Abstract
Over the past decade, Mass Administration of Medicines (MAM) has been a key strategy for controlling schistosomiasis and soil-transmitted helminthiasis (STHs) in Anambra State, Nigeria. This longitudinal study, conducted from 2017 to 2019, evaluated the impact of interventions for controlling schistosomiasis (SCH) and STHs in recipient communities. A total of 1,046 pupils aged 5 to 16 years were enrolled, with Kato-Katz and urine filtration methods used for faecal and urine sample analysis. A structured questionnaire was administered to 243 people to assess the contextual factors. At baseline, prevalence was 8% (82/1046), with A. lumbricoides (7.0%), T. trichiura (1.0%), Hookworm (0.1%), and S. haematobium (0.5%) observed. Co-infection was 1%. At follow-up, prevalence decreased to 6% (65/1046), with A. lumbricoides (2.0%), T. trichiura (2.2%), and S. haematobium (2%) observed, and co-infection was 0.2%. Infection levels varied by location (p > 0.05), with socio-economic status and inadequate WASH (Water, Sanitation, and Hygiene) infrastructure contributing to transmission risk. Most respondents (87%) earned less than $50 per month, and 39% practiced open defecation. The persistence of open defecation highlights critical gaps in WASH that undermine sustainable Neglected Tropical Diseases (NTD) control. Addressing cultural and economic challenges, alongside improving WASH infrastructure, is essential to sustain MAM’s impact.
Similar content being viewed by others
Introduction
Helminthiasis among pupils is a major impediment to their well-being and development. Over the past decade, Mass Administration of Medicines (MAM) has been a cornerstone in controlling schistosomiasis and soil-transmitted helminthiasis (STH) in Anambra State, Nigeria1,2,3. This longitudinal study, conducted from 2017 to 2019, evaluated the implementation and impact of these interventions in the affected communities, aiming to provide actionable insights for sustainable disease control and elimination. The study provides actionable insights for sustainable disease control and elimination, particularly in the context of ongoing global health challenges.
Schistosomiasis and soil-transmitted helminthiasis are public health concerns and significant impediments to socio-economic development4. Intestinal helminthiasis among pupils, for instance, is a major barrier to their well-being and cognitive development, leading to long-term economic consequences due to reduced educational attainment and productivity5,6. Schistosomiasis, with its debilitating effects, hinders physical and cognitive growth, thereby perpetuating cycles of poverty and economic disparity. Addressing these diseases is crucial for achieving broader health and economic goals, as emphasized in the World Health Organization (WHO) Neglected Tropical Diseases (NTD) Road Map 2021–20306.
The World Health Organization (WHO) Neglected Tropical Diseases (NTD) Road Map 2021–2030 heralds a paradigm shift from disease-specific interventions to integrated, cross-cutting approaches within existing health systems. This transition is essential for fostering the community sustainability of MDA programs6. The road map’s emphasis on mainstreaming interventions into health systems highlights the critical role of community engagement in achieving long-term success and sustainability. Strengthening program delivery by incorporating community perceptions and opinions is crucial for promoting equitable, person-centered service delivery7.
Active community participation is indispensable for the success of any control program, including MDA. Studies underscore that misunderstandings about an intervention or skepticism regarding its benefits can lead to low participation rates. Conversely, when communities understand and agree with the goals and methods of an intervention, their involvement and support increase significantly8,9,10. Therefore, community engagement is a pivotal, yet frequently overlooked, aspect of control and elimination programs. Effective community empowerment through knowledge, engagement, collaboration, and leadership, combined with improved infrastructure, can bring about sustainable changes that reduce the risk of acquiring schistosomiasis and STH6,7.
The COVID-19 pandemic has underscored the importance of community resilience and sustainable health interventions. The timing of this study, completed just weeks before the lockdown, highlights the critical need for robust community engagement strategies to ensure continuity and effectiveness of health programs in the face of global health crises. The pandemic’s impact on funding for NTD programs, particularly the significant cuts by the UK Foreign and Commonwealth Development Office (FCDO), has further emphasized the need for alternative funding mechanisms11. Public engagement and crowdfunding are now critical to ensuring the continuity of research and intervention programs for neglected tropical diseases12. Thus, community involvement is vital not only for program implementation but also for sustaining financial support in the long term12,13.
The WHO Regional Office for Africa’s Accelerated NTD Mapping Project, initiated in 2013, has highlighted the importance of epidemiological mapping for effective intervention planning14,15. Mapping provides essential data on disease distribution and endemicity, which is critical for targeting interventions and ensuring efficient resource utilization. Accurate mapping helps avoid wastage and ensures that treatment reaches the populations most in need6.
In Nigeria, schistosomiasis and STHs are major public health concerns, with mapping showing that 583 out of 774 local government areas (LGAs) are endemic for schistosomiasis1. Despite substantial progress in treatment coverage, challenges remain in ensuring the sustainability and effectiveness of MDA programs. The mapping of Soil-Transmitted Helminths (STH) and schistosomiasis in Anambra State was carried out in a phased manner between 2011 and 2013. The mapping of STH began in 2012 across Awka North, Ayamelum, Ekwusigo, Ihiala, and Orumba South, and continued in 2013 to include Aguata, Awka South, Dunukofia, Njikoka, Nnewi North, Nnewi South, and Oyi Local Government Areas (LGAs). Schistosomiasis mapping commenced earlier, in 2011, with Anambra West LGA, expanded in 2012 to Idemili North, Ogbaru, Onitsha South, and Orumba South, and extended further in 2013 to Awka South, Dunukofia, Njikoka, Nnewi North, Nnewi South, and Orumba South3. These mapping efforts identified endemic areas, leading to school-based chemotherapy with Albendazole and Praziquantel1,2,3. However, some communities were excluded from the intervention because they were classified as non-endemic1,3. No subsequent comprehensive mapping has been conducted in the state since then, raising concerns about potential misclassification of communities and restricted access to preventive chemotherapy (PC) programs. This oversight could mean that some populations at risk may have been excluded from necessary treatments. However, achieving sustainable disease control requires not just periodic treatment but also comprehensive measures to address the environmental and socio-economic factors that contribute to disease transmission.
Mass administration of medicines alone is unlikely to break the transmission cycle of schistosomiasis and STHs without complementary environmental measures such as improved sanitation and access to clean water. Health education is also critical for encouraging behaviours that reduce infection risk. Modelling studies suggest that community-wide MAM delivery, combined with high coverage and compliance, can potentially interrupt disease transmission. Nevertheless, achieving such outcomes requires overcoming significant challenges, including the risk of anthelmintic resistance and the premature dismantling of NTD programs due to funding constraints6,11.
The WHO NTD Road Map’s deadline of 2030 is now less than six years away, making it crucial to accelerate efforts to achieve the outlined targets. This urgency underscores the relevance of this study, which addresses the pressing need for a systematic evaluation of ongoing MAM programs in Anambra State. The limited evidence on the impact of ongoing Mass Administration of Medicines (MAM) programs in Anambra State underscores the need for surveillance, monitoring, and evaluation efforts. This knowledge gap not only hinders our understanding of the initiatives’ effectiveness but may also inadvertently deprive certain populations and communities of essential treatments.
This study aims to address the pressing need for a systematic evaluation of the ongoing MAM programs in Anambra State. It assesses the current prevalence of schistosomiasis and STHs, evaluates the efficacy of drug administration programs, and identifies socio-economic and behavioural factors influencing disease transmission. By understanding these factors, the study seeks to develop strategies for complete community involvement in managing and sustaining drug administration programs. Ultimately, this research aims to contribute to the control and possible elimination of schistosomiasis and soil-transmitted helminthiasis in the region, while also supporting the global effort to eliminate neglected tropical diseases, promote health equity, and improve access to quality healthcare.
Methods
Study area
Anambra State is located in the south eastern part of Nigeria with a landmass of about 4,844 km2 (1,870 sq. m) and a population of 4,177,828. It has geographical coordinates of 6°20′0′ North 7°0′0′ east. Anambra is the eighth-most populated state in the Federal Republic of Nigeria and the second-most densely populated state after Lagos State16. It has tropical rainforest vegetation, with two distinct seasons in the year, rainy (April to October) and the dry season (November to March). The area is rich in natural gas, crude oil, bauxite, and ceramic. It has an almost 100% arable soil. The state has many other resources in terms of agriculturally based activities such as fisheries and farming, as well as land cultivated for pasturing and animal husbandry. Open defecation practices are also very common as there is limited availability of functional toilet facilities and safe water supply in most of the towns within the state. Power supply is almost non-existent in these communities. Poorly equipped health centres, absence of standard laboratories and lack of trained health personnel indicate that public health outreaches in these communities are rare.
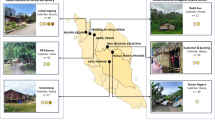
The study was carried out in three communities selected by the Ballot method17 namely; Achalla in Awka North Local Government Area (LGA), Ezinifite in Aguata LGA and Nsugbe in Anambra East LGA and covering the three senatorial zones of Anambra State selected (Fig. 1).
Study design
The longitudinal study was conducted from March 2017 to July 2019 in 9 public primary schools. The field study was conducted twice using the same cohorts first for baseline data collection and one year later for the follow-up data to accommodate the on-going mass administration of medicines by the Neglected Tropical Unit of Anambra State Ministry of Health in collaboration with the donor agency, Carter Centre.
This study focused specifically on primary school pupils, aligning with the Mass Administration of Medicines (MAM) programs implemented by Anambra State. These programs targeted primary school children due to the higher prevalence of helminth infections within this age group, as identified by earlier epidemiological assessments2,3. By concentrating on primary schools, the study aimed to optimize resource allocation and intervention effectiveness, providing valuable insights into factors influencing helminth prevalence. All methods were performed in accordance with the relevant guidelines and regulations.
Study population and sample size
Study participants and sampling
Within the 3 selected LGAs, public primary schools were chosen based on the following inclusion criteria:
-
a.
Size of the school’s population – large population preferred.
-
b.
Schools with pupils not dewormed in the last 3 months.
Based on the above criteria, 9 public primary schools, 3 from each selected LGAs were randomly selected from each LGA. Chosen by balloting17. These were Community, Egede and Udoka Primary Schools in Achalla; Akpunoji, Igwebuike and Ogbugbogu Primary Schools in Ezinifite and Central, Community and Development Primary Schools in Nsugbe. A scoping visit to meet and provide school teachers with the objectives and other detailed information on the study was done.
Selection of children
The study population consisted of 1046 pupils from 5 to 15 years from the selected public primary schools whose parents/guardians gave their consent. The assent of the pupils was also obtained.
The sample size of 1046 was determined using the formula18:
Where:
Where,
N = Population size, N = 2,182 (based on the total number of pupils in the three communities selected as obtained from Anambra State Universal Basic Education Board (ASUBEB).
Z = Z statistic for a level of confidence, Z = 1.96,
P = Expected proportion (in the proportion of one, P= 0.5819.
d = Precision (error of estimate), d = 0.05.
Substituting,
To account for a 75% response rate20 and a 25% non-response rate, an additional 14% of the sample size ( \(\:\frac{14}{100}\times\:917.2\)= 128.4+ 917.2 = 1,045.6) was calculated and included, resulting in a final sample size of 1046 participants to optimize precision and minimize potential withdrawals. Participant recruitment employed a stratified sampling approach18 with inclusion criteria including written parental/guardian consent, the pupil’s assent to participate and a minimum 3-year residency in the areas. Exclusion criteria included a nonresponsive study population, those unwilling to provide consent, and recent migrants or temporary residents of the area. Hence, a total of 1046 pupils aged 5–16 years from randomly selected public primary schools were enrolled for this study.
Parasitological examination
To determine the prevalence and intensity of helminth infections among the study population, we collected stool and urine samples from each child over two consecutive days during both baseline and follow-up surveys. Each sample was carefully collected and labelled with the participant’s serial number to ensure accurate tracking and analysis. All samples were freshly collected within the premises of the selected schools, ensuring immediate processing and minimizing the risk of sample degradation.
Urine collection and examination
Urine samples were collected between 10:00 AM and 12:00 PM, a time selected to optimize egg detection, as recommended by WHO guidelines21. Each pupil was provided with a sterile, labelled urine collection bottle and was instructed to provide a midstream urine sample of at least 10 ml, including the last few drops, which often contain the highest concentration of Schistosoma haematobium eggs. The collection was supervised by researchers and teachers to ensure compliance with protocols and to maintain the integrity of the samples.
Recognizing that menstruation could affect haematuria counts and potentially lead to false positive results22, we temporarily excluded menstruating females from these assessments. These individuals were marked and revisited once their menstrual periods had ended to collect accurate data. This system of regular communication allowed participants to inform the study team when their menstruation ceased, ensuring that all eligible participants could be included in the study.
Immediately after collection, urine samples were processed and examined on-site at the school premises. This on-site processing was essential due to the field study setting, ensuring that samples were analyzed promptly to maintain their integrity and prevent contamination or degradation. Samples were kept in sterile, airtight containers before analysis. Reagent strips (Urine-9 parameters, Accu-answer urinalysis laboratory test strips, Guilin Zhonghui Technology Co., Ltd., Guilin, China) were used to estimate the amount of blood present in the urine. For parasitological examination, 5 to 10 ml of urine was filtered through a 13 mm polycarbonate membrane filter (Sterlitech Corporation, NY, USA). The filter, which captured any S. haematobium eggs present, was then transferred to a clean, grease-free microscope slide. The slides were examined under a microscope at 40x magnification to identify and count S. haematobium ova. Eggs were identified based on their characteristic morphology: they are typically oval-shaped with a terminal spine21. Infection intensity was classified as light (1 to 49 eggs per 10 ml of urine) or heavy (more than 49 eggs per 10 ml of urine), following WHO guidelines21.
Stool collection and examination
Each pupil was given a universal sample bottle equipped with a small spoon for stool collection. Pupils were instructed to defecate on a clean piece of paper and to use the spoon to collect a sample of the stool, which was then placed inside the sample bottle and securely covered. These samples were freshly collected and immediately processed within the school premises. Stool samples were examined using the modified quantitative Kato-Katz method, following the protocols established by Martin and Beaver23.
Within the school premises, duplicate thick smears were prepared on microscope slides using 41.7 mg templates. The slides were allowed to clear for 10 to 25 min before examination under a microscope. This timing allowed for optimal visualization of helminth eggs. The number of eggs for each helminth species was recorded and multiplied by a factor of 24 to calculate the number of eggs per gram (EPG) of stool. The EPG was further adjusted using correction factors as described by Nawalinski et al.24 to estimate the intensity of infection accurately. Identification of intestinal parasites, including S. mansoni and other soil-transmitted helminths, was conducted according to WHO guidelines21.
Quality control
Field assistants received training in questionnaire administration and sample collection. Researchers ensured questionnaire completeness and adherence to recommended timeframes for urine and stool sample collection. All slides were initially examined by skilled laboratory technologists with expertise in parasitology. To ensure the accuracy and reliability of the results, a random 10% of the slides were re-examined by one of the laboratory technologists, who was blinded to the initial results and independently examined a subset of the slides. This double-checking process was implemented to verify the original egg count, maintain the integrity of the results, and minimize the risk of false positives. The results from both technologists’ examinations were then compared for consistency.
Assessment of the efficacy of drug administration programs
To evaluate the effectiveness of the Mass Administration of Medicines (MAM) programs in reducing schistosomiasis and soil-transmitted helminthiasis among school-aged children, we followed this approach:
-
1.
Baseline Data Collection: Stool and urine samples were collected from children before the MAM to establish the prevalence of infections, serving as a reference for post-treatment comparisons.
-
2.
Mass Administration of Medicines: Praziquantel and albendazole were administered simultaneously to target both schistosomiasis and soil-transmitted helminthiasis, ensuring comprehensive treatment.
-
3.
Follow-up Examinations: One-month post-treatment, follow-up stool and urine samples were collected from the same cohort of school-aged children to assess changes in helminth egg counts and determine the program’s impact on reducing helminth infections. All follow-up examinations were conducted within the one-year timeframe in the study locations chosen by the NTD Unit, Anambra State Ministry of Health, in collaboration with the Federal Ministry of Health (FMOH) and the donor agency (Carter Center), which provided the drugs for the mass treatment.
-
4.
Directly Observed Treatment: Drugs were administered under the supervision of healthcare personnel to ensure accurate dosing. Praziquantel was dosed at 40 mg/kg based on each child’s weight, and albendazole was provided as a single tablet25,26. Tablets were given orally, with instructions to swallow them with water, ensuring compliance and ease of intake.
-
5.
Homegrown School Feeding Program (HGSFP): Established in 2016, the HGSFP provides nutritious meals to children in all public primary schools in Anambra State. This initiative plays a crucial role in supporting the Mass Administration of Medicines (MAM) programs by ensuring that children receive a healthy meal before taking the medication. This not only helps to minimize potential side effects of the drugs but also enhances their effectiveness. Additionally, the HGSFP has significantly improved school enrollment and attendance, thereby increasing the number of children who can benefit from deworming treatments5.
Participants were observed for any adverse reactions post-administration, and they were also encouraged to report any side effects experienced. This monitoring helped to ensure the safety and well-being of the children and provided valuable information for future treatment protocols.
Egg Reduction Rate (ERR): The effectiveness of the treatment was assessed by calculating the ERR and comparing the number of helminth eggs in stool or urine samples before and after treatment25,27,28. ERR was determined using the formula27,28:
A higher ERR indicated a significant reduction in infection levels, reflecting successful treatment outcomes27.
Overall assessment of risk factors and community peculiarities
For the overall assessment of risk factors associated with Schistosomiasis and STH infections and the evaluation of the distinct characteristics of communities, surveillance visits, oral interviews, gender- sensitive focus group discussions of 8, and pre-tested questionnaires were employed. These assessments particularly focused on socio-demographic characteristics and behavioural variables chosen for the study.
Development of strategies for community involvement
Oral interview, focus group discussions and pre-tested questionnaire were employed for the overall assessment of the factors that could be responsible for ensuring the complete involvement of the people in the management and sustainability on the administration of medicines and the school feeding programme. Focus group discussions which was also gender sensitive were conducted in groups of four for all the parents and teachers that volunteered. All discussions were made in local languages (Igbo language) and simplified to the best understanding of all the participants in the selected communities.
Data analysis
Data analysis was performed using Minitab 17 software. The intensity of infection was categorized following WHO recommendations21. Descriptive statistics, including frequencies (n) and proportions (%), were calculated. Prevalence and 95% confidence intervals were determined for gender and age groups. Chi-square (χ2) tests were used to assess the association between prevalence and socio-demographic characteristics and behavioural variables. Kruskal-Wallis test in Minitab 17 were employed for variations and comparisons of arithmetic means. Additionally, Odds Ratios (ORs) were calculated to assess associations between binary outcomes and predictor variables. Multinomial Logistic Regression was used to model relationships with multiple categorical outcomes. The level of significance was set at p < 0.05.
Results
A total of 1046 pupils (523 males and 523 females) with a mean age of 9.25 ± 0.126 years had complete parasitological data and were also administered questionnaire and were included for all subsequent analyses (Table 1).
At baseline, 8.4% of male pupils and 7.3% of female pupils were infected, with an OR of 1.172 (95% CI: 0.746–1.842, p = 0.490), showing no statistically significant difference in infection risk between genders. The highest infection rate was observed in pupils aged 9–12 years (10%), followed by those aged 5–8 years (6%) and 13–16 years (3.4%). Odds ratios were 3.153 (95% CI: 0.964–10.311, p = 0.058) for ages 5–8 and 1.852 (95% CI: 0.547–6.273, p = 0.322) for ages 9–12, with no significant differences between age groups (Table 1).
At follow-up/surveillance, a year later, results showed that the overall prevalence of helminth infection was 6% (65/1046) with 5.0% of male pupils and 7.0% of female pupils being infected, with an OR of 1.439 (95% CI: 0.865–2.394, p = 0.161). No significant differences were observed in the prevalence of helminth infection based on gender and age (P > 0.05) among the pupils in the study area. There was Co-endemicity of urogenital schistosomiasis and soil-transmitted helminthiasis among the pupils was also observed. The following helminth ova were identified respectively; A. lumbricoides 2% (20/1046) and T. trichiura 2.2% (23/1046) only while for Schistosoma spp., only Schistosoma haematobium ova 2% (20/1046) was seen. Co-infection of 0.2% (2/1046) between A. lumbricoides and S. haematobium was also observed (Table 2).
Although there was a decline in the overall prevalence of helminth infection by 2%, there was an increase in the total number of infected pupils with S. haematobium by 1% (15/1046) while a decline in the number of infected pupils with STHs by 3% (32/1046) was seen.
Result showed that the overall prevalence of Helminth infection (Schistosomiasis and Soil-transmitted helminthiasis) varied significantly at baseline (P = 0.00) and follow-up (P = 0.01) respectively with respect to location (Table 3).
At baseline, the overall helminth infection prevalence varied significantly across locations. Achalla recorded the highest prevalence (17.4%), followed by Nsugbe (4.0%) and Ezinifite (1.1%). Achalla showed significantly higher odds of infection compared to Ezinifite (OR: 3.423; 95% CI: 1.062–11.028; p = 0.04), while Nsugbe exhibited the highest odds (OR: 19.587; 95% CI: 7.067–54.285; p = 0.00). At follow-up, overall prevalence declined across all locations. Achalla reduced to 9.0%, Nsugbe to 6.0%, and Ezinifite remained low at 3.0%. Despite the reduction, Nsugbe continued to show significantly higher odds of infection compared to Ezinifite (OR: 2.982; 95% CI: 1.523–5.839; p = 0.00) (Table 3).
For species-specific prevalence, Achalla consistently recorded the highest rates at baseline. Ascaris lumbricoides prevalence was 15% (57/390) and Trichuris trichiura prevalence was 2% (6/390), both significantly higher than in other locations (p = 0.00 and p = 0.01, respectively). Similarly, Schistosoma haematobium prevalence was highest in Achalla (1%; 3/390), compared to Nsugbe (0.4%; 1/281) and Ezinifite (0.3%; 1/375). Significant differences in Ascaris lumbricoides prevalence were observed across locations at both baseline (p = 0.00) and follow-up (p = 0.034) (Table 3).
The prevalence of species-specific Schistosoma infection showed that individual infections were generally low (< 5%) with only S. haematobium found both at baseline and follow-up study respectively. At baseline, S. haematobium infection intensities were primarily of heavy infection except in a pupil with a light infection intensity. At Follow-up, S. haematobium infection intensities were mainly of light infection except in 7 pupils with heavy intensity of infection. (Table 4).
This study also confirmed the evidence of female genital schistosomiasis (FGS) seen in a female pupil that had a heavy intensity of infection and this was published29.
At baseline, there was moderate intensity of infection with A. lumbricoides only among the pupils in Achalla. The intensity of parasite load amongst children who were positive for T. trichiura and Hookworm was of light intensity at baseline for the three communities studied. Follow-up study showed that there was light intensity of infection for all STHs infection seen in all the locations studied (Table 5).
For S. haematobium, the pre-treatment egg count ranged from 23.962 to 52.818 eggs, with a mean of 38.39 eggs (± 14.4280). Following treatment, the post-treatment egg count ranged from 37.974 to 57.686 eggs, with a mean of 47.83 eggs (± 9.856). This resulted in an ERR that ranged from − 42.52 to 58.51%. For STH infection, the results revealed a high ERR for A. lumbricoides (95.84%), indicating a significant reduction in egg count after treatment. For T. trichiura, the ERR was comparatively lower (7.81%), suggesting a lesser reduction in egg count. Remarkably, a 100% ERR was observed for Hookworm, signifying the elimination of detectable eggs post-treatment (Fig. 2).
The assessment of the risk factors associated with Urogenital Schistosomiasis and Soil-Transmitted Helminth (STH) infections revealed significant socio-demographic and behavioral variables among the study population. Results showed that among the 1046 pupils examined, 8% (82/1046) were found to be infected with helminthiasis. Among the infected pupils, 93.9% (77/82) do not wash hands regularly, 91.5% (75/82) lack knowledge about helminthiasis transmission, 62% (51/82) practice open defecation in the bush, 92.7% (76/82) have not been dewormed for more than 6 months, 82.9% (68/82) have a preference for swimming, 11% (9/82) use a stream as their primary water source, and 89% (73/82) practice defecation/urination near the stream. The study found that hand washing habits (P = 0.046), knowledge about helminthiasis (P = 0.0031), type of toilet used (P = 0.002), duration since last deworming (P = 0.026), swimming habits (P = 0.00), source of water supply (P = 0.039), and defecation/urination near streams (P = 0.021) were significantly associated with the prevalence of these infections (Fig. 3). Furthermore, observations (Figs. 4, 5 and 6) provided additional context to these findings.
Assessment of the best ways to ensure complete community involvement in the management and sustainability of medicine administration for the control and possible elimination of schistosomiasis and soil-transmitted helminthiasis revealed the following: Among the 243 respondents, 87% earn less than $50 per month, 39% practice open defecation, and 71% advocated for the continuation of Mass Administration of Medicines, with 67% appreciating that it was free of charge. Regarding sustainability, 98% were satisfied that the government is providing funds (Table 6).
Furthermore, Oral interview and Focus group discussions provided additional context to these findings. For example,
One respondent who sold food at the local market shared,
“I am the breadwinner of the family and any money I get goes to feeding and paying their school fees. I cannot afford to give my children ‘ogwu okpo’ (anthelminthic drugs) every three or six months. Where will I get the money?’’ (Female Respondent A).
Another respondent said,
“Who has time to take drugs when you are not sick? I only take the medicines when the government shares them and I keep them until anyone falls sick, then I give the medicines to them.’’(Male Respondent A).
Another respondent a local CHEW had this to say,
“We need more awareness of the importance of regular deworming. Many of us in the community are unaware of or neglect it.’’ (Female Respondent B).
A concerning 39% reported open defecation practices.
One respondent gave the following reason,
“We do not have water, we have to buy or fetch from the stream. It is easier to go to the bush or farmlands, that way, we save more water.” (Female Respondent C).
Another respondent, a teacher also echoed the lack of water as a reason for open defecation, adding that,
“Even the school lacks water, and the pupils have to go a reasonable distance just to get water. The toilet facilities are very poor too. So what do you expect us to do? We know that open defecation is bad, and hygiene is important, but all the initiatives we have had so far have fallen short because there is no water.” (Female Respondent D).
The majority (71%) of respondents favoured Mass Administration of Medicines (MAM), primarily due to its free administration.
A respondent reported,
“I like that the drugs are free. For most of us, these free drugs are the health care we can afford. It is a good thing.” (Male Respondent B).
In contrast, one respondent said,
“I don’t trust these drugs. Most of them might be expired or something worse. I prefer to go to the chemist and buy exactly what I want. I think it is safer.” (Male Respondent C).
A majority of the respondents, 81% believed that school feeding programs substituted their child’s ration at home.
A respondent reported,
“The food my children are given in school helps a lot. I don’t have to worry about feeding them when they get back from school.” (Female Respondent E).
Another respondent had this to say,
“I like the school feeding oo. But I will still feed my children when they get back. I think the school feeding has added to what they get. My children look plumper and fresher. I like it.” (Female Respondent F).
While 75% suggested that the government should fund MAM for sustainability, 98% objected to contributing funds themselves.
In this regard a vocal respondent shared,
“If we had the money, we would be buying these drugs ourselves. Already we are struggling with a lack of many necessary things even roads. These free drugs are the least of what the government owes us.” (Male Respondent D).
Respondents recommended the use of Community Drug Distributors (CDDs)/Community Health Extension Workers (CHEWs) for drug administration, with a preference for female CDDs/CHEWs (60%).
One respondent shared,
“I like to see females who we know sharing these drugs (CHEWs). We trust them more.” (Male Respondent E).
Concerning preference, one respondent shared,
“I don’t know why, but I just like to be treated by the female nurses. They are also gentler with children. And when they come to your house, you don’t have to worry that you are in some sort of trouble.” (Female Respondent G).
Regarding child treatment practices, 58% relied on chemists, 38% visited herbalists, and only 4% sought treatment at hospitals.
One respondent shared,
“Using herbs or going to the chemist to mix drugs is cheaper. Hospital treatments are very costly and health centres are not always open, because they do not have drugs.” (Male Respondent F).
Discussion
This study confirmed that helminth infections remain prevalent in communities in Anambra State despite ongoing Mass Administration of Medicines (MAM) efforts. Among the primary school pupils sampled, the overall prevalence of helminth infection was relatively low at 8% at baseline and 6% during follow-up, which aligns with similar studies across sub-Saharan Africa that have reported reductions in infection rates following MAM initiatives30,31. Our findings reveal that although MAM has reduced the overall burden of infection, several underlying factors contribute to reinfection, making it clear that MAM alone is insufficient for sustainable control. The persistence of helminths highlights the need for a more integrated approach that goes beyond medication distribution to address environmental, behavioural, and socio-cultural factors.
Key contributors to the continued presence of helminths include inadequate water and sanitation infrastructure, lack of comprehensive health education, and socio-cultural barriers to drug uptake. Many communities, particularly those in peri-urban and rural areas, still struggle with poor access to clean water and proper sanitation, conditions that facilitate the transmission of helminths. The persistence of open defecation, mentioned by 39% of respondents as a necessity due to lack of water, highlights critical gaps in WASH (Water, Sanitation, and Hygiene) infrastructure that undermine sustainable NTD control efforts. Without addressing these foundational WASH issues, achieving the WHO 2030 Road Map goals for NTD elimination will remain a significant challenge. Additionally, the low levels of health literacy and persistent cultural beliefs about medicine further impede the success of MAM programs, as many people remain hesitant to participate fully in these campaigns. This calls for more robust, culturally sensitive community engagement to foster trust and increase compliance. This issue mirrors findings from Kenyan research, where community-specific challenges have contributed to the persistence of helminth infections despite regular MAM32. This shows that there is a need to understand contextual cultural values as this has a great influence on the uptake of any health intervention in the communities29,33. This will help to determine the best culturally congruent approach to the provision of healthcare in these endemic communities that will be implemented. This study therefore emphasizes the importance of socio-cultural factors, such as mistrust in health programs and misinformation, which have been less explored in previous studies. Addressing these socio-cultural issues is crucial for improving community participation and compliance.
A notable challenge to MAM effectiveness identified in this study was compliance, with some children initially pretending to take medication but subsequently spitting it out. This issue was mitigated by implementing Directly Observed Therapy (DOT) and reinforcing the importance of adherence through education, improving compliance, and aligning with successful Ugandan studies that applied similar behavioural interventions34. Such adherence strategies are crucial for maximizing MAM impact and reducing helminth transmission.
Additionally, while MAM campaigns have achieved measurable success in reducing infection rates, the study found that reinfection remains a significant issue, especially in peri-urban areas where one might expect lower transmission due to better infrastructure. This finding challenges assumptions that urbanization alone leads to better health outcomes and suggests that localized factors, such as intermittent access to sanitation and unregulated water sources, may play a larger role in these communities than previously acknowledged.
Co-endemicity of urogenital schistosomiasis and soil-transmitted helminthiasis was observed, albeit with a low co-infection rate of 0.2% for A. lumbricoides and S. haematobium. Although low, this co-infection rate suggests a need for integrated control programs to address overlapping environmental and behavioural factors contributing to these infections5,35,36. Combined interventions are more effective for co-endemic areas37,38. Targeted preventive chemotherapy in such areas is essential to mitigate potential health impacts associated with co-infections. Encouragingly, a declining trend in helminth prevalence was observed throughout this study, consistent with recent studies5,39, likely due to the ongoing MDA efforts facilitated by organizations like the Carter Center in partnership with federal and state health ministries. However, A. lumbricoidesprevalence remained notably higher than other STHs at both baseline (7%) and follow-up (2%), a pattern consistent with other studies5,36 that identify this parasite as particularly resilient. This persistence suggests a need for targeted interventions to specifically address A. lumbricoides, which may respond less effectively to standard MAM protocols.
Concerningly, an increase in the prevalence of Schistosoma haematobiumwas observed during the follow-up, likely due to the lack of MAM coverage in non-schisto-endemic areas. Untreated infections in even a single individual can perpetuate transmission within a community, underscoring the need for comprehensive mapping and inclusion of all at-risk areas to prevent untreated communities from acting as reservoirs for infection40,41. Expanding MAM to all communities with potential exposure could significantly reduce the risk of transmission and reinforce the progress made by MAM efforts.
Finally, this study was limited to primary school pupils, as MAM programs in Anambra State specifically target this high-risk age group. Secondary school students were excluded to streamline resource management and intervention delivery. Expanding future studies to include older age groups, such as secondary school students, would allow for a broader understanding of helminth infection dynamics and provide insights for more comprehensive public health strategies that build on these findings. This could support more inclusive, sustainable interventions that contribute to lasting reductions in infection rates and align with global neglected tropical disease (NTD) elimination goals.
The longitudinal nature of the study is a key strength, as it allowed for the tracking of infection rates and community responses to MAM over an extended period, providing a more in-depth understanding of the factors at play than a cross-sectional approach would allow. Additionally, the study’s large sample size and its representation of diverse communities across Anambra State strengthen the generalizability of the findings. However, the study’s limitation to a single state means that broader regional patterns remain unexplored, and future research should expand to different geographic areas in Nigeria or other sub-Saharan African countries to validate these findings.
The practical implications of this study are significant. The persistence of helminth infections suggests that policymakers must adopt a more holistic strategy. While MAM remains a vital tool, it should be integrated with WASH initiatives, community health education, and tailored local interventions to reduce environmental risk factors and improve drug uptake. Additionally, involving local leaders and health workers in education campaigns could help dispel myths and encourage wider participation in future MAM rounds. Further, implementing community-based surveillance systems could enable health authorities to monitor infection and reinfection rates more effectively, allowing for adaptive interventions based on real-time data.
In conclusion, this study contributes to the broader discourse on helminth control by highlighting the limitations of MAM as a standalone intervention and advocating for a more integrated approach. The findings suggest that health policies must focus on environmental, socio-cultural, and behavioural factors in addition to medical interventions to achieve sustainable reductions in helminth prevalence. Future research should consider broader population groups, such as secondary school students, to better understand infection dynamics and inform more comprehensive public health strategies. The study consequently advocates the need for the government to introduce vocational programs and improve basic social amenities in these communities, as these efforts will help to improve the financial status and overall well-being of community members. By addressing both the health and socioeconomic needs of affected populations, these strategies hold promise for enhancing the long-term effectiveness and sustainability of helminth control efforts in Anambra State and similar settings.
Data availability
All relevant data supporting the findings of this study are included in the manuscript.
References
Federal Ministry of Health. Neglected Tropical Diseases Nigeria Multi – Year Master Plan, 2015–2020. Abuja, Nigeria: Federal Ministry of Health; (2015).
Federal Ministry of Health. Nigeria NTD situation Snapshot. Abuja, Nigeria: Federal Ministry of Health; (2019).
State Ministry of Health. Situational Analysis on Schistosomiasis and Soil-transmitted Helminth infection, Anambra State NTDs Report. Awka, Anambra: State Ministry of Health; (2019).
Montressor, A., Albonico, M., Chritsulo, L., Crompton, D. W., Diary, A., Engel, D., Gabriel, A., Gyorlcos, T. N., Mbabazi, P., Ottesen, E., Savioli, L. & Yajima, A. Helminth control in school-age children: a guide for managers of control programmes. Second edition. Geneva: World Health Organization, Geneva, 75Pp. http://w\vw.who.int/gho/en/ (2011)
Aribodor, O. B. et al. Status of intestinal helminth infection in schools implementing the home-grown school feeding program and the impact of the program on pupils in Anambra state, Nigeria. Acta Parasitol. 66, 1528–1537 (2021).
World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021 – 2030. Geneva, p. 196. https://www.who.int/publications/i/item/9789240010352. (2021).
Zawolo, G. et al. Alternate and Enhanced Community Engagement for the Liberian Neglected Tropical Disease Programme: Community Perspectives on Mass Drug Administration. Countdown, 1-8 (2018).
Musuva, R. M. et al. Community knowledge, attitudes and practices on schistosomiasis in north-western Tanzania. Acta Trop.128, 391–398 (2014).
Mwanga, J. R. & Lwambo, N. J. Pre- and post-intervention perceptions and water contact behaviour related to schistosomiasis in north-western Tanzania. Acta Trop. 128, 391–398 (2013).
Kosinski, K. C. et al. Effective control of Schistosoma haematobium infection in a Ghanaian community following installation of a water recreation area. PLoS Negl. Trop. Dis. 6, el 709 (2012).
Anderson, R. M. et al. Responding to the cuts in UK AID to neglected tropical diseases control programmes in Africa. Trans. R. Soc. Trop. Med. Hyg. 117(3), 237–239 (2023).
Shrestha, P. et al. Public engagement and crowdfunding in health research: a practical guide. Geneva: World Health Organization, 30Pp. https://apo.who.int/publications/i/item/9789240039087 (2021).
Shirin, M. et al. The role of community participation for sustainable integrated neglected tropical diseases and water, sanitation and hygiene intervention programs: A pilot project in Tanzania. Soc. Sci. Med. 202, 28–37 (2018).
World Health Organization. Accelerating work to overcome the global impact of neglected tropical diseases - a roadmap for implementation. Geneva: World Health Organization, 42 Pp. (2012).
World Health Organization. Investing to overcome the global impact of neglected tropical diseases: third WHO report on neglected diseases 2015. World Health Organization, Geneva, Switzerland, pp.1–90. http://www.who.int/mediacentre/fact- sheets/fs366/en/ . (2015).
National Population Commission. Population of Anambra State. www.npc.org.ng (2006).
Rao, K. V. Biostatistics: A manual of Statistical Methods for use in Health, Nutrition and Anthropology. (2nd ed.), 754 Pp. (Rajkamal Electronic Press, 2007).
Naing, L., Winn, T. & Rusli, B. N. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 1, 9–14 (2006).
Aribodor, D. N. et al. Analysis of schistosomiasis and soil-transmitted helminths mixed infections among pupils in Enugu State, Nigeria: Implications for control. Infect. Dis. Health 24(2), 98–106 (2019).
Naing, N. N. Determination of Sample Size. Malaysia J Med Sci. 2003; 10(2):84–86.
World Health Organization. Manual of Basic Techniques for a Health Laboratory. (2nd ed.) 384Pp. (World Health Organization, 2003).
Savioli, L., Gabrielli, A. F., Montresor, A., Chitsulo, L. & Engels, D. Schistosomiasis control in Africa: 8 years after World Health Assembly Resolution 54.19. J. Parasitol. 136, 1677–1681(2009).
Martin, L. K. & Beaver, P. C. Evaluation of Kato Katz thick smear technique for quantitative diagnosis of helminth infections. Am. J. Trop. Med. Hyg. 77, 382–391(1968).
Nawalinski, T. A., Schad, G. A. & Choudhury, A. B. Hookworm burdens and faecal egg counts: an analysis of the biological basis of variation. Trans. R. Soc. Trop. Med. Hyg. 79, 812–825 (1978).
Scherrer, A. U. et al. Sequential analysis of helminth egg output in human stool samples following albendazole and praziquantel administration. Acta Trop. 109(3), 226–231(2009).
World Health Organization. Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. Geneva: World Health Organization; 2017; p. 87.
Levecke, B. et al. The optimal timing of post-treatment sampling for the assessment of anthelminthic drug efficacy against Ascaris infections in humans. Int. J. Parasitol. Drugs Drug Resist. 8(1), 67–69 (2018).
Aboagye, I. F. & Addison, Y. A. A. Praziquantel efficacy, urinary and intestinal schistosomiasis reinfection - a systematic review. Pathog. Glob. Health 117(7), 623–630 (2023).
Aribodor, O. B. et al. Urinary schistosomiasis and primary evidence of female genital schistosomiasis among pupils in Nsugbe community, Anambra State, Nigeria. N. J. Parasitol. 42(2), 394–402 (2021).
Dorkenoo, A. M. et al. Progress from morbidity control to elimination as a public health problem of schistosomiasis and the status of soil-transmitted helminth infection in Togo: a second impact assessment after ten rounds of mass drug administration. Parasit Vectors 16(1), 314 (2023).
Leta, G. T. et al. National mapping of soil-transmitted helminth and schistosome infections in Ethiopia. Parasit Vectors 13(1), 437 (2020).
Nikolay, B. et al. Understanding heterogeneity in the impact of national neglected tropical disease control programmes: evidence from school-based surveys in Kenya. PLoS Negl Trop Dis. 9(9), e0004108 1-20 (2015)
Winkelman, M. Cultural awareness, sensitivity and competency. 217Pp. (Eddie Bower’s Publishing, 2009).
Odongo-Aginya, E., Taylor, M. G., Sturrock, R. F., Chimbari, M. J. & Fenwick, A. Evidence that cercariae of Schistosoma mansoni do not penetrate the skin of humans wearing sandals. Ann. Trop. Med. Parasitol. 91(4), 437–439 (1997).
Salawu, S. A., Asaolu, S. O. & Sowemimo, O. A. Co-infections with Schistosoma haematobium and soil-transmitted helminths among school-aged children in Saki, Oyo State, Nigeria. J. Public Health Epidemiol. 6(12), 417–423 (2014).
Aribodor, O. B., Ekwunife, C. A., Sam-Wobo, S. O., & Aribodor, D. N. Risk factors and socio-demographic determinants of intestinal helminthiasis among children in schools that implemented the homegrown school feeding program in Ekwulobia, Anambra State, Southeast Nigeria. Int. J. Trans. Med. Res. Pub. Health 2(1), 1–10 (2018).
Hanson, C. et al. Integrated implementation of programs targeting neglected tropical diseases through preventive chemotherapy: identifying best practices to roll out programs at national scale. Am. J. Trop. Med. Hyg. 86(3), 508–513 (2012).
Matthys, B. et al. History of helminth control in sub-Saharan Africa: a review on the integration of preventive chemotherapy with other control strategies. Infect. Dis. Poverty 9, 19–23 (2011).
Ohiolei, J. A., Isaac, C. & Omorodion, O. A. A review of soil-transmitted helminthiasis in Nigeria. Asian Pac. J. Trop. Dis. 7(12), 841–848 (2017).
Kabatereine et al. Progress towards countrywide control of schistosomiasis and soil-transmitted helminthiasis in Uganda. Trans. R. Soc. Trop. Med. Hyg. 100(3), 208–215 (2006).
Karunamoorthi, K., Almalki, M. J. & Ghailan, K. Y. Schistosomiasis, a neglected tropical disease of poverty: a call for intersectoral mitigation strategies for better health. J. Health Res. Rev. 5, 1–12 (2018).
Acknowledgements
The study appreciates the support of the Traditional Rulers, Community Leaders, Parents/Guardians, Head Teachers, Staff and pupils for their cooperation. We also acknowledge the technical support provided by the technologists during the field studies.
Funding
This study was funded by Joint World Health Organization (WHO)-Regional Office for Africa (AFRO) /TDR/EDCTP Small Grants Scheme for implementation research on infectious diseases of poverty and Needs Assessment Fund for Academic Staff Training. The contents of the study are sole responsibility of the authors and do not in any way represent the views of the funders. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
O.B.A. developed the concept. O.B.A., S.S., C. O. and E.O. designed the study methodology, including data collection methods. O.B.A. and C.O. developed essential software tools for data analysis. O.B.A., S. S., and C. O. Conducted literature searches, analysis, and reporting.O.B.A. C. O., S.S. and A.B. were responsible for the Project Administration, and Coordination. The initial manuscript was drafted by O.B.A. All the authors have read and approved the final submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Ethical clearance and approval was obtained from the Ethical Committee of Anambra State owned Chukwuemeka Odumegwu Ojukwu University Teaching Hospital in Awka, the State Capital with approval letter number COOUTH/AC/Vol. XI/00013. Permission for access to the schools in order to carry out the study was obtained from the Anambra State’s Commissioner of Basic Education with approval letter number MOE/SCHD/1673/Vol.1/17, Universal Basic Education Board (ASUBEB) and Educational Secretary in charge of primary schools in the study area. Parents, guardians, teachers and other key stakeholders including traditional ruler and town union leaders were sensitized during advocacy visits to the community selected for the study. Informed consent (written and verbal) was obtained from all subjects and/or their legal guardian(s). To ensure the willingness of pupils to participate, assent was obtained from them at every step by providing age-appropriate information, and addressing questions and concerns, all while maintaining open communication throughout the study. There was an emphasis that participation was voluntary and participants are free to withdraw from the study at any time without further obligations. Permission to take photographs was sought and obtained from the participants and parents. Written consent was obtained, explicitly acknowledging that the images might be utilized to illustrate risk factors in scientific publications. All infected pupils included in the study underwent treatment with Praziquantel and Albendazole.
Consent for publication
All authors of this manuscript have given their approval for its publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aribodor, O.B., Okaka, C., Sam-Wobo, S. et al. Factors contributing to helminth prevalence after repeated mass administration of medicines in Anambra State, Nigeria. Sci Rep 15, 3721 (2025). https://doi.org/10.1038/s41598-024-83063-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83063-6