 post-SAH, but was decreased on day 28 post-SAH (Fig. 7g). Interestingly, the expression levels of proteins associated with hydrocephalus according to previous studies showed the same trends, as they were higher in the Single 90 group than in the other groups.
Increase in hydrocephalus-related protein expression in SAH model mice. (a) Representative images of Western blot showing the protein levels of TGF-β1, smad2/3, p-smad2/3, p38, CTGF, and AQP4 in the periventricular parenchyma in the sham, Single 60, Single 90, and Double groups. (b) Diagram of periventricular tissue sampling. (c) Quantification of TGF-β1 levels in each group on day 3 and 28 post-SAH(n = 6). (d) Quantification of p-smad2/3 levels in each group on day 3 and 28 post-SAH(n = 6). (e) Quantification of p38 levels in each group on day 3 and 28 post-SAH(n = 6). (f) Quantification of CTGF levels in each group on day 3 and 28 post-SAH(n = 6). (g) Quantification of AQP4 levels in each group on day 3 and 28 post-SAH(n = 6). The values were presented as the mean ± SD. *: p < 0.05 versus the sham group; one-way analysis of variance followed by the lease significant difference test.
Discussion
In this study, a novel mouse model of hydrocephalus after SAH was established by injecting autologous blood into the cisterna magna. Compared with 2 other models involving autologous blood injection, our novel mouse model presented more severe histological injury, neurocognitive deficits, acute hydrocephalus, and chronic hydrocephalus after SAH. Furthermore, the new model exhibited a lower mortality rate and a greater incidence of hydrocephalus than did the double blood injection model.
Many studies have focused on SAH in the past few decades, and various animal models have been established. Three animal models have been used to replicate clinical SAH: the endovascular perforation model, the cisterna magna injection model, and the chiasmatic cistern injection model18,28,29. The endovascular perforation model, which has been used in various studies, involves the use of intracranial endovascular perforation to induce bleeding; the pathophysiological changes observed in this model are similar to those observed in SAH patients and include acute intracranial pressure elevation, decreased cerebral perfusion pressure, brain tissue injury, and vascular damage caused by transient hemorrhage after vascular rupture30. However, SAH that develops after endovascular perforation is more severe than SAH that develops after blood injection, and the mortality rate is greater. Moreover, the experimental animals that survive modeling exhibit significant differences in the severity of hemorrhage and brain damage. In the SAH models involving cisterna magna and chiasmatic cistern injection, the animal’s own blood is injected directly into the cisterna magna or chiasmatic cistern to simulate SAH pathology31. Although autologous blood injection models cannot accurately simulate the acute injury process during hemorrhage, the rate and volume of hemorrhage can be adjusted in these models28,32,33. Moreover, an increasing number of studies have indicated that autologous blood injection models also exhibit pathophysiological changes, such as blood‒brain barrier injury, cerebral vasospasm, delayed cerebral ischemia, cerebral edema, and neuronal apoptosis31,34,35. Considering that the degree of SAH is associated with the incidence of hydrocephalus, we chose the autologous blood injection method, in which the bleeding volume can be controlled, to model hydrocephalus after SAH in this study. A single cisterna magna injection of 60µL of blood results in mild neurocognitive impairment on day 10 after SAH31,36. The use of a single injection of 300µL of blood from rats with cognitive deficits to model SAH was found to significantly increase the number of apoptotic neurons in all the brain regions examined37. However, owing to differences in procedural details, the source of blood, and blood injection speed, tissue injury manifestations and neurological dysfunction were found to vary significantly among SAH models established by different strategies, and pairwise comparisons of study findings are limited38. In this study, mice received one or two injections of blood into the cisterna magna to establish an SAH model. In the Single 60 group, the incidence of hydrocephalus was quite unstable, and mild ventriculomegaly and neurological deficits were observed. Moreover, the mortality rate of the double blood injection group was high, and the operation was more difficult. Therefore, we increased the volume of blood injected to 90µL and evaluated the incidence of hydrocephalus and manifestations of tissue damage in this novel model. The new model has an acceptable mortality rate. In terms of tissue damage, we demonstrated via histologic staining that the new model exhibited similar or even more severe tissue damage than previously reported models36,39,40. Meanwhile, our cisterna magna injection model bears certain similarities to clinical subarachnoid hemorrhage originating from the posterior part of the circle of Willis. By comparing different models, we were able to effectively analyze various forms and severities of SAH, thereby better reflecting multiple clinical scenarios. This design further enhances the potential translational value of the new model and its relevance to clinical applications.
There are few reports on the incidence of hydrocephalus in animal SAH models. In a rat model of SAH induced by intravascular perforation, the incidence of acute hydrocephalus within 24 h was found to be 44%, and approximately 40% of the animals presented increased intracranial pressure associated with ventricular dilation on day 21 after SAH41. Furthermore, the incidence of hydrocephalus among rats with SAH that received two injections of autologous blood was shown to be 44.6%21. To date, no relevant studies have reported the incidence of acute and chronic hydrocephalus in mouse models of SAH. In this study, we determined the incidence of hydrocephalus in each group on the basis of the diagnostic criteria for hydrocephalus reported by Okubo42. The incidence of hydrocephalus in the Single 90 group on days 3 and 28 after SAH was 72.36% and 89.65%, respectively, which was higher than that in the Single 60 and Double groups. Extensive research has consistently demonstrated that ventricular dilation during hydrocephalus triggers structural alterations in the ventricular wall and periventricular tissue, ultimately resulting in neurological and cognitive deficits. Our study indicated that with the development of ventricular enlargement, the degree of white matter damage and cognitive dysfunction in SAH model mice also increased. This finding underscores the consistency of the features of our novel model with the clinical manifestations of hydrocephalus.
Strong evidence suggests a strong association between TGF-β and hydrocephalus after stroke, especially in the context of SAH24,43,44. Dong et al.21reported that the expression of TGF-β1 in the cerebrospinal fluid and brain parenchyma increased significantly on the 21st day after SAH in rat models. In their study, the protein levels of smad2/3, p-smad2/3, and CTGF in the superficial layers of the rat brain also increased. In addition, many studies have established a robust association between the TGF-β1/p38 signaling pathway and the development of hydrocephalus8,45,46. In our new hydrocephalus mouse model, the expression levels of TGF-β1, smad2/3, p-smad2/3, p38, CTGF, and AQP4 showed the same trends as those previously reported in models of hydrocephalus after SAH17,18,20,47,48. AQP4 serves as the primary water transporter in the brain and is intimately involved in cerebral edema progression and cerebrospinal fluid regulation following SAH. Elevated AQP4 has been reported to be associated with increased cerebral edema after SAH, but upregulating AQP4 expression can reduce the severity of hydrocephalus49,50. Thus, the regulatory pattern and mechanistic role of AQP4 in post-SAH hydrocephalus remain a subject of ongoing debate. Our findings revealed a marked upregulation of AQP4 expression at 3 days post-SAH, followed by a significant decline at 28 days relative to the 3-day level. The dynamic change in AQP4 expression is multi-sourced. We hypothesize that early AQP4 elevation was the result of the progression of cerebral edema and activation of the glymphatic system. The decrease of AQP4 expression on day 28 was mainly associated with the reduction of early acute brain injury and cerebral edema after SAH. However, the effect of glymphatic system injury on AQP4 should not be underestimated. While AQP4 upregulation is generally thought to mitigate hydrocephalus, our model suggests that progressive hydrocephalus is likewise associated with elevated AQP4, potentially due to disrupted AQP4 polarization following glymphatic system impairment.
The elevation of the expression of these proteins in all the groups indicated that the proteins are involved in the development of hydrocephalus after SAH; however, there was a difference in the extent to which the expression of the proteins changed, which may have been related to interactions between these proteins or related signaling pathways as well as the involvement of other inflammatory factors and proteins. The decrease in the level of AQP4 on day 28 post-SAH may have been related to the alleviation of acute brain injury and cerebral edema in the early period after SAH.
The results presented above suggest that our novel mouse model exhibits typical features of hydrocephalus in terms of imaging, neuronal apoptosis, white matter damage, neurological deficits, and changes in protein expression and may represent an ideal model for studying hydrocephalus after SAH.
There are several potential limitations in our study. First, only hydrocephalus after SAH induced by the injection of blood into the cisterna magna was evaluated in this study. Second, although the scope of our evaluation was broad, the number of time points studied was insufficient; imaging, histological analysis, and molecular biology assays were only performed on days 3 and 28 after SAH. Finally, as the intracranial pressure was not monitored during this study, not all of the clinical features of hydrocephalus were adequately assessed.
Conclusion
In the present study, we established a novel model of hydrocephalus after SAH and characterized the model in terms of histological changes, imaging findings, neurological function, and molecular biological changes. The results indicated that the mouse SAH model established by a single injection of 90µL of autologous blood into the cisterna magna exhibits histological and neurological changes typical of post-SAH hydrocephalus and is an ideal model for studying the mechanism of post-SAH hydrocephalus and for validating the efficacy of potential therapeutic methods.
Materials and methods
Animals
A total of 251 healthy adult male C57BL/6 mice (22–28 g, 6–8 weeks old) from GemPharmatech (Chengdu) Co., Ltd. were used and performed in strict accordance with the experimental protocol and procedures approved by Institutional Animal Care and Use Committee of North Sichuan Medical College (NO.2023045), which are based on the recommendations of the National Institutes of Health and the ARRIVE Guidelines (https://arriveguidelines.org). Administration lasted for 28 days. All the mice were fed, observed, and weighed daily, and they were maintained under controlled conditions at 23–25 °C and 70% humidity on a 12-h light/dark cycle and given free access to normal mouse chow and filtered water.
Experimental design
We established a sham group, Single 60 group (60µL of blood were injected into the cisterna magna), Single 90 group (90µL of blood were injected into the cisterna magna), and Double group (60µL of blood was injected into the cisterna magna first, and then 30µL was injected into the cisterna magna 24 h later via the same procedure) to characterize our hydrocephalus model. The mice were randomly assigned to the experimental groups by generating random numbers. The SAH scoring system was used to assess the severity of SAH at 2 h after the surgical procedure. A modified Garcia scoring system was used to assess disease severity on days 1, 3, 7, 14, 21, and 28 after SAH. The Morris water maze test was performed on days 23 to 28 after SAH to compare cognitive function over the long term. Magnetic resonance imaging (MRI) was conducted on days 3 and 28 after SAH to measure the ventricular volume. Hematoxylin and eosin (HE), Nissl, and TUNEL staining were used to assess tissue damage, neuronal necrosis and apoptosis on days 3 and 28 after SAH. To observe white matter damage, transmission electron microscopy (TEM), silver staining, and immunofluorescence were performed on days 3 and 28 after SAH. A Western blot analysis was conducted to evaluate hydrocephalus-related protein expression levels on days 3 and 28 after SAH (Fig. 1a). All scoring was completed by independent investigators who were blinded to the subgroup assignments.
SAH model
The mice were anesthetized with 1% pentobarbital sodium (35 mg/kg, intraperitoneally) and then placed in the supine position on a heating pad, and their body temperature was maintained at 36.5 ± 0.5 °C. SAH was induced via cisterna magna injection of autologous blood as previously described28,51. The mice were placed on an inclined board at a 30° angle in a stable position with their heads downward. An approximately 5 mm vertical incision was then made along the midline of the occipitocervical junction to expose the occipital bone and neck muscles. The neck muscles were subsequently dissected to expose the atlantooccipital fascia. After the mouse tail was disinfected, autologous blood was collected from the tail with a 100µL microinjection needle and washed with 833 U/ml heparin sodium. 60µL of blood and 90µL of blood were injected into the cisterna magna in the Single 60 group and Single 90 group, respectively. In the Double group, 60µL of blood was injected into the cisterna magna first, and then 30µL was injected into the cisterna magna 24 h later via the same procedure (Fig. 1b). Sham-operated mice were subjected to the same operation except the injection of blood. Mice that died during the experiment were replaced with other mice.
SAH score
The mice were euthanized via deep anesthesia with 1% pentobarbital sodium at 2 h after SAH. The brain was removed after perfusion with physiological saline and 4% paraformaldehyde (Servicebio, Wuhan, China). The SAH score was determined according to the previously described SAH scoring system15. The basal cistern was divided into six segments, and a score from 0 to 3 was assigned to each segment on the basis of the number of subarachnoid blood clots. The total score for the six segments was calculated as the SAH score, with a higher score indicating an increased blood volume.
Neurobehavior assessment
Short-term neurological deficits after SAH were evaluated via the modified Garcia (ranging from 0 to 18) scoring system, as described in a previous study52. In total, six tests were performed to evaluate short-term neurological function, including spontaneous activity, symmetrical movement of the four limbs, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch. A higher score indicated better short-term neurological function.
The Morris water maze test was performed on days 23 to 28 after SAH to assess long-term neurological function, including spatial learning and memory, as described in a previous study53. In brief, the mice were placed in a circular tank (0.5 m deep and 1.3 m in diameter) kept at a constant temperature in a quiet and dark environment. The maze was divided equally into four quadrants, and a 10 cm platform was submerged 1 cm below the surface of the water in the target quadrant. The mice were allowed to search for the submerged platform for 60 s. In the acquisition phase, the mice were trained in the maze four times per day for five days (from days 23 to 27 after SAH). The escape latency and swimming distance in the Morris water maze test were recorded via ANY-maze video tracking software (Stoelting, Wisconsin, USA) on day 28 after SAH. The target platform was removed, and each mouse was subjected to a 60 s probe trial. The escape latency, time spent in the target quadrant, and number of crossings of the target quadrant were recorded.
MRI
The mice were anesthetized with a mixture of 2% isoflurane and air during MRI. MRI was performed on days 3 and 28 after SAH in a 7.0-T Varian MR scanner (Bruker, Massachusetts, USA) with a T2 sequence, a view field of 50 mm×50 mm, a matrix of 256 × 256, and a 1 mm slice thickness. The ventricular volume and Evans ratio were measured and calculated as previously described29. The ventricular area over all slices was summed and multiplied by the section thickness to calculate the ventricular volume. The Evans ratio was calculated as the maximum width of the lateral ventricles divided by the maximum width of the brain. Two independent investigators analyzed all the images via ImageJ software (National Institutes of Health, Bethesda, MD, USA). Hydrocephalus was defined as a ventricular volume greater than the mean ventricular volume plus three times the standard deviation (SD) of the sham group42.
Histological staining
Coronal brain slices (1 mm) were prepared for immunofluorescence staining. Briefly, the slices were incubated with 0.3% Triton X-100 (Biosharp, Guangzhou, China) for 10 min and 5% BSA (Bosterbio, Wuhan, China) for 1 h at room temperature; the sections were incubated with a rabbit anti-MB (1:4000, Abcam, Cambridge, US) primary antibody overnight at 4 °C. After three washes in PBS (Zsbio, Beijing, China), the slices were incubated for 2 h with secondary antibodies at room temperature. The slices were then incubated with DAPI (Beyotime, Shanghai, China) for 5 min. The sections were visualized and imaged via a fluorescence microscope (Olympus, Tokyo, Japan).
For Nissl staining, after the brain tissue sections were deparaffinized and hydrated, crystal violet staining solution was used to stain the sections for 60 min at 56 °C. The sections were rinsed with deionized water for 10 min. The signals were visualized via a Nissl staining kit (Solarbio, Wuhan, China). To detect apoptotic neurons in the frontal motor cortex, TUNEL staining was performed via a TUNEL apoptosis kit (Servicebio, Wuhan, China) according to the manufacturer’s instructions. The sections were sequentially immersed in proteinase K (Solarbio, Beijing, China), 3% H2O2methanol solution, and labeling solution. The sections were subsequently incubated with HRP-streptavidin solution for 30 min and color development was performed with DAB. Then, the nuclei were stained with hematoxylin, routinely dehydrated, and covered with neutral gum. ImageJ software was used to count and analyze the positive cells under a microscope (Olympus, Tokyo, Japan). HE and silver staining were performed as previously described54; the sections were immersed in aqueous hematoxylin or silver ammonia solution after deparaffinization. An independent researcher observed the stained sections under a microscope.
TEM
The mice were sacrificed on days 3 and 28 after SAH and perfused with normal saline and 4% paraformaldehyde (Servicebio, Wuhan, China) as previously described29. Briefly, the frontal cortex or corpus callosum was removed, immersed in 2.5% glutaraldehyde (Servicebio, Wuhan, China) at 4 °C overnight and postfixed in 1% osmium tetroxide for 1 h. Then, the samples were dehydrated in graded ethanol solutions for 10 min and embedded in 70% acetone for 10 min. After being embedded with epoxy resin, the samples were cut into sections and stained with 2% uranyl acetate (Ribiology, Shanghai, China) and lead citrate. The sections were visualized and imaged with a transmission electron microscope (HITACHI, Tokyo, Japan).
Western blotting
Western blotting was performed as described previously55. The mice were euthanized on days 3 and 28 after SAH. Brain tissue was coronal resected from 0 to 1.0 mm posterior to bregma, followed by a lateral cut of 1*1*2 mm in size in the ventricular wall to obtain periventricular parenchyma (Fig. 7b). And the brain tissues were stored in liquid nitrogen before being thoroughly ground on ice. Then, 1 ml of RIPA lysis buffer (Sigma‒Aldrich, MO, USA) was added to lyse the brain tissue for 30 min. The lysates were centrifuged at 12,000 × g for 20 min at 4 °C, and this process was repeated three times. The supernatant was then collected. A standard BCA assay (Bio-Rad) was conducted to determine the protein concentration in each sample. Proteins from each sample were separated by SDS–polyacrylamide gel electrophoresis and electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). The membranes were blocked with 5% skim milk for 60 min at room temperature before they were incubated overnight at 4 °C with the following primary antibodies: anti-TGF-β1 (1:1000, Abcam, Cambridge, US), anti-Smad2/3 (1:1000, Abcam, Cambridge, US), anti-pSmad2/3 (1:500, Abcam, Cambridge, US), anti-p38 (1:100, Abcam, Cambridge, US), anti-CTGF (1:1000, Abcam, Cambridge, US), and anti-AQP4 (1:1000, Abcam, Cambridge, US). Then, secondary antibodies were added, and the membranes were incubated at 37 °C for 60 min. Finally, the immunoblot bands were visualized via a Millipore ECL Western Blotting Detection System (Millipore, Billerica, MA, USA). The expression level of each protein was normalized to that of β-actin. Relative protein levels were determined via ImageJ software.
Statistical analysis
Statistical analysis was performed via SPSS 22.0 statistical software (IBM, Armonk, NY, USA), and graphs were created via GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA; www.graphpad.com). The experimental data are expressed as the means ± SDs. Comparisons between the two groups were made using Student’s t-test to confirm that the data met the assumption of normal distribution. A one-way analysis of variance (ANOVA) with Tukey’s post-hoc test was used to assess the significance of the differences between the groups. A two-way repeated measures ANOVA analysis was used to assess the significant differences of the time course of body weight and neurobehavioral impairment, setting an alpha level of 0.05 and selecting a two-tailed test.
Ethics approval
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of North Sichuan Medical College.
Data availability
The data are available from the corresponding author on reasonable request.
References
Claassen, J. & Park, S. Spontaneous subarachnoid haemorrhage. Lancet 400 (10355), 846–862 (2022).
Neifert, S. N. et al. Aneurysmal Subarachnoid Hemorrhage: the last decade. Translational Stroke Res. 12 (3), 428–446 (2021).
Werring, D. J. & Banerjee, G. Convexity subarachnoid haemorrhage. Lancet 401 (10372), 193–194 (2023).
Lawton, M. T. & Vates, G. E. Subarachnoid hemorrhage. N Engl. J. Med. 377 (3), 257–266 (2017).
Maher, M., Schweizer, T. A. & Macdonald, R. L. Treatment of spontaneous subarachnoid hemorrhage: guidelines and gaps. Stroke 51 (4), 1326–1332 (2020).
Chen, S., Luo, J., Reis, C., Manaenko, A. & Zhang, J. Hydrocephalus after Subarachnoid Hemorrhage: pathophysiology, diagnosis, and treatment. Biomed. Res. Int. 2017, 8584753 (2017).
Xu, H. New concept of the pathogenesis and therapeutic orientation of acquired communicating hydrocephalus. Neurol. Sci. 37 (9), 1387–1391 (2016).
Hochstetler, A., Raskin, J. & Blazer-Yost, B. L. Hydrocephalus: historical analysis and considerations for treatment. Eur. J. Med. Res. 27 (1), 168 (2022).
Kahle, K. T., Kulkarni, A. V., Limbrick, D. D. Jr. & Warf, B. C. Hydrocephalus in children. Lancet 387 (10020), 788–799 (2016).
Yang, Y. C. et al. Cerebrospinal fluid predictors of shunt-dependent hydrocephalus after hemorrhagic stroke: a systematic review and meta-analysis. Neurosurg. Rev. 45 (3), 1847–1859 (2022).
Chen, L. et al. Risk factors of shunt-dependent hydrocephalus after subarachnoid hemorrhage: a systematic review and meta-analysis based on observational cohort studies. Neurosurg. Rev. 47 (1), 421 (2024).
Xie, Z. et al. Predictors of Shunt-dependent Hydrocephalus after Aneurysmal Subarachnoid Hemorrhage? A systematic review and Meta-analysis. World Neurosurg. ; (2017). 106:844 – 60.e6.
O’Kelly, C. J., Kulkarni, A. V., Austin, P. C., Urbach, D. & Wallace, M. C. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: incidence, predictors, and revision rates. Clinical article. J. Neurosurg. 111 (5), 1029–1035 (2009).
Bederson, J. B. et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 40 (3), 994–1025 (2009).
Scollato, A., Gallina, P., Di Lorenzo, N. & Bahl, G. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery 63 (6), E1209 (2008). author reply E.
Mao, X., Enno, T. L. & Del Bigio, M. R. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur. J. Neurosci. 23 (11), 2929–2936 (2006).
Gonzalez-Marrero, I. et al. Altered expression of AQP1 and AQP4 in Brain barriers and Cerebrospinal Fluid May affect Cerebral Water Balance during Chronic Hypertension. Int. J. Mol. Sci. ;23(20). (2022).
Peng, D., Fu, M., Wang, M., Wei, Y. & Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer. 21 (1), 104 (2022).
Lee, P., Monaco, E. A. 3rd, Friedlander, R. M. , Blocking TGF-β activity and associated inflammation may halt hydrocephalus. Neurosurgery 73 (6), N13–N14 (2013).
Yan, H. et al. Decorin alleviated chronic hydrocephalus via inhibiting TGF-β1/Smad/CTGF pathway after subarachnoid hemorrhage in rats. Brain Res. 1630, 241–253 (2016).
Dong, C. et al. Icariside II attenuates chronic Hydrocephalus in an experimental subarachnoid hemorrhage rat model. J. Pharm. Pharm. Sci. 21 (1), 318–325 (2018).
Kitchen, P. et al. Targeting Aquaporin-4 subcellular localization to treat Central Nervous System Edema. Cell 181 (4), 784–99e19 (2020).
Peng, S., Liu, J., Liang, C., Yang, L. & Wang, G. Aquaporin-4 in glymphatic system, and its implication for central nervous system disorders. Neurobiol. Dis. 179, 106035 (2023).
Kahle, K. T. et al. Paediatric hydrocephalus. Nat. Rev. Dis. Primers. 10 (1), 35 (2024).
Zaksaite, T., Loveday, C., Edginton, T., Spiers, H. J. & Smith, A. D. Hydrocephalus: a neuropsychological and theoretical primer. Cortex 160, 67–99 (2023).
Güresir, E., Schuss, P., Borger, V. & Vatter, H. Rat cisterna magna double-injection model of subarachnoid hemorrhage - background, advantages/limitations, technical considerations, modifications, and outcome measures. Acta Neurochir. Supplement. 120, 325–329 (2015).
Boyko, M. et al. The neuro-behavioral profile in rats after subarachnoid hemorrhage. Brain Res. 1491, 109–116 (2013).
Pedard, M., El Amki, M., Lefevre-Scelles, A., Compere, V. & Castel, H. Double direct injection of blood into the Cisterna Magna as a model of subarachnoid hemorrhage. J. Vis. Exp. 2020(162).
Liu, C. et al. Inhibition of neuronal necroptosis mediated by RIP1/RIP3/MLKL provides neuroprotective effects on kaolin-induced hydrocephalus in mice. Cell. Prolif. 54 (9), e13108 (2021).
Lee, J. Y., Sagher, O., Keep, R., Hua, Y. & Xi, G. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery 65 (2), 331–343 (2009). discussion 43.
Schüller, K., Bühler, D. & Plesnila, N. A murine model of subarachnoid hemorrhage. J. Visualized Experiments: JoVE. 81, e50845 (2013).
Fang, Y. et al. A new perspective on cerebrospinal fluid dynamics after subarachnoid hemorrhage: from normal physiology to pathophysiological changes. J. Cereb. Blood Flow. Metab. 42 (4), 543–558 (2022).
Liu, S. et al. Endovascular perforation model for subarachnoid hemorrhage combined with magnetic resonance imaging (MRI). J. Visualized Experiments: JoVE 2021(178).
Bhattacharya, A., Choi, W. W. Y., Muffat, J. & Li, Y. Modeling Developmental Brain diseases using human pluripotent stem cells-derived Brain Organoids - Progress and Perspective. J. Mol. Biol. 434 (3), 167386 (2022).
Papaioannou, V., Czosnyka, Z. & Czosnyka, M. Hydrocephalus and the neuro-intensivist: CSF hydrodynamics at the bedside. Intensive Care Med. Exp. 10 (1), 20 (2022).
Turan, N. et al. Neurocognitive outcomes in a Cisternal Blood Injection Murine Model of Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 29 (11), 105249 (2020).
Zhang, D. et al. Akt specific activator SC79 protects against Early Brain Injury following subarachnoid hemorrhage. ACS Chem. Neurosci. 7 (6), 710–718 (2016).
Guresir, E., Schuss, P., Borger, V. & Vatter, H. Rat cisterna magna double-injection model of subarachnoid hemorrhage - background, advantages/limitations, technical considerations, modifications, and outcome measures. Acta Neurochir. Suppl. 120, 325–329 (2015).
Prunell, G. F., Mathiesen, T., Diemer, N. H. & Svendgaard, N. A. Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery 52 (1), 165–175 (2003). discussion 75 – 6.
Piepgras, A., Thomé, C. & Schmiedek, P. Characterization of an anterior circulation rat subarachnoid hemorrhage model. Stroke 26 (12), 2347–2352 (1995).
Lackner, P. et al. Chronic hydrocephalus after experimental subarachnoid hemorrhage. PLoS One. 8 (7), e69571 (2013).
Okubo, S., Strahle, J., Keep, R. F., Hua, Y. & Xi, G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke 44 (2), 547–550 (2013).
Li, Y. et al. Choroid plexus mast cells drive tumor-associated hydrocephalus. Cell 186 (26), 5719–38e28 (2023).
Li, Z., Zhang, H., Hu, G. & Zhang, G. Post-traumatic hydrocephalus: an overview of classification, diagnosis, treatment, and post-treatment imaging evaluation. Brain Res. Bull. 205, 110824 (2023).
Yue, X. J. et al. Transforming growth factor-β1 induces fibrosis in rat meningeal mesothelial cells via the p38 signaling pathway. Mol. Med. Rep. 14 (2), 1709–1713 (2016).
Karimy, J. K. et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 23 (8), 997–1003 (2017).
Wang, X. et al. Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nat. Commun. 9 (1), 551 (2018).
Zhang, S., Chen, D., Huang, C., Bao, J. & Wang, Z. Expression of HGF, MMP-9 and TGF-β1 in the CSF and cerebral tissue of adult rats with hydrocephalus. Int. J. Neurosci. 123 (6), 392–399 (2013).
Rizwan Siddiqui, M. et al. Erythropoietin-mediated activation of aquaporin-4 channel for the treatment of experimental hydrocephalus. Child’s Nerv. System: ChNS : Official J. Int. Soc. Pediatr. Neurosurg. 34 (11), 2195–2202 (2018).
Szczygielski, J. et al. Changes in Posttraumatic Brain Edema in Craniectomy-Selective Brain Hypothermia Model are Associated with modulation of Aquaporin-4 level. Front. Neurol. 9, 799 (2018).
Kamp, M. A. et al. Evaluation of a murine single-blood-injection SAH model. PLoS One. 9 (12), e114946 (2014).
Cao, Y. et al. Metformin alleviates delayed Hydrocephalus after Intraventricular Hemorrhage by inhibiting inflammation and fibrosis. Translational Stroke Res. 14 (3), 364–382 (2023).
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1 (2), 848–858 (2006).
Uchihara, T. Silver diagnosis in neuropathology: principles, practice and revised interpretation. Acta Neuropathol. 113 (5), 483–499 (2007).
Shen, D. et al. Metformin preserves VE-Cadherin in Choroid Plexus and attenuates Hydrocephalus via VEGF/VEGFR2/p-Src in an Intraventricular Hemorrhage Rat Model. Int. J. Mol. Sci. ;23(15). (2022).
Acknowledgements
We would like to express our deepest gratitude to the Neurosurgery Department of North Sichuan Medical College for their invaluable guidance, support, and encouragement throughout this study. We also extend our sincere thanks to the Institute of Neurological Diseases for providing the necessary resources and facilities that made this research possible. Our appreciation goes to Affiliated Hospital of North Sichuan Medical College and North Sichuan Medical College for their financial support, which was crucial for the successful completion of this project. We are also grateful to everyone for their contributions, insightful discussions, and constructive feedback. Lastly, we thank our families and friends for their continuous support and understanding during the course of this research.
Funding
This work was supported by Doctoral start-up fund of Affiliated Hospital of North Sichuan Medical College, Grant No. 2023-2GC005 (to Long Zhao); Innovation and Entrepreneurship Training Program for College Students in Sichuan Province, Grant No. S202210634079 (to Xuehai Deng), Grant No. S202310634019 (to Junchi Wang), Grant No. 202210634009 (to Jianlin Ding).
Author information
Authors and Affiliations
Contributions
Study design and supervision: LZ, XPT; animal model, SAH grade and behavioral test: XHD, JLD, LZ, CL; MRI and HE staining: XHD, JLD, JCW; TUNEL staining, Nissl staining, and Silver staining: XHD, JLD, CL; Immunofluorescence staining: XHD, JLD, JCW, WDL; Western blot: LZ, QYD, CL; Data collection and analysis: XHD, JLD, CL, ZW; Manuscript draft: XHD, JLD, LZ, CL; TEM: XHD, JLD, LZ; Project administration and manuscript revision: XHD, LZ, XLC, WDL; All authors read and approved the final version of the manuscript. [Please add funding information after this paragraph] funding This work was supported by Doctoral start-up fund of Affiliated Hospital of North Sichuan Medical College, Grant No. 2023-2GC005 (to Long Zhao); Innovation and Entrepreneurship Training Program for College Students in Sichuan Province, Grant No. S202210634079(to Xuehai Deng), Grant No. S202310634019(to Junchi Wang), Grant No. 202210634009 (to Jianlin Ding).
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest are associated with this manuscript.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deng, X., Ding, J., Liu, C. et al. Progressive histological and behavioral deterioration of a novel mouse model of secondary hydrocephalus after subarachnoid hemorrhage. Sci Rep 14, 31794 (2024). https://doi.org/10.1038/s41598-024-82843-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82843-4