Abstract
Red blood cell distribution width (RDW) is considered to be associated with the prognosis of patients with severe infections. This study aims to investigate the relationship between RDW and prognosis in osteomyelitis patients admitted to the ICU. Through a retrospective cohort study, relevant records of severe osteomyelitis patients were collected from the Medical Information Mart for Intensive Care (MIMIC-IV) database. Patients were divided into low-level and high-level groups based on the median RDW. The primary outcome was the one-year mortality rate of ICU patients. Univariate and multivariate Cox models were used to determine the relationship between RDW and mortality rate in osteomyelitis patients, with hazard ratios (HRs) and 95% confidence intervals (CIs) calculated. Kaplan–Meier survival curves were used to analyze patient survival status. Restricted Cubic Spline (RCS) was used to detect the nonlinear relationship between RDW and mortality rate. Stratified analysis was performed to explore the interaction between different subgroups. The study included a total of 1109 patients, among which 134 (12.08%) died within one year. Results from both univariate and multivariate Cox regression analyses indicated a significant association between elevated RDW levels (HR = 2.128, 95% CI 1.324–3.420) and an increased risk of mortality in patients with osteomyelitis. Kaplan–Meier survival curves demonstrated that the survival time of patients in the low RDW group was greater than that of those in the high RDW group (p < 0.0001). RCS analysis revealed a non-linear increase in the strength of the correlation between patient mortality rate and continuous changes in RDW. Subgroup analysis also observed a relationship between RDW levels and mortality rates in patients with osteomyelitis. Elevated RDW levels are correlated with an increased risk of mortality in osteomyelitis patients admitted to the ICU.
Similar content being viewed by others
Introduction
Osteomyelitis is an inflammatory response process accompanied by bone tissue destruction, caused by infective factors1. Literature reports2 indicate that prior to the use of antibiotics, the mortality rate of severe infections in osteomyelitis was as high as 20%, with an incidence rate of 45–50%. In recent years, the incidence of osteomyelitis has continued to rise due to the increase in infections caused by methicillin-resistant Staphylococcus aureus (MRSA), surgical implants, and the number of accidental traumas3,4. The diagnosis of osteomyelitis typically relies on imaging studies and laboratory cultures. Important methods for diagnosing osteomyelitis include blood tests, X-rays, CT scans, MRI, or radionuclide bone scans, as well as bone cultures and/or abscess cultures. Among these, intraoperative tissue pathology examination of the lesion is considered the “gold standard” for diagnosing osteomyelitis. In the ICU, early identification of high-risk factors for poor prognosis in critically ill patients with osteomyelitis is becoming increasingly important. Previously, we commonly used C-reactive protein (CRP), procalcitonin, and erythrocyte sedimentation rate (ESR) for the diagnosis, differential diagnosis, and treatment of osteomyelitis4. However, there is currently limited research on the prognosis of osteomyelitis.
The red cell distribution width (RDW) is considered as an indicator of erythrocyte volume heterogeneity in peripheral blood5,6. There is currently evidence suggesting that RDW is a novel biomarker for inflammation and oxidative stress7. Higher RDW values have been identified as an independent risk factor for poor prognosis in critically ill patients8. Therefore, we hypothesize that RDW may have prognostic value in osteomyelitis patients.
Currently, there is limited research regarding the association between red cell distribution width (RDW) and prognosis in osteomyelitis patients admitted to the intensive care unit (ICU). Therefore, the aim of this study is to investigate the correlation between RDW and the mortality rate of osteomyelitis patients admitted to the ICU.
Materials and methods
Sources of data
This study is a retrospective observational investigation that extracted medical records of osteomyelitis patients from the MIMIC-IV database. Clinical information of patients hospitalized in the ICU from 2008 to 2019 was retrieved from the MIMIC-IV database, which is a publicly available database of patients admitted to the Beth Israel Deaconess Medical Center (BIDMC) ICU and is freely accessible to researchers. After completing the online training course, the Collaborative Institutional Training Initiative, the authors received a certificate (No. 38821147) and access to the clinical database. The MIMIC database is a publicly available anonymised database, and ethical committee approval was not required.
Data collection and definitions
This study extracted the following information: demographic details, vital signs, complications, laboratory parameters, treatment, and severity scores. Demographic information included demographic characteristics such as gender, age, BMI, height, weight, marital status, race, admission and discharge times, time of death, and duration of ICU stay. Vital signs encompassed systolic blood pressure, diastolic blood pressure, mean arterial pressure, oxygen saturation, heart rate, respiratory rate (RR), temperature, and blood glucose.Complications included hypertension, diabetes, chronic obstructive pulmonary disease (COPD), kidney injury, and sepsis. Laboratory parameters encompassed white blood cell count (WBC), neutrophil-to-lymphocyte ratio, hemoglobin, albumin, platelet count, lactate, creatinine, international normalized ratio (INR), PaO2, partial pressure of carbon dioxide (PCO2), C-reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), calcium, chloride, potassium, sodium, magnesium, red cell distribution width(RDW), wound microbiota detection, and others. Severity scores included the Sequential Organ Failure Assessment (SOFA) score. Treatments included mechanical ventilation and antibiotic use. All data were collected within the first 24 h of the patient’s admission to the ICU.
Inclusion criteria: (1) Diagnosis of osteomyelitis; (2) First admission to the ICU. Exclusion criteria: (1) ICU stay less than 24 h. The primary outcome was the all-cause mortality rate within one year, determined by the admission time and recorded death time in the database. For patients with multiple admissions, we used data from the first hospitalization; for repeated examinations, data from the first examination within the first 24 h of admission were used.
Missing data handling
In this research, patients or variables with more than 50% missing values were excluded from the analysis. Subsequently, the missing values in the database were addressed using the “norm. predict” method in Multivariate Imputation by Chained Equation (MICE), wherein each variable is imputed conditionally on all other variables. This approach helped to mitigate the impact of missing data and ensured a more comprehensive and reliable analysis of the dataset.
Statistical analysis
Data extraction and processing were performed using the PostgreSQL database management system, and statistical analysis was conducted using R software (version 4.2.3). The extracted patients were divided into two groups based on the median RDW value: the high RDW group (RDW > 14.2%) and the low RDW group (RDW ≤ 14.2%). All data extracted from the database were compared between the survival group and the deceased group, as well as between the high RDW group and the low RDW group. A univariate COX regression model was employed to determine the correlation between RDW and mortality rate.To evaluate the independent impact of RDW on mortality rate, we established three models to assess the independent influence of RDW levels on all-cause mortality. Model 1 was a univariate Cox regression model (unadjusted); Model 2 adjusted for age, gender, race, marital status, BMI, and weight; Model 3 adjusted for Model 2, SPO2, heart rate, respiratory rate, SBP, DBP, MBP, temperature, renal impairment, diabetes, hypertension, COPD, creatinine, white blood cells, CRP, albumin, INR, platelets, calcium, potassium, sodium, chloride, magnesium, pH, lactate, ANION_GAP, ICU duration, hemoglobin, pathogen detection, antibiotic use, SOFA score; Kaplan–Meier survival curve analysis was used to analyze the survival status of the high and low RDW groups. Restricted Cubic Spline (RCS) curves were employed to detect the nonlinear relationship between RDW and sepsis mortality rate. Stratified analysis was conducted using COX regression models to obtain hazard ratios (HR) and 95% confidence intervals (95% CI) to explore interactions among different subgroups. The normality of continuous variables was assessed using the Shapiro–Wilk test. As the continuous variables did not follow a normal distribution, they were described using the median (interquartile range) [M(Q1, Q₃)]. Between-group comparisons for continuous variables were conducted using non-parametric tests (Mann-Whitney U test or Kruskal–Wallis test). Categorical variables were presented as counts (percentages), and between-group comparisons were performed using the Pearson chi-square test.
Results
Characteristics of the study cohort
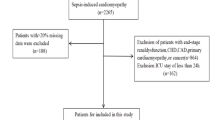
The flow chart of the study is shown in Fig. 1. 4865 cases of patients with sepsis were selected from the database. Excluding non-first-time admission patients (N = 2238) and patients with ICU stay less than 24 h (N = 1474), as well as patients with incomplete data (N = 44), a total of 1109 patients were included in the study. The average age of the patients was 64.7 years (ranging from 65.2 to 74.1 years), with 691 males and 418 females, accounting for approximately 62.3% of the total number of patients. The patients were divided into two groups according to the median RDW, and the baseline characteristics of these patients are shown in Table 1. The high RDW group exhibited statistically significant differences (P < 0.05) from the low RDW group in terms of age, weight, BMI, diastolic blood pressure, mean arterial pressure, hypertension, body temperature, creatinine, WBC, CRP, calcium ion, magnesium ion, INR, ANION GAP, marital status, PCO2, hemoglobin, albumin, diabetes, renal impairment, SOFA score, sepsis, ICU stay duration, 90-day mortality, and 365-day mortality. In the study, there were 134 deaths and 975 survivors within one year. The characteristics comparison between the death group and the survival group is shown in Supplementary Table 1.
Association between RDW and mortality
The results of univariate analysis of covariates and mortality rates can be found in Supplementary Table 2. Table 2 presents the results of the correlation analysis between RDW and 90-day and 365-day mortality rates. The data is presented as hazard ratios (HR) with 95% confidence intervals (CI). We developed three models to assess the independent impact of RDW levels on all-cause mortality using univariate and multivariate Cox proportional hazards models. In the unadjusted model (Model 1), the effect size and 95% CI for 365-day all-cause mortality were 1.222 (1.168–1.278), indicating that a unit difference in RDW was associated with a 22.2% increased risk of death (P < 0.001). In the minimally adjusted model (Model 2), the risk of death increased by 25.3% due to a unit change in RDW, with an effect size and 95% CI of 1.253 (1.188–1.323) (P < 0.001). In the fully adjusted model (Model 3), which accounted for differences in RDW units (adjusted for all variables shown in Table 1), the risk of death increased by 13%, with an effect size and 95% CI of 1.130 (1.039–1.229) (P < 0.01). Similar trends were observed when analyzing RDW as a categorical variable (Table 2). The results for 90-day mortality were consistent with those for 365-day mortality, indicating stability and reliability.
Survival status of the patients with different admission RDW levels
Figure 2 shows the Kaplan–Meier curves for patients with osteomyelitis. We conducted the Kaplan–Meier survival analysis based on RDW as a categorical variable, with all-cause mortality as the outcome variable. The K–M survival curves demonstrate that the survival time within one year is higher in G1 compared to G2 (p < 0.0001), as shown in Fig. 2. The survival probability of patients with osteomyelitis decreases with time. The survival analysis results for 90 days are consistent with those for 365 days.
Dose–response relationship between red cell distribution width and mortality
Based on the RCS regression model, the dose-response relationship between RDW and mortality rates in osteomyelitis is illustrated in Fig. 3. We observed a non-linear dose-response relationship between RDW and 90-day and 365-day mortality rates (nonlinear P < 0.01) (Fig. 3a,b). As RDW continues to change, the strength of correlation with 90-day and 365-day mortality rates shows a non-linear increase.
Subgroup analysis
Figure 4 shows the subgroup analysis results of the relationship between RDW and one-year mortality rate, using age, sex, race, marital status, hypertension, diabetes, acute kidney injury, COPD, pathogen detection, antibiotics, sepsis, albumin, CRP, and hemoglobin as stratification variables. The trend of the effect size was observed and a forest plot of the data was generated (Fig. 4). The results showed that the interaction tests of the stratification variables mentioned above were not statistically significant (P for interaction > 0.05).
Discussion
In this study, we found a relationship between increased RDW at ICU admission and adverse outcomes in osteomyelitis patients. After adjusting for confounding factors, we found that an elevated RDW at ICU admission was significantly associated with 90-day and 365-day mortality rates. An increased RDW at ICU admission was identified as an independent risk factor for adverse outcomes in osteomyelitis patients.
Existing research on osteomyelitis primarily focuses on treatment modalities and prognosis, emphasizing the importance of factors such as site of infection, clinical examination findings, types of pathogens, and the detection of acute-phase reactants (APRs) such as C-reactive protein (CRP), procalcitonin, and erythrocyte sedimentation rate (ESR) for early diagnosis, differential diagnosis, and antibiotic management of osteomyelitis3,4,5,6.
However, there is limited research on the correlation between RDW and prognosis in osteomyelitis. Irem Eldem et al.7analyzed the changes in RDW and other acute inflammatory markers during the hospitalization process of children with osteomyelitis. They found that RDW gradually increased during the three stages of admission, treatment, and post-treatment, showing a weak negative correlation with acute-phase reactants (APRs). However, in our study, we extracted the initial RDW at ICU admission and found that there were statistically significant differences in demographic data, clinical signs, APRs, length of ICU stay, 90-day mortality, 365-day mortality, and other indicators between the high RDW group and the low RDW group. Univariate and multivariate Cox regression analysis results showed that RDW was an independent risk factor for 90-day and 365-day mortality rates in severely ill osteomyelitis patients. This finding is consistent with some studies8,9,10, which indicate that higher RDW levels indicate a higher risk of mortality in severe infectious diseases. In surgical ICU patients, an elevated RDW is significantly associated with mortality. Some studies suggest that RDW can serve as a marker of inflammatory response11,12.
The mechanisms underlying the correlation between RDW and osteomyelitis prognosis remain unclear. Based on the existing literature, the potential mechanisms are as follows: (1) Inflammatory Response: Osteomyelitis induces an inflammatory response, where inflammatory factors such as interleukin-1, interleukin-6, tumor necrosis factor-alpha, and interferon-gamma may inhibit the marrow’s responsiveness to erythropoietin (EPO), affecting the maturation process of red blood cells and leading to increased heterogeneity in red blood cell volume13,14. (2) Oxidative Stress: Under oxidative stress conditions, excessive reactive oxygen species may cause lipid and protein oxidation of red blood cell membranes, affecting their morphology, function, and lifespan, which in turn leads to an increased red cell distribution width (RDW)15,16. (3) Anemia: Osteomyelitis may lead to localized or systemic anemia, where the generation and metabolism of red blood cells can be affected, thus influencing their size and morphology and resulting in elevated RDW17. (4) Malnutrition: Patients with osteomyelitis may experience malnutrition due to chronic disease consumption, loss of appetite, or malabsorption, leading to deficiencies in essential nutrients like iron, folate, and vitamin B12, which affect red blood cell production and maturation, resulting in increased RDW18. (5) Abnormal Erythropoiesis and Iron Metabolism: Inflammatory cytokines increase red blood cell apoptosis, promote deformability of red blood cell membranes, reduce erythropoietin production, decrease iron bioavailability, and contribute to erythropoietin resistance, all of which alter erythropoiesis and consequently increase RDW19,20,21,22. In our study, we found that the CRP level in the high RDW group (73.2 [25.0; 136] mg/L) was higher than that in the low RDW group (52.3 [11.4; 131] mg/L), p < 0.01. The white blood cell count was also higher in the high RDW group compared to the low RDW group, showing statistically significant differences, p < 0.01.
Red cell distribution width (RDW) is a parameter that reflects the heterogeneity of red blood cell volume in peripheral blood and indicates the degree of uniformity in red blood cell size23,24,25. Therefore, in previous literature, RDW has been commonly used to differentiate the etiology of anemia26,27,28. In this study, we found that the hemoglobin levels in the high RDW group were lower than those in the low RDW group, with statistically significant differences (11.2 [9.50; 12.6] g/dL, 12.6 [11.4; 13.9] g/dL, P < 0.001). After adjusting for age, sex, race, marital status, weight, BMI, SPO2, heart rate, respiratory rate, SBP, DBP, MBP, body temperature, renal impairment, diabetes, hypertension, COPD, creatinine, white blood cells, CRP, albumin, INR, platelets, calcium, potassium, sodium, chloride, magnesium, pH, lactate, anion gap, ICU duration, hemoglobin, pathogen detection, antibiotic use, and SOFA score, we found that a high RDW level was an independent risk factor for increased 90-day and 365-day mortality rates in ICU-admitted patients with osteomyelitis, with P-values less than 0.01. In the three models adjusted for different variables, we found that as RDW increases, the risk of mortality also rises. This further suggests that RDW is an independent predictor of prognosis in critically ill patients with osteomyelitis.
Through Kaplan–Meier survival curve analysis, we also demonstrated that within each RDW level group, the survival time at 90 days and 365 days for patients in the low RDW group was higher than that of the high RDW group (p < 0.0001). The level of RDW is positively correlated with both short-term and long-term all-cause mortality. This finding is consistent with some literature indicating an independent correlation between RDW and mortality rates in critically ill patients admitted to the ICU29,30,31. Wang et al.‘s study suggested a correlation between RDW and both mortality rate and total hospital stay in ICU patients, highlighting the independent association of high RDW levels with adverse outcomes in ICU patients. They proposed RDW as an effective clinical monitoring indicator for the prognosis of ICU patients32. Recent studies have also reported on the relevance of inflammatory factors to RDW17,33. Consistent with these findings, our study revealed similar results, showing statistically significant differences in CRP and WBC levels between the high RDW group and the low RDW group (P < 0.01). To exclude bias introduced by other factors, our study performed subgroup analyses stratified by age, sex, race, marital status, hypertension, diabetes, acute kidney injury, COPD, pathogen detection, antibiotic use, sepsis, albumin, CRP, and hemoglobin. Our subgroup analysis demonstrated that the predictive value of high RDW for mortality was consistent across the pre-specified subgroups. The forest plot results indicated no significant interaction among the stratified variables across the subgroups. This finding suggests that an increase in RDW is associated with a high risk of all-cause mortality, regardless of the presence of anemia or common comorbidities such as diabetes and hypertension.
The clinical significance of this study is as follows: it is the first to explore the independent relationship between RDW and mortality rates in critically ill patients with osteomyelitis; the results of this study contribute to evaluating RDW as an effective tool for risk stratification of critically ill osteomyelitis patients upon admission; this study may provide support for future research in establishing predictive models for patient mortality. Additionally, RDW can be quickly and easily obtained from routine laboratory tests upon admission. Therefore, RDW may serve as a simple and user-friendly biomarker for predicting mortality risk in critically ill osteomyelitis patients.
This study has some limitations. First, the data were obtained from the MIMIC-IV database. While the large sample size is an advantage, some information contained in this database may be outdated or missing, such as PCT, ESR, and other inflammatory markers. Second, this is a single-center retrospective study and the digital records are incomplete. The low incidence of abnormal values in the database may also have resulted in bias. Third, we only selected RDW measurements taken within 24 h after admission, without monitoring dynamic trends in RDW levels.
Conclusion
According to our research findings, high RDW in critically ill osteomyelitis patients is independently associated with prognosis and mortality rates. RDW is a simple, inexpensive, and routine laboratory parameter that can be used for prognostic monitoring and risk assessment in certain critically ill patients. However, large-scale multicenter prospective studies are needed to confirm these research results.
Data availability
The dataset used in this study can be found on the online website of the MIMIC-IV database. Anyone who meets the requirements for database usage can access the database.
References
Rupp, M. et al. Terminology of bone and joint infection. Bone Joint Res. 10(11), 742–743. https://doi.org/10.1302/2046-3758.1011.BJR-2021-0371 (2021).
Horch, R. E., Taeger, C. D., Steinau, H. U., Kneser, U. & Schnürer, S. Osteomyelitis: Behandlungskonzepte aus Sicht der plastischen Chirurgie [Osteomyelitis: treatment concepts from the plastic surgeon’s point of view]. Chirurg 84(11), 962–969. German. https://doi.org/10.1007/s00104-013-2623-8 (2013).
Arnold, S. R. et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J. Pediatr. Orthop. 26(6), 703–708. https://doi.org/10.1097/01.bpo.0000242431.91489.b4 (2006).
Masters, E. A. et al. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 20(7), 385–400. https://doi.org/10.1038/s41579-022-00686-0 (2022).
Conrad, D. A. Acute hematogenous osteomyelitis. Pediatr. Rev. 31(11), 464–471. https://doi.org/10.1542/pir.31-11-464 (2010).
Markanday, A. Acute phase reactants in infections: evidence-based review and a guide for clinicians. Open. Forum Infect. Dis. 2(3), ofv098. https://doi.org/10.1093/ofid/ofv098 (2015).
Eldem, I., Almekdash, M. H., Almadani, O., Levent, F. & Al-Rahawan, M. M. Red blood cell distribution width as a potential inflammatory marker in pediatric osteomyelitis. Proc. (Bayl Univ. Med. Cent) 36(4), 443–447. https://doi.org/10.1080/08998280.2023.2209921 (2023).
Purtle, S. W., Moromizato, T., McKane, C. K., Gibbons, F. K. & Christopher, K. B. The association of red cell distribution width at hospital discharge and out-of-hospital mortality following critical illness*. Crit. Care Med. 42(4), 918–929. https://doi.org/10.1097/CCM.0000000000000118 (2014).
Sičaja, M. et al. Red blood cell distribution width as a prognostic marker of mortality in patients on chronic dialysis: a single center, prospective longitudinal study. Croat. Med. J. 54(1), 25–32. https://doi.org/10.3325/cmj.2013.54.25 (2013).
Martínez-Velilla, N., Ibáñez, B., Cambra, K. & Alonso-Renedo, J. Red blood cell distribution width, multimorbidity, and the risk of death in hospitalized older patients. Age (Dordr). 34(3), 717–723. https://doi.org/10.1007/s11357-011-9254-0 (2012).
Hart, S. J. et al. Detection of iron deficiency in children with Down syndrome. Genet. Med. 22(2), 317–325. https://doi.org/10.1038/s41436-019-0637-4 (2020).
Peng, S., Li, W. & Ke, W. Association between red blood cell distribution width and all-cause mortality in unselected critically ill patients: analysis of the MIMIC-III database. Front. Med. (Lausanne). 10, 1152058. https://doi.org/10.3389/fmed.2023.1152058 (2023).
Zimmerli, W. Clinical practice. Vertebral osteomyelitis. N. Engl. J. Med. 362(11), 1022–1029. https://doi.org/10.1056/NEJMcp0910753 (2010).
McHenry, M. C., Easley, K. A. & Locker, G. A. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin. Infect. Dis. 34(10), 1342–1350. https://doi.org/10.1086/340102 (2002).
Claessen, B. E. et al. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. JACC Cardiovasc. Interv. 4(9), 974–981. https://doi.org/10.1016/j.jcin.2011.07.005 (2011).
Allen, L. A. et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J. Card. Fail. 16(3), 230–238. https://doi.org/10.1016/j.cardfail.2009.11.003 (2010).
Lippi, G. et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 133(4), 628–632. https://doi.org/10.5858/133.4.628 (2009).
Patel, K. V. et al. Red cell distribution width and mortality in older adults: a meta-analysis. J. Gerontol. Biol. Sci. Med. Sci. 65(3), 258–265. https://doi.org/10.1093/gerona/glp163 (2010).
Scharte, M. & Fink, M. P. Red blood cell physiology in critical illness. Crit. Care Med. 31(12 Suppl), S651–S657. https://doi.org/10.1097/01.CCM (2003).
Felker, G. M. et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM program and the Duke Databank. J. Am. Coll. Cardiol. 50(1), 40–47. https://doi.org/10.1016/j.jacc.2007.02.067 (2007).
Laftah, A. H. et al. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem. J. 397(1), 61–67. https://doi.org/10.1042/BJ20060215 (2006). Epub 2006/03/29.
Pierce, C. N. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion 20, 83–90. https://doi.org/10.1191/0267659105pf793oa (2005).
Pinna, A. et al. Red cell distribution width (RDW) and complete blood cell count-derived measures in non-arteritic anterior ischemic optic neuropathy. Int. J. Med. Sci. 18(10), 2239–2244. https://doi.org/10.7150/ijms.53668 (2021).
Soni, M. & Gopalakrishnan, R. Significance of RDW in predicting mortality in COVID-19—An analysis of 622 cases. Int. J. Lab. Hematol. 43(4), O221–O223. https://doi.org/10.1111/ijlh.13526 (2021).
Cetin, S. et al. RDW value may increase the diagnostic accuracy of MPS. Sisli Etfal Hastanesi Tip Bulteni. 55(1), 76–80. https://doi.org/10.14744/semb.2019.58159 (2021).
Danese, E., Lippi, G. & Montagnana, M. Red blood cell distribution width and cardiovascular diseases. J. Thorac. Dis. 7(10), E402–E411. https://doi.org/10.3978/j.issn.2072-1439.2015.10.04 (2015).
Kim, K. M. et al. Red cell distribution width is a risk factor for hip fracture in elderly men without anemia. J. Bone Miner. Res. 35(5), 869–874. https://doi.org/10.1002/jbmr.3963 (2020).
Xiang, L., Zhang, M., Wu, H. & Xie, D. The expression and prognostic value of ischemia modified albumin (IMA), red blood cell distribution width (RDW), and lipoprotein (LP) in patients with diabetes mellitus complicated with coronary heart disease. Ann. Palliat. Med. 10(4), 4463–4471. https://doi.org/10.21037/apm-21-425 (2021).
Fontana, V. et al. Can red blood cell distribution width predict outcome after cardiac arrest? Minerva Anestesiol. 84(6), 693–702. https://doi.org/10.23736/S0375-9393.17.12102-4 (2018).
Fontana, V. et al. Red cell distribution width after subarachnoid hemorrhage. J. Neurosurg. Anesthesiol. 30(4), 319–327. https://doi.org/10.1097/ANA.0000000000000459 (2018).
Fujita, B. et al. Red cell distribution width and survival in patients hospitalized on a medical ICU. Clin. Biochem. 48(16–17), 1048–1052. https://doi.org/10.1016/j.clinbiochem.2015.07.011 (2015).
Wang, F. et al. Red cell distribution width as a novel predictor of mortality in ICU patients. Ann. Med. 43(1), 40–46. https://doi.org/10.3109/07853890.2010.521766 (2011).
Vayá, A., Sarnago, A., Fuster, O., Alis, R. & Romagnoli, M. Influence of inflammatory and lipidic parameters on red blood cell distribution width in a healthy population. Clin. Hemorheol. Microcirc. 59(4), 379–385. https://doi.org/10.3233/CH-141862 (2015).
Funding
Ningbo Public Welfare Fund Project (2023S125), Ningbo Top Medical and Health Research Program (2022020405).
Author information
Authors and Affiliations
Contributions
Y.L.: Participate in research design. Y.Z.: Responsible for data collection and analysis. Y.L.: Writing a manuscript. Y.Z.: Assist in editing and reviewing manuscripts. S.D.: Help revise the manuscript. All authors have contributed to the article and agree to the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The establishment of MIMIC-IV (v2.0) was approved by the institutional review boards of the Beth Israel Deaconess Medical Center (Boston, MA) and Massachusetts Institute of Technology (Cambridge, MA). Thus, this study was granted a waiver of informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Zheng, Y. & Ding, S. Association between red blood cell distribution width and mortality in severe osteomyelitis patients. Sci Rep 15, 360 (2025). https://doi.org/10.1038/s41598-024-82891-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82891-w