Abstract
Treatment of postpneumonectomy empyema remains challenging, especially in presence of bronchopleural fistula. We analysed clinical outcome data of patients with and without bronchopleural fistula undergoing an accelerated empyema treatment concept. From November 2005 to July 2020, all patients with postpneumonectomy empyema were included. Therapy consisted of repeated surgical debridement of the pleural cavity, evaluation for loco-regional flap, negative pressure wound therapy and definitive closure after installation an antibiotic solution in the cavity. Primary endpoint was perioperative mortality, focusing on comparison between patients with (= group A) and without bronchopleural fistula (= group B). Secondary endpoints were empyema resolution/recurrence and length of stay. 58 patients underwent the treatment concept: 19 (32.8%) with bronchopleural fistula. Patients’ mean age was 62.7 ± 11.5 years. Nine patients (15.5%) deceased within 30 days: 3 (15.8%) in group A, 6 (15.4%) in group B. 90-days mortality tends to be lower in group A (n = 3 (15.8%)) compared to group B (n = 11 (28.2%)) (p = 0.078). Incidence of postoperative complication was 63.2% (n = 12) in group A compared to 56.4% (n = 22) in group B (p = 0.316). Postpneumonectomy empyema resolution was 100% in the cohort. 3 patients (15.8%) in the group A and 3 (7.7%) in group B (p = 0.175) developed an empyema-recurrence, successfully managed with the treatment concept again. Mean hospital length of stay was lower in group A (24.6 ± 9.5 days vs 27.2 ± 24.3 days in group B; p = 0.329). With our accelerated treatment concept, postpneumonectomy empyema with bronchopleural fistula could effectively and safely be treated while maintaining integrity of the chest wall.
Clinical Registration Number: KEK-ZH-NR: 2021-01114.
Similar content being viewed by others
Introduction
Postpneumonectomy empyema (PPE) remains a serious complication with an incidence of 2–16% and mortality of around 20% according to current literature1,2,3,4. Prognosis mainly depends on the underlying disease (malignant versus non-malignant). In addition, presence of a bronchopleural-fistula (BPF) has an important impact, due to spillage to the remaining lung, showing an increase of mortality up to 71%5,6. Furthermore, severe infections frequently lead to a septic shock. In often immunocompromised/-suppressed cancer patients controlling this life-threatening situation is even more demanding7.
Main problem in PPE is the combination of a large sized infected pleural cavity and poorly vascularized infected tissue, making conservative treatment with antibiotics solely inefficient.
Key aspect in management of any infected cavity is surgical debridement (“ubi pus ibi evacua”). In PPE, surgical strategy mainly depends on the presence of a BPF. A wide spectrum of surgical approaches have been described ranging from single-staged lavage by video-assisted thoracoscopy (VATS)8, open debridement followed by continuous antibiotic irrigation9, open window thoracostomy (OWT)10, Clagett thoracostomy11 to thoracoplastic procedures12. In countries without high levels of tuberculosis or other infectious diseases, no technique has set itself as a gold standard and consensus in the best approach for management of PPE with or without BPF is still lacking.
In 1995, our team introduced an accelerated treatment concept for PPE, consisting in repeated scheduled mechanical debridement and packing of the pleural cavity with povidone-iodine-soaked towels under suction by chest drain (corresponding to a negative pressure wound therapy) and temporarily closure of the chest. When there is pathogen growth in the microbiologically analysed debridement samples and the pleural cavity is macroscopically clean, it is filled with an antibiotic solution and definitive closure follows3 (Fig. 1). We demonstrated that this approach is effective for PPE. The aim of this study is to update our report of short and long-term outcome of the Zurich approach for PPE, with focus on comparison between patients with BPF and without BPF.
Patients and methods
Study design
This is a retrospective, monocentric cohort study, from November 2005 until July 2020, which included all patients developing PPE and undergoing the Zurich treatment concept.
Ethical consideration
The study protocol was submitted to an independent Ethics Committee for approval (KEK-ZH-NR: 2021-01114). The study was carried out in accordance with principles enunciated in the current version of the Declaration of Helsinki and the guidelines of Good Clinical Practice (GCP). Premise was existing general consent and/or biobank appendix, which in case of death was given by the contacted relatives.
Study endpoints
Primary endpoint was:
-
perioperative mortality (in-hospital mortality, 30- and 90- days mortality) in patients with BPF (group A) compared to patients without BPF (group B).
Secondary endpoints were:
-
postoperative morbidity (rated using the Clavien-Dindo classification13) in patients with BPF (group A) compared to patients without BPF (group B);
-
evaluation of overall length of stay in group A patients with BPF compared to patients without BPF group B;
-
empyema resolution and recurrence;
-
5-year survival in patients with BPF group A compared to patients without BPF group B.
Data collection
For baseline characteristics of our cohort, we collected data on gender, age, cardio-vascular (i.e., coronary heart disease) or pulmonary (i.e., COPD, bronchial asthma) comorbidities, aetiology of pneumonectomy and in case of underlying malignancy, prior treatment. Peri- and postoperative data on surgery, morbidity, length of hospital stay, and survival were collected.
Diagnostic procedure
Diagnosis of PPE was based on the following arguments, from the ERS/ESTS guidelines on the management of pleural infection in adults14:
-
combination of clinical signs, i.e.:
-
cough (with or without sputum)
-
fever
-
reduced general health condition
-
dyspnoea;
-
-
radiologic features e.g., contrast enhancement of the pleura on thorax computed tomographic scan;
-
pleural fluid analysis: macroscopic appearance (pus), pH < 7.2 and/or presence of bacteria in culture or direct examination.
Diagnostic work up and surgical management
Contrast enhanced chest computed tomography was performed upon admission in every patient. The diagnostic workup was completed by bronchoscopy to search for a BPF, and aspiration samples were sent for microbiology analysis. In the presence of BPF, the defect size was estimated in relation to the instruments.
Broad-spectrum antimicrobial therapy was administered upon admission and adapted according to microbiological finding.
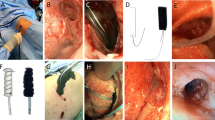
If patients’ clinical performance-status was compatible with a surgical intervention under general anaesthesia, the first surgical debridement was carried soon after PPE diagnosis. The surgical protocol has been previously described3,15 (Fig. 1). Under general anaesthesia and with a double-lumen endotracheal tube, radical debridement of the pleural cavity, consisting of curettage of all necrotic and fibrinous infected tissue, was followed by irrigation with diluted povidone-iodine solution (1:10). If needed, size of BPF was measured with a sterile measuring tape. In case of an early small BPF in an acute PPE (occurred within 90 days after pneumonectomy), direct closure was attempted with interrupted sutures (3–0 or 4–0 PDS sutures) reinforced by a muscle- or omentum-flap. Choice of flap was done by patients’ physical constitution.
Larger late-occurring BPF in chronic PPE (more than 90 days after initial pneumonectomy) were closed by a parachute technique, preferentially using the omentum as a patch to cover the defect.
The pleural cavity was then packed with povidone-iodine-soaked dressings (diluted 1:10) at the end of the first intervention. Negative pressure (-75 mmHg) wound therapy was applied by placing a chest tube between the towels and the chest wall.
Thoracotomy was temporarily closed, and the patient was extubated in the OR The antiseptic packing was changed every 48 to 72 h under general anaesthesia until the chest cavity was deemed macroscopically clean (presence of vascularized granulation tissue) and there was no pathogen growth in the microbiologically analysed debridement samples. Before definitive closure of the chest wall, the pleural space was obliterated with an antibiotic solution adapted to the microbiologic report.
Statistical analysis
Continuous variables are reported as mean with standard deviation for normally distributed data and median with 95% confidence interval (CI) for non-normal distributed data. Categorial variables are shown as frequencies and percentages: independent-sample T-test was performed to compare the two groups. 5-year survival rate was evaluated by Kaplan–Meier curves. Comparison between group A and group B was made by log rank-test. Evaluation of risk factors was performed by COX-regression. Statistical analysis was performed using IBM SPSS Statistics Version 29 (SSPS Inc, Chicago, IL).
Results
Baseline profiles
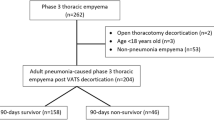
From November 2005 until July 2020, a total of 261 pneumonectomies were performed in our department. Among these patients, 52 patients developed PPE resulting in a PPE-incidence of 19.9%. Six patients underwent pneumonectomy (PE) in an external institution and were referred for further PPE management.
Baseline clinical characteristics of all 58 patients included are presented in Table 1: 76% were male and mean age at time of surgery was 62.7 ± 11.5 years. 63.8% (n = 38) of PPE were classified as acute PPE. BPF was present in 32.8% (n = 19) of the cases, and 16 of those BPF were right sided (84.2%).
Most common indication for pneumonectomy in both groups was non-small-cell lung cancer (NSCLC) (total n = 24), followed by pleural mesothelioma (PM) (total n = 22). Of the 6 patients with benign disease, 3 were operated for destroyed lung due to an infection (tuberculosis n = 2, aspergillosis n = 1), one recurrent major haemoptysis, one for myofibroblastic tumour (n = 1) and one for persistent pneumonia.
Thirty-three patients (56.9%) were operated on basis of a diagnosis of PPE after assessment of the clinical presentation, blood sample and radiographic imaging, without prior puncture or chest tube drainage.
Surgical details
Peri- and post-operative characteristics are detailed in Table 2. Different flaps were used to cover the BPF: In most cases (n = 13, 68.4%) an omentum-flap was used. A muscle flap from M. latissimus dorsi or M. serratus anterior were each used in one case. A combination of these muscle flaps with omentum was used again in one case. In the remaining 3 patients with BPF (15.7%), the lesion was sutured over directly.
In none of the patients with underlying malignancy could a tumor recurrence be detected as cause of BPF.
All surgeries could be performed under general anaesthesia. In group A, more scheduled revision-interventions after the first rethoracotomy tend to be necessary compared to group B (4.2 ± 1.8 vs. 2.4 ± 1.7, respectively; p = 0.217).
Primary endpoint
Within 30 days, 9 patients (15.5%) deceased: 3 (15.8%) in group A and 6 (15.4%) in group B (p = 0.938). Death within 90-days after surgery was also not significantly different in group A (n = 3 (15.8%)) compared to group B (n = 11 (28.2%)) (p = 0.078).
An overview of cause of death is presented in Table 3. Cause of 30-day mortality was as follow: in 3 cases therapy was stopped at patients’ explicit wish, despite improvement of health status (underlying carcinoma n = 2, underlying invasive pulmonal aspergillosis n = 1). The remaining 6 patients died of multiple system organ failure (preoperative sepsis n = 2), abdominal surgery due to colon ileus (n = 1), acute pulmonary embolism (n = 1), tumour-progression (PM, n = 1) and non-manageable bleeding out of the superior vena cava (n = 1).
Cause of death for 90-days mortality was tumour-progression (n = 4) and a systemic infection after amputation of the lower limp due to peripheral artery disease (n = 1). Main reason for death in the further follow-up (n = 27) was tumour-progression (group A n = 8; group B n = 12).
Secondary endpoints
Outcomes were similar in the two groups (shown in Table 2). Sixty-four complications occurred in 34 patients, resulting in an incidence of postoperative complications of 56.8%: 63.2% (n = 12) in the group A and 56.4% (n = 22) in group B (p = 0.316). All complications were classified according to the Clavien-Dindo classification: Grade I 19 (26.0%), grade II 22 (30.1%), grade IIIa/IIIb 7 (9.6%) and grade IVa/IVb 16 (21.9%) (shown in Table 4).
Mean hospital length of stay was lower in the group A compared to group B (24.6 ± 9.5 days vs 27.2 ± 24.3 days; p = 0.329).
PPE was resolved in 100% (n = 58) of the cases. There were 6 patients (10.3%) with a recurrence of PPE: 3 (15.8%) in group A and 3 (7.7%) in group B (p = 0.175). Recurrence-free interval was 61.3 ± 51.6 days in group A and 35.3 ± 17.0 days in group B (p = 0.227). All recurrences were managed again with the accelerated treatment concept: 3.0 ± 0.0 scheduled re-surgeries were necessary in group A, 4.3 ± 3.51 in group B, respectively (p = 0.273).
5-year survival was 33.4% (n = 5) in group A versus 29.4% (n = 6) in group B (p = 0.064) (Fig. 2).
Microbiological analysis
We were able to identify pathogenic germ in 46 patients (79.3%). 60.9% (n = 28) out of these cohort, showed mono-microbial infection. Most common pathogen was coagulase-negative Staphylococcus (n = 12, 20.7%) followed by streptococcus viridans (n = 9, 15.5%). One or more fungi were detected in 11 patients: Candida species (n = 12), Aspergillus species (n = 2). In the remaining 12 patients, no pathogen could be identified.
Discussion
By analysing outcomes of 58 patients with PPE undergoing the Zurich accelerated treatment concept, between November 2005 and July 2020, we found no difference in early and late post-operative outcomes when comparing patients with BPF and patients without BPF.
The postoperative morbidity rate of 58.6% was comparable to other techniques like Clagett procedure with a reported postoperative complication rate of 55%2, without mutilating consequences for the patient. There was no significant difference between both groups in our study (63.2% vs. 56.4%, respectively; p = 0.316). The high rate of morbidities classified as Clavien-Dindo IV was mainly due to single organ failure: respiratory insufficiency (n = 5) and renal insufficiency (n = 5). In 3 cases septic shock occurred with need to transfer to the ICU accordingly. In 5 of the 7 morbidities classified as Clavien-Dindo III abdominal surgery was necessary due to colon ileus (n = 1), abdominal compartment syndrome (n = 1), acute pancreatitis (n = 1), acute cholecystitis (n = 1) and incarceration of the small bowl (n = 1). In none of these 5 cases BPF was covered with an omentum flap, so there was no direct relation to out accelerated treatment concept.
With our accelerated treatment concept, we achieved a PPE resolution of 100%, which is higher than other techniques like Clagett procedure with a resolution rate of 75–88%16. PPE was also resolved before death of the 9 patients, who deceased within 30 days after surgery. PPE recurrence occurred in 3 cases (15.8%) of the BPF group and in 3 cases (7.7%) of the other group (p = 0.175). In the BPF group, PPE relapse was due to recurrent BPF in 2 patients (10.5%), corresponding to a lower rate than described in the literature with 18%2. The third case was due to tumour progress (underlying PM). In group B, 1 patient developed an esophago-pleural fistula, which was treated with an omentum-flap plastic. Aetiology for PPE recurrence in the other 2 cases remains unknown.
Perioperative mortality in patients with PPE and BPF was lower than described in literature, ranging from 25 to 71%6,7,8,9. In our study, death within 90-days after surgery was not significantly different between group A and group B (n = 3 (15.8%) vs. n = 11 (28.2%) respectively; p = 0.078). The higher 90-day mortality in group B mainly is due to systemic tumour progression (n = 4) of PM. Similarly, 5-year survival probability was not significantly different in group A compared to group B (n = 5 (33.4%) vs. n = 6 (29.4%); p = 0.064). Not surprisingly, main reason for mortality in both groups was systemic tumour progression (total n = 20). Due to high inhomogeneity of the cohort and small cohort size, no definitive conclusion can be drawn from these results. But of all deaths, only one case had a possible relationship to the treatment concept: In the seventh scheduled re-intervention, an uncontrollable bleeding of the superior vena cava occurred. Due to underlying PM and already septic condition, decision was made against a vascular reconstruction.
Thus, with our accelerated treatment concept, BPF could be managed correctly without an increased mortality compared to patients without BPF. Presence of BPF had no significant impact on short term survival and was therefore not a risk factor as described in other reports17.
Incidence of BPF in our cohort was 32.8%. BPF was more frequent after right-sided pneumonectomy (56.9%). This has already been described previously. The right bronchus is most commonly supplied only by one bronchial artery, and not by two bronchial arteries like the left side which may lead to a higher incidence of BPF18. Other probable risk factors are the larger diameter of the right main bronchus as well as lack of mediastinal coverage compared to the left side19. Furthermore, 50.2% had neoadjuvant therapy for underlying malignancy, which is another known risk factor20,21. Possibly, the increase in neoadjuvant treatment concepts for NSCLC and PM is also the reason for the rate of 19.9% PPE after pneumonectomy performed in our department in the study period. In none of the patients with underlying malignancy could a tumor recurrence be detected as cause of BPF.
For BPF, reinforcement of the bronchial stump was mainly performed by omentum flap (n = 13, 68.4%). Due to its well-documented ability of neovascularization which favours healing in septic environment20, it’s the most used technique in our department: moreover, it doesn’t affect quality of life like more radical options represented by the Clagett intervention. But depending on patients’ constitution and history (i.e., prior visceral surgery), thickness of the omentum may not be sufficient to cover BPF. Therefore, they need to be combined with a muscle flap (n = 2), or the muscle flap is used alone (n = 4). In 3 patients, BPF could be sutured over directly due to small lesion of the bronchial stump.
In PPE patients, hilum sometime appears fuse. However, the hilum could be prepared in such a way that the flap could be sutured over the defect using the parachute technique. Even in complex cases, we experienced no problems regarding this step during surgery.
Despite patients being classified as high risk due to PPE and comorbidities, all operations were performed without complications under general anaesthesia. Numbers of scheduled re-interventions (4.2 vs. 2.4; p = < 0.001) and consecutive duration until definitive chest wall closure was higher in the BPF-group (12.1 vs. 7.1 days; p = 0.002). As the microbiological analyses sometimes require more than 72 h, scheduled re-interventions were also carried out before the definitive microbiological analyses were received. In some cases, the definitive reports showed no further microbial growth, meaning that a re-operation could have been possibly avoided.
Overall, delay until definitive chest wall closure was way shorter than in OWT procedures with a delay reported ranging from 45 days to 36 months2,12,22. Furthermore, with our technique the integrity of the chest wall can be preserved without aesthetic impairment for the patients.
Hospital stay was significantly shorter in the BPF group (24.6 days vs. 27.2 days; p = 0.329). This seems surprising knowing the increased morbidity associated with BPF23. But it is explainable by the fact that in 4 patients from group B, PPE occurred during hospitalisation for pneumonectomy, resulting in a hospital stay from 51 to 132 days.
Overall, hospital stay was shorter than what is reported in other studies including patients with BPF9,10,24 and, especially in case of Clagett procedure (30 days)2 or thoracomyoplasty (34 days)25. This is an additional argument to justify that our protocol seems adapted for management of PPE, especially in presence of BPF. Despite several interventions within a short period, the accelerate treatment concept is well tolerated by patients. This is because of the short duration of the repetitive intervention, early extubation, mobilisation and intensive physiotherapy between the surgeries3.
Main limits of this study are represented by its retrospective design. Remains also the question of overtreatment in the absence of BPF, where the vacuum assisted closure therapy maybe is not mandatory. In addition, the accelerated treatment concept could be performed via a thoracoscopic approach in selected cases. A thoracoscopic management of PPE without BPF has already shown as an effective method8,26. Different authors already have shown that cleaning of the infected pleural cavity is possible in a 1-step intervention, as far as no major BPF (> 3 mm) is present3,8,27,28. Moreover, the accelerated treatment has also been proposed as empyema prevention in high-risk procedures like salvage-pneumonectomy or completion pneumonectomy in a septic environment29. Only prospective trials could answer those questions. But it is difficult to set up as number of pneumonectomies have been decreasing in Switzerland, and worldwide, due to epidemiological changes in lung cancer.
Conclusion
PPE, especially combined with BPF, remains a challenge with necessity of individually tailored but aggressive treatment to prevent lethal outcome. Our accelerated treatment concept has shown to be particularly adapted for management of acute and chronic PPE with BPF. Moreover, it prevents patients from body deforming interventions like OWT in the Clagett procedure. Further investigations are needed to better define indications of this protocol, especially in a prevention concept.
Data availability
The data underlying this article were accessed from the University Hospital of Zurich. The derived data generated in this research will be shared on reasonable request to the corresponding Author.
Abbreviations
- BPF:
-
Bronchopleural fistula
- NSCLC:
-
Non-small-cell lung cancer
- PM:
-
Pleural mesothelioma
- OWT:
-
Open window thoracostomy
- PPE:
-
Postpneumonectomy empyema
- VATS:
-
Video-assisted thoracoscopy
References
Deschamps, C. et al. Empyema and bronchopleural fistula after pneumonectomy: Factors affecting incidence. Ann. Thorac. Surg. 72(1), 243–247 (2001) (discussion 248).
Zaheer, S. et al. Postpneumonectomy empyema: Results after the Clagett procedure. Ann. Thorac. Surg. 82(1), 279–286 (2006) (discussion 286-7).
Schneiter, D. et al. Accelerated treatment of postpneumonectomy empyema: A binational long-term study. J. Thorac. Cardiovasc. Surg. 136(1), 179–185 (2008).
Misthos, P. et al. Surgical management of late postpneumonectomy bronchopleural fistula: The transsternal, transpericardial route. Respiration 73(4), 525–528 (2006).
Hicham, H. et al. Postpneumonectomy empyema: Risk factors, prevention, diagnosis, and management. Asian Cardiovasc. Thorac. Ann. 28(2), 89–96 (2020).
Sirbu, H. et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: Incidence, risk factors, and management. Ann. Thorac. Cardiovasc. Surg. 7(6), 330–336 (2001).
Dagher, G. A. Are patients with cancer with sepsis and bacteraemia at a higher risk of mortality? A retrospective chart review of patients presenting to a tertiary care centre in Lebanon. BMJ Open 7(3) (2017).
Gossot, D. et al. Thoracoscopic management of postpneumonectomy empyema. Ann. Thorac. Surg. 78(1), 273–276 (2004).
Ng, T. et al. Treatment of postpneumonectomy empyema with debridement followed by continuous antibiotic irrigation. J. Am. Coll. Surg. 206(6), 1178–1183 (2008).
Goldstraw, P. Treatment of postpneumonectomy empyema: The case for fenestration. Thorax 34(6), 740–745 (1979).
Clagett, O. T. & Geraci, J. E. A procedure for the management of postpneumonectomy empyema. J. Thorac. Cardiovasc. Surg. 45, 141–145 (1963).
Regnard, J. F. et al. Open window thoracostomy followed by intrathoracic flap transposition in the treatment of empyema complicating pulmonary resection. J. Thorac. Cardiovasc. Surg. 120(2), 270–275 (2000).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240(2), 205–213 (2004).
Bedawi, E. O., Ricciardi, S., Hassan, M., et al, ERS/ESTS statement on the management of pleural infection in adults. Eur. Respir. J. 61(2) (2022).
Schneiter, D. et al. Accelerated treatment for early and late postpneumonectomy empyema. Ann. Thorac. Surg. 72(5), 1668–1672 (2001).
Stafford, E. G. & Clagett, O. T. Postpneumonectomy emphema. Neomycin instillation and definitive closure. J. Thorac. Cardiovasc. Surg. 63(5), 771–775 (1972).
Darling, G. E. Risk of a right pneumonectomy: Role of bronchopleural fistula. Ann. Thorac. Surg. (2005).
Zanotti, G. & Mitchell, J. D. Bronchopleural fistula and empyema after anatomic lung resection. Thorac. Surg. Clin. 25(4), 421–427 (2015).
Wright, C. D. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: Incidence, risk factors, and management. J. Thorac. Cardiovasc. Surg. 112(5), 1367–1371 (1996).
Li, S. et al. Neoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: A systematic meta-analysis of 14 912 patients. Jpn. J. Clin. Oncol. 46(6), 534–546 (2023).
Hu, X.-F. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann. Thorac. Surg. 96(2), 419–424 (2013).
Fukui, T. et al. Simple chest closure of open window thoracostomy for postpneumonectomy empyema: A case report. Surg. Case Rep. 5(1), 1–5 (2019).
Miller, D. L. Completion pneumonectomy: Factors affecting operative mortality and cardiopulmonary morbidity. Ann. Thorac. Surg. 74(3), 876–883 (2002).
Perentes, J. Y. et al. Vacuum-assisted closure device for the management of infected postpneumonectomy chest cavities. J. Thorac. Cardiovasc. Surg. 149(3), 745–750 (2015).
Seify, H. et al. Single-stage muscle flap reconstruction of the postpneumonectomy empyema space: The Emory experience. Plast. Reconstr. Surg. 120(7), 1886–1891 (2007).
Scarci, M. et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur. J. Cardiothorac. Surg. 48(5), 642–653 (2015).
Bagan, P. et al. Postpneumonectomy empyema treated with a combination of antibiotic irrigation followed by videothoracoscopic debridement. J. Thorac. Cardiovasc. Surg. 132(3), 708–710 (2006).
Hollaus, P. H. et al. Videothoracoscopic debridement of the postpneumonectomy space in empyema. Eur. J. Cardiothorac. Surg. 16(3), 283–286 (1999).
Schneiter, D. et al. Prevention of recurrent empyema after pneumonectomy for chronic infection. Eur. J. Cardiothorac. Surg. 21(4), 644–648 (2002).
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
G.M.: Conceptualisation, Data curation, Formal analysis, Investigation, Methodology, Visualisation, Writing—original draft, H.E. Methodology, Visualisation, Writing – review & editing S.H.: Writing – review & editing C.C.: Writing – review & editing O.L.: Writing – review & editing I.O.: Supervision, Writing – review & editing D.S.: Conceptualisation, Project administration, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
Isabelle Opitz: Roche (Institutional Grant and Speakers Bureau), AstraZeneca (Advisory Board and Speakers Bureau), MSD (Advisory Board), BMS (Advisory Board), Medtronic (Institutional Grant), Intuitive (Proctorship). The other authors have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Monsch, GM., Etienne, H., Hillinger, S. et al. Accelerated treatment concept in postpneumonectomy empyema with bronchopleural fistula. Sci Rep 14, 31837 (2024). https://doi.org/10.1038/s41598-024-83334-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83334-2