Abstract
In order to provide some references for vein approach selection in adrenal vein sampling (AVS), this retrospective study analyzed 325 cases of primary aldosteronism (PA) patients who underwent AVS via the upper extremity vein approach, comparing the differences in complications and visual analogue scale (VAS) scores through median cubital vein (MCV), basilic vein (BV), and cephalic vein (CV). The results indicated no significant difference in the incidence of venous spasm (right MCV vs. right BV vs. left MCV vs. left BV: 4.2 vs. 5.9 vs. 5.3 vs. 0.0%, p>0.05) and VAS scores between AVS performed using the MCV and the BV. However, the right CV access was associated with a relatively higher incidence of venous spasm (right CV: 20% vs. right MCV: 4.2%, p<0.05) and higher rate of thrombosis formation (right CV: 5.7% vs. right MCV: 0.0%, p<0.05) than right MCV, accompanied by more severe pain. The study suggests that bilateral MCV and BV are both viable options vein access for AVS. When neither the MCV nor the BV on one side is accessible, it may be more prudent to opt for the MCV or BV on the contralateral side rather than the CV.
Similar content being viewed by others
Introduction
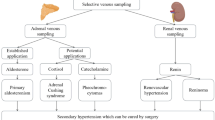
Primary aldosteronism (PA) is one of the most common types of secondary hypertension, accounting for about 4–7% of all newly diagnosed hypertensive patients1. Among them, idiopathic adrenal hyperplasia and aldosterone-producing adenoma account for more than 95% of PA cases, and there are differences in the choice of treatment strategies according to the subtype of PA. The preferred treatment for patients with unilateral PA is adrenalectomy, while bilateral lesions are generally treated with aldosterone receptor antagonists2. However, CT or MRI might lead to misdiagnosis and mistreatment, thus, adrenal vein sampling (AVS) is considered as the gold standard for subtype diagnosis of PA3,4.
In previously studies, most of the AVS procedures were performed via the femoral vein5,6. Recently, AVS via the upper extremity vein approach has been rapidly promoted, which increased the success rate, shortened the learning curve, and reduced the incidence of complications7,8. According to these studies, the right median cubital vein (MCV) is generally used as the preferred puncture vein, while the basilic vein (BV) and cephalic vein (CV) can be alternatives if the MCV is absent or unpuncturable7.
However, due to the differences in anatomical characteristics of the antecubital vessels, it is suggested that operations through different vein access might bring different patient experiences and clinical outcomes in peripherally inserted central catheter (PICC) practices9. Thus, the present study intends to review the data of 325 cases of AVS via the upper extremity vein approach in our center, comparing the differences in complications and pain scores between AVS procedure performed through different vein access, to provide some references for the vein access selection in AVS.
Methods
Data collection
The present study was conducted in accordance with the tenets of the Declaration of Helsinki and approved by Institutional review board of The Second Affiliated Hospital of Chongqing Medical University. This study was also conducted in accordance with the local legislation and institutional requirements. Given the retrospective design of this research, the need for informed consent was waived by our hospital institutional review board. Patients diagnosed with PA according to current guidelines and undergoing AVS via the upper extremity vein route from September 2019 to July 2024 at our center were included10. Surgical records, nursing records, and digital subtraction angiography (DSA) images were meticulously reviewed.
Any adverse events related to venous access during the AVS procedure were documented, including venous spasm (defined as the inability to advance the catheter even despite the administration of a vasodilator), bleeding incidents, and thrombosis. Moreover, self-reported patient pain perception was quantified using the visual analogue scale (VAS). All data were obtained from medical records in a fully anonymized and de-identified manner prior to analysis. None of the researchers had access to identifying information. Personal identification numbers were scrambled to protect patients’ privacy.
AVS procedure
All AVS procedures were performed between 8:00 am and 12:00 am. After the patient rested in a supine position for 2 h, they were transferred to the catheterization laboratory using a gurney. After disinfection and draping of both upper extremities, the right MCV was selected as the primary venous access for puncture. If the right MCV was not exposed or difficult to puncture, the right BV, right CV, left MCV, left BV, and left CV was selected as alternative puncture vessels, sequentially. All nurses responsible for venous puncture had PICC qualifications.
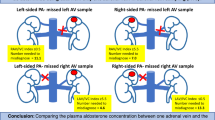
Following successful puncture, a 5 F sheath was inserted, and 2000 IU to 3000 IU of heparin, adjusted according to body weight, was administered. A 230 cm guidewire was advanced to the inferior vena cava. Subsequently, a 5 F MP (Cook Medical) and a 5 F TIG catheter (Terumo Corporation) was introduced along the guidewire for the cannulation of the right and left adrenal veins, respectively. Six milliliters of samples were collected from bilateral adrenal veins and inferior vena cava respectively to measure aldosterone and cortisol level. All of the AVS procedures were performed by a single operator (Dr. J.Q.) without ACTH stimulation. A successful AVS procedure was defined by a cortisol concentration in the sampling vessel that exceeded twice the cortisol concentration in the inferior vena cava.
In case of vascular spasm during the procedure that could not be relieved after he administration of drugs (e.g., sodium nitroprusside), and inability to advance the catheter, or if the patient experienced intolerable pain, the AVS procedure was continued on the contralateral upper extremity. The sequence for vein access selection was as follow: MCV, BV, and CV. If the puncture could not be successful completed in both upper extremity veins, the right femoral vein route was utilized for AVS. The patient’s pain perception throughout the entire AVS procedure was assessed using the VAS scale.
Statistics methods
Data analysis was conducted using SPSS 27.0 software (SPSS Inc., Chicago, USA). Quantitative data that conform to a normal distribution were expressed as mean ± standard deviation, while quantitative data that did not conform to a normal distribution were expressed as Median (P25-P75). Intergroup comparisons were made using the independent samples Kruskal-Wallis test, with post hoc pairwise comparisons adjusted for significance using the Bonferroni method. Count data were expressed as n (%), and intergroup comparisons were made using the chi-square (χ2) test or Fisher’s exact test. A p-value < 0.05 was considered statistically significant.
Results
A total of 325 PA patients (Male 137, Female 188) who underwent AVS via the upper extremity vein approach were included. The baseline characteristics of the study population are presented in (Table 1). A total of 213 procedures were performed via the right MCV, 51 cases via the right BV, 35 via the right CV, 19 via the left MCV, and 7 via the left BV. Based on the pre-determined criteria, the bilateral sampling procedure was successfully performed in 297 cases.
Complications analysis
As shown in Table 2, venous spasm was observed in 20 cases (including 9 cases in the right MCV, 3 cases in the right BV, 7 cases in the right CV, and 1 case in the left MCV). Notably, the incidence of venous spasm in the right CV was comparatively higher (p = 0.008). The AVS procedures were successfully completed by utilizing the contralateral antecubital vein for puncture in all cases of venous spasm. Venous thrombosis occurred in 2 cases, both of which were presented with persistent upper extremity pain post-AVS via the right CV access. Additionally, localized hemorrhage events were observed in 2 cases: one involving the right MCV, and the other involving the right BV. All hemorrhage events were successfully managed with conservative treatment.
VAS score analysis
As demonstrated in Table 3, The VAS score for venous access in the right MCV, right BV, right CV, left MCV, and left BV venous access were as follows: 3 (2,3), 3 (2,3), 3 (3,5), 3 (2,3), and 3 (2, 3), respectively. Notably, the VAS score for the right CV route was significantly higher than other routes (H-value = 13.833, p = 0.008).
Discussion
The success rate of AVS via the femoral vein exhibits significant variation across different centers, thereby limiting its broader clinical application. The majority of these failures can be attributed to the unsuccessful cannulation of the right adrenal vein7. In most patients, the right adrenal vein is oriented at an angle towards the inferior vena cava. Consequently, AVS performed via the upper extremity route may be more suitable and has seen increasing adoption in recent years8,11,12.
The MCV is the thickest and most prominent and robust vessel in the cubital fossa, which is considered to be a relatively safer site for venipuncture13,14. Consequently, it is currently one of the most frequently utilized venous access for upper extremity AVS. However, in some patients, variations in the MCV or its unclear exposure can impede the completion of the puncture and catheterization procedure15. In such cases, the right CV or BV may be selected, but directly choosing the contralateral upper extremity vein as the AVS route is also a feasible option. The present study revealed there was no significant difference in the incidence of vasospasm between the bilateral MCV and BV access, nor in the VAS scores. While considering that most MCVs were directly drain into the BV, this is relatively straightforward to comprehend, and suggesting that AVS via either the MCV or the BV accesses may be equally appropriate.
However, in comparison to MCV and BV, the incidence of venous spasm via the CV access is relatively higher. Additionally, all thrombotic events in our study were observed in cases with CV access, thus suggesting an increased risk of vascular injury associated with AVS via CV access. The possible reasons might be as follows: (1) the CV has a greater number of valves compared to the MCV and BV16; (2) the angle at which the CV flows into the axillary vein is larger, impeding the smooth advancement of the catheter post-puncture; (3) the CV typically exhibits an initial thickening followed by thinning and relative tortuosity, while some studies also suggested that its diameter is smaller than that of the BV17. These factors not only predispose individuals to venous spasm but may also contributors to increased intraoperative pain perception and higher VAS scores. In fact, while during adrenal vein sampling (AVS) procedures, a higher incidence of slight or mild spasms was observed when utilizing the CV approach, yet the catheter could still be advanced without necessitating a change in venous access. This could also account for the higher VAS scores in the CV group.
In conclusion, the findings of this retrospective observational study indicate no significant difference in the incidence of complications and patient pain scores between AVS performed using the bilateral MCV and the BV. However, AVS conducted through the CV access is associated with a relatively higher incidence of venous spasm and a potentially higher rate of thrombosis formation, accompanied by more severe patient pain. These findings suggest that both the MCV and the BV are viable options for routine puncture in AVS. Furthermore, when the MCV or BV on one side is inaccessible, it may be more prudent to opt for the MCV nor the BV on the contralateral side rather than the CV.
Limitations
Firstly, this study is a single-center retrospective investigation. Secondly, the sample size of cases involving the left upper extremity veins was relatively small, potentially leading to statistical imprecision. Thirdly, in this study, the determination of vasospasm relied primarily on operator assessment. More objective evidence, such as imaging data, could strengthen the assessment. In addition, the potential impact of various clinical characteristics (e.g., gender, BMI, and age) on the overall results was not further analyzed. At last, although it is generally accurate to rely on experience to determine the target vein in most cases, previous studies have shown that there are additional veins in the forearm, such as the median antebrachial vein18. In light of these considerations, we are committed to refining our research methodology in future studies to mitigate these limitations.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Xu, Z. et al. Chongqing primary aldosteronism study (CONPASS) group. Primary aldosteronism in patients in China with recently detected hypertension. J. Am. Coll. Cardiol. 75, 1913–1922 (2020).
Reincke, M. et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 9, 876–892 (2021).
Magill, S. B. et al. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J. Clin. Endocrinol. Metab. 86, 1066–1071 (2001).
Younes, N., Larose, S., Bourdeau, I., Therasse, E. & Lacroix, A. Role of adrenal vein sampling in guiding surgical decision in primary aldosteronism. Exp. Clin. Endocrinol. Diabetes 131, 418–434 (2023).
Vonend, O. et al. Adrenal venous sampling: evaluation of the German conn’s registry. Hypertension 57, 990–995 (2011).
Ladurner, R. et al. Accuracy of adrenal imaging and adrenal venous sampling in diagnosing unilateral primary aldosteronism. Eur. J. Clin. Invest. 47, 372–377 (2017).
Dong, H. et al. Adrenal venous sampling via an antecubital approach in primary aldosteronism: A multicenter study. J. Clin. Endocrinol. Metab. 109, e274–e279 (2023).
Jiang, X. et al. A novel method of adrenal venous sampling via an antecubital approach. Cardiovasc. Intervent Radiol. 40, 388–393 (2017).
Sandhu, J. Peripheral devices. Tech. Vasc. Interv. Radiol. 1, 140–147 (1998).
Funder, J. W. et al. The management of primary aldosteronism: Case dtection, diagnosis, and treatment: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 1889–1916 (2016).
Xu, J. et al. A feasibility study on percutaneous forearm vein access for adrenal venous sampling. J. Hum. Hypertens. 31, 76–78 (2017).
Yu, Y. et al. Evaluation of adrenal vein anatomy by adrenal venous sampling in patients with primary aldosteronism in Chinese. J. Clin. Hypertens. (Greenwich) 26, 912–920 (2024).
Mukai, K. et al. Safety of venipuncture sites at the cubitalfossa as assessed by ultrasonography. J. Patient Saf. 16, 98–105 (2020).
Mikuni, Y., Chiba, S. & Tonosaki, Y. Topographical anatomy of superficial veins, cutaneous nerves, and arteries at venipuncture sites in the cubital fossa. Anat. Sci. Int. 88, 46–57 (2013).
Yammine, K. & Erić, M. Patterns of the superficial veins of the cubital fossa: A meta-analysis. Phlebology 32, 403–414 (2017).
Shima, H. et al. Anatomical study of the valves of the superficial veins of the forearm. J. Craniomaxillofac. Surg. 20, 305–309 (1992).
Mazzola, J. R., Schott-Baer, D. & Addy, L. Clinical factors associated with the development of phlebitis after insertion of a peripherally inserted central catheter. J. Intraven. Nurs. 22, 36–42 (1999).
Lee, H. et al. Variations of the cubital superficial vein investigated by using the intravenous illuminator. Anat. Cell. Biol. 48, 62–65 (2015).
Acknowledgements
We express our sincere gratitude to Qiuhua Xue, Yun Yang, Mingying Long, and the whole nursing team of Cardiovascular Department for their valuable contributions to the AVS procedures.
Author information
Authors and Affiliations
Contributions
J. Q., and XM. X. planned and designed the study. J. Q. wrote the main manuscript text. FR. Z., N. Z., and SH. Z. mainly performed the vein puncture. R. H., J. S., WF. L., and D. S. analyzed and interpreted the data. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, F., Zhu, N., Zhao, S. et al. Optimal vein access selection in adrenal vein sampling via upper extremity approach: a retrospective analysis of 325 cases. Sci Rep 15, 337 (2025). https://doi.org/10.1038/s41598-024-83377-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83377-5