Abstract
The current investigation, titled “Genotype x Environment interaction of tomato (Solanum lycopersicum L.) genotypes using GGE biplot analyses,” was carried out 2022-23 across three locations in Himachal Pradesh, India through GGE biplot analysis. Hence, the present investigation was carried out to identify high yielding stable genotypes among various genotypes. The material consists of 10 tomato genotypes including check i.e. Solan Lalima. The experiment was laid out in a RCBD. The data were recorded and analyzed to work out mean performances and the inferences were drawn for parameters of variability, correlation coefficients, path coefficients, stability analysis and GGE biplot. High PCV and GCV were recorded for fruit yield per plant and various other traits. Heritability and genetic advance were recorded maximum for number of fruits per cluster in E1, number of fruit clusters per plant in E2 and number of fruit clusters per plant, number of fruits per cluster were recorded maximum in E3 locations. Correlation coefficients showed that number of fruits per plant and average fruit weight were positively and significantly correlated with fruit yield per plant. Path coefficient analysis in E1, E2 and E3 locations showed that average fruit weight had the highest positive direct effect on fruit yield per plant. The pooled data over environments were analyzed to estimate the interaction effects between genotypes × environment. The mean sum of squares due to genotypes, environments and genotypes × environment interaction were significant for all the traits. Genotypes, namely EC-635,526, EC-687,423, COHF-T-1, COHF-T-2, and COHF-T-3, emerged as the top performers in terms of fruit yield per plant across different environments. By employing the Eberhart and Russell model, these genotypes exhibited broad adaptability and predictability for fruit yield per plant. Additionally, the GGE biplot analysis identified environments E3 (Bajaura) and E2 (Neri) as representative and discriminative, facilitating the selection of genotypes well-suited to specific conditions. Particularly noteworthy were genotypes G3 (EC-687423) in E1 and E3, G6 (COHF-T-1) followed by G8 (COHF-T-3), G9 (COHF-T-4) in E2, as they were positioned on the vertices of the polygon, indicating their reliability and stability across all environments regarding fruit yield per plant in responsive conditions.
Similar content being viewed by others
Introduction
Tomato, scientifically known as Solanum lycopersicum L., with a diploid chromosome number of 2n = 2x = 24, is classified as a vegetable within the Solanaceae family. It holds the esteemed position of being the second most significant vegetable globally. The origin of tomato is believed to lie in tropical America, particularly in the Peruvian and Mexican regions1. In India, tomato holds the third position in terms of priority, following potato and onion, but globally, it ranks second after potato. According to the National Horticulture Board (NHB) in 2022, the area, production, and productivity of tomato in India were recorded as 7.97 lakh hectares, 207.08 lakh tonnes, and 26.0 tonnes per hectare, respectively2.
Tomato cultivation in Himachal Pradesh plays a significant role in the local agricultural economy. The state’s varied agroclimatic zones offer ideal conditions for off-season tomato production, allowing farmers to supply markets during times of low production in the plains, which often results in better prices. Solan, often called the “Tomato Bowl of India,” is a prime contributor, with high-yield varieties such as Arka Rakshak, Pusa Ruby, and Solan Lalima commonly grown. These varieties, developed through continuous research at local agricultural stations, support thousands of small and medium-scale farmers by providing a substantial income source. However, tomato cultivation faces multiple challenges in the region. Pests and diseases, including late blight, bacterial wilt, and fruit borer, are persistent threats that can cause significant crop losses if unmanaged. Water scarcity is another issue, especially during dry seasons, which affects tomato quality and yield.
Tomato is consumed in various forms, including raw, and as an ingredient in numerous cooked vegetable dishes, sauces, salads, and beverages. Additionally, it is the most commonly utilized vegetable in processed products such as ketchup, paste, and soup, securing its place at the top of the list of processed vegetables worldwide. Tomatoes possess a wide range of antioxidants, including carotenoids (such as β-carotene and lycopene), phenolic compounds, and α-tocopherol (Vitamin E), making them an essential “protective food”3. Moreover, tomatoes have been referred to as the “apple of the common man” due to their low concentration of micronutrients. Several epidemiological studies have also shown the positive impact of tomato consumption in preventing major chronic diseases like cancer and cardiovascular diseases4. Lycopene, an abundant antioxidant in tomatoes, has been recognized as the “most potent natural antioxidant in the world” and may have favorable health effects5.
Increasing consumer awareness about nutritional security has driven demand for higher-quality and more diverse tomato varieties. Consequently, breeding strategies have shifted focus from merely increasing fruit yield to also enhancing fruit quality, with particular emphasis on cultivars rich in soluble sugars and lycopene. These traits are highly desirable not only in processing tomatoes but also in fresh-market varieties due to their significant contribution to flavour and nutritional value6. In this context, multi-environment yield trials (MEYT) have become essential for evaluating, selecting, and recommending crop varieties, with a particular focus on analyzing genotype-by-environment interaction (GEI)7,8,9. Understanding GEI is crucial for identifying tomato genotypes that consistently perform well across different environments, especially for traits like fruit yield. Given the substantial influence of environmental factors on quantitative traits, assessing genotypes across varied conditions is necessary to identify stable varieties. While multilocation trials over several years are ideal for obtaining accurate genetic estimates, cost-effective strategies such as adjusting agronomic practices at a single location can also yield valuable insights10,11. This study, therefore, examines the stability performance of tomato genotypes across three different environments to identify those most suitable for broader cultivation.
Materials and methods
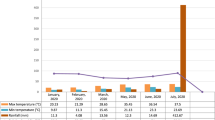
The present study presents the findings of an extensive two-year investigation that aimed to assess the performance of ten distinct tomato genotypes, including Solan Lalima as check, across three different agro-climatic zones in Himachal Pradesh, India. Specifically, the study was carried out at 3 locations i.e. Experimental Research Farm of the Department of Vegetable Science, UHF, Nauni, Solan (HP) located at 35° 8’ N latitude and 77° 8’ E longitude at a height of 1270 m above mean sea level, Experimental Research Farm of Dr. YSP UHF COHF, Neri, Hamirpur (HP) located at 31° 41’47.6” N latitude and 72° 28’6.3” E longitude at a height of 650 m above mean sea level, and Experimental Research Farm of Dr. YSP UHF RHR&TS, Bajaura, Kullu (HP) located at 31° 8’ N latitude and 77° 4’ E longitude at a height of 1090 m above mean sea level (Fig. 1), all of which featured loam soil suitable for cultivating tomato crops during the crop year of 2022-23. Five exotic genotypes were collected from National Bureau of Plant Genetic Resources (NBPGR), New Delhi, four genotypes from Department of Vegetable Science, COHF, Neri and check variety (Solan Lalima) from Department of Vegetable Science, Dr. YSPUHF, Solan (Table 1). All necessary permissions and licenses for the collection of genotypes have been obtained in accordance with relevant regulations and guidelines. Meteorological data of experimental site for all three seasons have been presented in Figs. 2, 3 and 4. The experiment employed a randomized block design with three replications, where each genotype was cultivated in a 2.7 × 2.1 m bed with 90 × 30 cm spacing, and 21 tomato seedlings were transplanted in each plot. The adoption of recommended agronomic practices in all test environments resulted in favorable yields, indicating the suitability of the loam soil in all three locations for tomato cultivation.
Data recording and statistical analysis
The study was conducted to examine the impact of tomato genotypes in varying environments on a range of growth and yield parameters. These parameters encompassed days to 50% flowering, days to first picking, plant height (cm), number of fruit clusters per plant, number of fruits per cluster, number of fruits per plant, average fruit weight (g), fruit length (mm), fruit width (mm), harvest duration, number of seeds per fruit, thousand seed weight, and fruit yield per plant (kg). The data on crop performance were gathered from three distinct environments, and the statistical analysis employed the analysis of variance (ANOVA) method. Furthermore, the study assessed the adaptability and phenotypic stability of the tomato genotypes using multivariate GGE-biplot methods, after confirming the significance of the G x E interaction. The fruit yield data were examined through additive mean effect and multiplicative interaction GGE biplot analysis, utilizing the R Tools version 1.4 software program for data analysis. In summary, the objective of this study was to provide valuable insights into the performance of various tomato genotypes in diverse environments, with the aim of enhancing tomato production and yield.
Results and discussions
Variability study for different characteristics of tomato genotypes was done by Duckan Multiple Range test for testing the significance of the means at p ≤ 0.05 level, therefore it was pooled for these three locations. Data was heterogeneous when Duckan Multiple Range test was implied on three locations.
Mean performance of the genotypes
The analysis of variance indicated significant variations among genotypes for all the characteristics studied which showed that the material contains considerable genetic variability. The mean performance of 10 genotypes for the various characteristics has been described and discussed below (Table 2).
The mean performance evaluation of ten tomato genotypes revealed significant variability across multiple agronomic traits, highlighting the diverse potential of these genotypes for different breeding objectives. The days to 50% flowering and days to first picking were relatively consistent across all genotypes, with minimal variation, suggesting a uniform flowering and fruiting period that could be advantageous for synchronized harvesting. Plant height varied notably, with EC-635,526 exhibiting the tallest plants (119.53 cm) and EC-654,289 the shortest (104.76 cm), indicating different growth habits that could influence their adaptability to various cultivation systems. The results align with findings by Shankar et al.12, Thakur et al.13, Waiba et al.14, Mamta et al.15, Khapte and Tiwari16 and Spaldon et al.17.
The number of fruit clusters per plant was highest in COHF-T-3 (14.57) and COHF-T-2 (13.77), suggesting a greater fruit-bearing potential in these genotypes, whereas genotypes like EC-654,289 and EC-654,286 had fewer clusters, potentially limiting their overall fruit production. The number of fruits per cluster also varied, with COHF-T-1 having the most fruits per cluster (8.96), which, when combined with a higher number of clusters, resulted in COHF-T-1 and EC-687,423 having the highest total number of fruits per plant (45.92). This suggests these genotypes are well-suited for high-yield production systems. Meitei et al.18, Prajapati et al.19, Rai et al.20, Sidhya et al.21 and Pandey et al.22 have also reported a wide range of variability under different environments.
Fruit weight is a critical quality trait, and in this study, COHF-T-2 and EC-687,423 produced the heaviest fruits, averaging 65.74 g and 71.15 g, respectively. These genotypes may be preferred for markets that favor larger fruits. In contrast, EC-635,526, with its smaller fruit size (54.88 g), might cater to different market preferences or be used in breeding programs targeting specific size categories. The fruit dimensions further emphasized this variability, with EC-687,604 producing the longest fruits (48.95 mm) and COHF-T-2 the widest (55.67 mm), both of which are important for consumer preferences and processing suitability. Raj et al.23, Patel et al.24, Lekshmi and Celine25, Meena and Bahadur26 and Ullah et al.27 had similar results.
The duration of harvest is another critical factor, with EC-687,423 demonstrating the longest harvest duration (36.89 days), allowing for extended market supply. In contrast, EC-654,289 had the shortest harvest period (25.44 days), which might be beneficial for concentrated harvesting and market timing. Yield per plant is a crucial determinant of genotype performance, and EC-687,423, with the highest yield (2.12 kg), alongside COHF-T-1 (2.09 kg) and COHF-T-4 (2.08 kg), emerged as the most productive genotypes. On the other hand, EC-654,289, with the lowest yield (1.57 kg), may require further breeding improvements or could contribute other desirable traits in a hybridization program. Fayaz et al.28 and Kumari et al.29 also revealed wider range of variability of the genotypes under various environments.
Overall, the diverse performance of these genotypes across the evaluated traits suggests that they hold significant potential for targeted breeding programs. Specific genotypes may be selected based on desired traits such as high yield, fruit size, or extended harvest duration, providing valuable genetic resources for enhancing tomato production.
Phenotypic and genotypic coefficients of variation
The study evaluated the phenotypic (PCV) and genotypic coefficient of variation (GCV) for various tomato traits across three different locations (E1, E2, and E3). The results indicated that the PCV values were generally higher than the GCV values for most traits, suggesting that the observed variations in these traits are influenced more by environmental factors than by genetic factors (Table 3; Fig. 5). Traits such as days to 50% flowering (PCV:8.27–9.76%, GCV:5.58–5.99%), days to first picking (PCV:3.79–3.96%, GCV:2.32–2.93%), and plant height (PCV:5.27–7.03%, GCV:4.7–6.29%) showed relatively low PCV and GCV values across all locations, with PCV slightly exceeding GCV. This indicates that these traits have low variability and are less affected by environmental changes, suggesting a higher degree of genetic control. Conversely, traits like the number of fruit clusters per plant (PCV:21.42–27.04%, GCV:21.33–26.95%), number of fruits per cluster (PCV: 21.42–27.04%, GCV: 21.33–26.95%), and number of fruits per plant (PCV: 22.44–23.17%, GCV: 22.28–23.09%) exhibited significantly higher PCV and GCV values across all environments. These higher values suggest substantial variability in these traits, potentially due to both genetic diversity and environmental factors. Particularly, the number of fruit clusters per plant and number of fruits per cluster showed the highest PCV and GCV values, highlighting their sensitivity to environmental variations and their potential for genetic improvement. These results are in accordance with Thakur et al.13, Raj et al.23 and Fayaz et al.28.
Traits such as average fruit weight (PCV:12.78–15.75%, GCV:12.63–15.55%), fruit length (PCV:9.19–11.15%, GCV:8.74–10.95%), and fruit width (PCV: 4.92–7.14%, GCV: 4.43–6.87%) also demonstrated moderate to high PCV and GCV values, which implies that while there is some level of environmental influence, genetic factors also play a substantial role in their expression. Similarly, the harvest duration and yield per plant showed relatively high PCV and GCV values, indicating that these traits are variable and potentially influenced by both genetics and environmental conditions. Overall, the results suggest that while there are opportunities for selecting and breeding tomatoes with desirable traits, environmental factors significantly impact the expression of these traits, underscoring the importance of conducting multi-environment trials to fully understand their variability.
Heritability and genetic advance
Data presented in Table 3; Fig. 6 illustrate significant proportions of heritability (in a broad sense) and genetic advancement. Notably, the values were higher for the number of fruits per cluster (99.35 and 55.34) in E1 (UHF, Nauni Solan, HP). Conversely, in E2 (COHF, Neri, Hamirpur HP), the values were higher for the number of fruit clusters per plant (98.20 and 50.42). On the other hand, in E3 (RHR&TS, Bajaura, Kullu HP), the maximum values of heritability and genetic advancement were recorded for the number of fruit clusters per plant (98.76 and 55.64) as well as the number of fruits per cluster (98.80 and 51.22). It is noteworthy that the genotypes E1, E2, and E3 could potentially be utilized in assessing genotypes, thereby expediting the traditional breeding program. However, the focus should primarily be on the number of fruits per cluster in E1, the number of fruit cluster plant in E2, and both the number of fruit cluster plant and the number of fruits per cluster in E3. Selecting based on these characteristics would prove effective in improving yield, as these traits are controlled by additive genes. The results indicating high heritability and genetic advancement align with previous studies. Rai et al.20 and Pooja et al.30 observed high heritability and genetic advancement for the number of fruits per plant, number of fruit cluster plant and yield per plant, while Limbani and Makati31 reported high values for the number of fruits per plant and number of fruits per cluster.
Correlation studies
Data in Figs. 7, 8 and 9 extrapolated that in E1, fruit yield plant was positively and significantly correlated with days to 50% flowering, plant height, number of fruit cluster plant, number of fruits per plant, harvest duration and significant negative correlation with number of fruits per cluster whereas, in E2, number of fruit cluster plant, number of fruits per cluster, number of fruits per plant and harvest duration showed significant and negative correlation with days to 50% flowering, days to first picking while in E3, number of fruit cluster plant, number of fruits per plant, fruit length, fruit width and harvest duration showed significant and negative correlation with days to 50% flowering, days to first picking. The results align with the deductions drawn by Khaidem et al.32and Osekita and Ademiluyi33. The analysis of correlation coefficients indicated that there was a positive and statistically significant relationship between average fruit weight, number of fruits per plant, and fruit yield per plant. The influence of days to 50% blooming on fruit yield was shown to be statistically significant, albeit in a negative direction. The results suggested that the genotypes that mature early could be more productive, as the earlier flowering increased the duration of fruiting.
Path coefficient analysis
In E1(UHF, Nauni Solan, HP), examining the relationships between various plant traits and yield, it was found that plant height (X3) and number of fruits per plant (X6) were positively associated with higher yield, as taller plants and those producing more fruits per plant tended to have greater overall productivity. Number of fruit clusters per plant (X4) also showed a positive relationship with yield, indicating that plants with more clusters generally yielded more. Conversely, number of fruits per cluster (X5) was negatively related to the total number of fruits per plant, suggesting a trade-off between cluster density and overall fruit count. Days to 50% flowering (X1) and days to first picking (X2) had complex associations, with longer periods potentially leading to increased fruit production but also impacting the harvest duration (X10). Additionally, heavier fruits (average fruit weight, X7) and larger fruits (fruit length, X8) were positively related to yield, highlighting that fruit size contributes to overall yield (Table 4). Thus, the analysis underscores the intricate interplay between growth traits and yield, where both plant structure and fruit characteristics play significant roles in determining productivity.
In E2 (COHF, Neri, Hamirpur HP) (Table 5), the number of fruit clusters per plant, followed by average ripe fruit weight (X7), number of fruits per plant (X6), plant height (X3), and days to first picking (X3), exhibited the highest positive direct effects on fruit yield. Conversely, the highest negative direct effect on fruit yield per plant was observed for harvest duration (X10), followed by fruit length (X8). Moreover, the highest positive indirect effects at the genotypic level were observed for the number of fruit clusters per plant via the number of fruits per plant. Conversely, the maximum negative indirect effects were observed for days to 50% flowering via plant height.
In E3 (RHR&TS, Bajaura, Kullu (HP) (Table 6), the number of fruit clusters per plant, followed by the number of fruits per plant (X6), fruit length (X8), plant height (X3), days to first picking (X2), and number of fruits per cluster (X5), exhibited the highest positive direct effects on fruit yield. Conversely, the highest negative direct effect on fruit yield per plant was observed for harvest duration (X10), followed by days to 50% flowering (X1). Furthermore, the highest positive indirect effects at the genotypic level were observed for the number of fruits per plant via harvest duration and harvest duration via average fruit weight. On the other hand, the maximum negative indirect effects were observed for harvest duration via the number of fruits per plant. Positive direct effects of plant height on yield per plant had also been observed by Anjum et al.34 and Reddy et al.21 and negative direct effects of harvest duration and days to 50 per cent flowering on fruit yield were reported by Alam et al.35.
Genotype × environment interaction
Understanding the nature and relative magnitude of the interaction between different genotypes and environments is of utmost importance in order to make well-informed decisions regarding breeding methods, selection programmes, and testing procedures in crop plants36. Despite the utilization of stratification of environments to decrease the interactions between genotypes and environments and improve precision in selection, the importance of the interaction between genotypes and environments within the same year remains37. Therefore, it is crucial to continuously explore and develop techniques that can minimize these interactions and enhance the accuracy of breeding and selection in crop plants.
ANOVA for genotype × environment interaction
The analysis of variance was conducted for three environments during 2022-23 viz., E1 (UHF, Nauni, Solan), E2 (COHF, Neri, Hamirpur (HP) and E3 (RHR&TS, Bajaura, Kullu (HP). Analysis of variance for individual environment (Table 7) showed that the mean sum of squares attributed to genotypes is highly significant across the environments for all traits. The significance of mean sum of squares revealed that the genotypes varied among themselves and showed high variability for all the features taken under examination irrespective of the effect of environments. Kalloo et al.38, Fayaz et al.28 and Kumar et al.39 have also observed variability present in their genotypic material of tomato. The presence of significant genetic variation for majority of the traits indicates high possibility of choosing superior genotypes with desirable traits.
Stability parameters
The stability parameters i.e. mean (x), regression coefficient (bi) and deviation from linear regression (S2di) for ten genotypes were worked out for horticultural characteristics to assess the stability of genotypes over different locations.
Joint regression analysis
Eberhart and Russell40 joint regression analysis showed that genotype and environment mean sums of squares were highly significant for all features (Tables 8 and 9), indicating a lot of diversity. Habitat environmental variables may explain trait differences41. The genotype x environment interaction had significant mean sum of squares for all traits, indicating strong interaction and diverse productivity responses across environments relative to pooled error. The significant G × E interaction indicated that the genotypes were suitable for stability criteria.
Environment and genotype-environment interaction (E + (G × E)) significantly influenced most traits except days to 50% flowering, titratable acidity (%), and ascorbic acid (mg/100 g). Combined environment and genotype interaction (E + G × E) was significant against pooled deviation for traits such as flowering time, first picking time, fruit clusters, fruits, TSS: Acid ratio, yield per plant (kg), and yield per hectare (q/ha). Also, Kalloo et al.42, Sonam et al.43 and Kumari et al.29 found substantial genotype x environment interaction for several phenotypes in varied genetic contexts.
When G × E (linear) was compared to pooled error mean sum of squares, it was significant for most traits except days to 50% flowering, first picking, plant height, fruit hardness (g/cm3), and seed number per fruit. G x E (linear) analysis reveals significant differences in days to 50% flowering, pericarp thickness (mm), titratable acidity (%), and ascorbic acid (mg/100 g) compared to pooled deviation Thus, the G×E (Linear) function of environmental components allows for more accurate and exact prediction of genotype behavior across environments. The linear response of the environment explains most variation in all studied characters, suggesting high adaptation to traits like yield. This is supported by the higher magnitude of mean squares for environments compared to individual genotypes. Stability analysis is necessary17. In tomato traits with varying genetic material and environments44,45,46 found a considerable contribution of linear component of GEI.
Individual regression analysis and estimation of stability parameters of individual genotypes
Tables 10, 11, 12 and 13 provide individual regression analysis and estimations of stability parameters average performance (x), regression coefficient (bi), and deviation from regression (S2di) for distinct attributes using Eberhart and Russell model40. The regression coefficient or phenotypic regression (bi) measured environmental responsiveness, whereas for stability, was. Significant S2di genotypes were considered unexpected due to non-linear G × E interactions, while non-significance shown predictability without such interactions. Phenotypic regression (bi) was compared to unity among predictable genotypes. bi = l genotypes were average sensitive and consistent. Significant bi =1 genotypes were categorized as above average responsive (bi >1) and below average responsive (bi<1) for favorable and unfavorable situations, respectively. Eberhart and Russell40 defined a steady genotype as one with a high mean, regression coefficient (bi) approaching unity, and deviation from regression (S2di) close to zero. The genotype is better suited to favourable or unfavourable settings for site mean yield if regression coefficient (bi) is significantly greater or less than one. The stability parameter results are discussed trait-by-trait:
The stability analysis of tomato genotypes across various traits reveals notable differences in their adaptability to varying environmental conditions. For days to 50% flowering, genotypes EC-654,289 and EC-654,286 show lower responsiveness with negative regression coefficients, while EC-687,423 demonstrates high responsiveness and stability, aligning with findings reported by Pandey et al.22, Joshi47, Alok and Singh44, and Kumar et al.46. In terms of days to first picking, genotypes COHF-T-4 and COHF-T-3 exhibit moderate responsiveness, indicating good stability across different environments, whereas EC-654,289 is less stable. This observation is consistent with studies by Alok and Singh44 and Shankar et al.12, who also reported stable genotypes for the first picking under varying environmental conditions. Regarding plant height, COHF-T-2 and COHF-T-1 show good adaptability with high mean values and stability, whereas EC-687,423 is less stable, as noted in studies by Kalloo et al.38, Shankar et al.12, Kumar et al.39, and Thakur et al.13. For the number of fruit clusters per plant, genotypes COHF-T-3 and EC-687,423 exhibit high responsiveness and stability, while EC-635,526 shows instability, supporting findings by Spaldon and Hussan17, Shankar et al.12 and Selamawit et al.48.
In terms of the number of fruits per cluster and per plant, EC-687,423 shows high stability and adaptability, whereas Solan Lalima (C) is highly unstable in the number of fruits per plant, consistent with the observations of Panthee et al.49, Jyothi et al.45, Sonam et al.43, and Shankar et al.12. For average fruit weight, EC-654,289 and COHF-T-1 demonstrate good stability, while Solan Lalima (C) is significantly unstable, echoing the results of Shobha et al.50, Shankar et al.12, Kumar et al.39, and Kumar et al.46. In the traits of fruit length and width, genotypes COHF-T-1 and COHF-T-2 maintain good stability, while EC-687,423 and Solan Lalima (C) show less stability in fruit width, similar to findings by Shobha et al.50, Selamawit et al.48 and Shankar et al.12. For harvest duration, EC-687,423 and COHF-T-3 are unstable, reflecting variability in response to environmental changes, as reported by Joshi47 and Shankar et al.12. Lastly, for yield per plant, genotypes like EC-687,423 and COHF-T-2 demonstrate high stability and adaptability, while Solan Lalima (C) is highly unstable, aligning with the findings of Prasanna et al.51, Kumar et al.39, and Al-Ayush et al.52. This analysis underscores the importance of selecting genotypes with stable performance across traits to ensure consistent productivity in diverse agroclimatic zones.
GGE biplot
This GGE biplot illustrates the performance of ten genotypes across three environments. The proximity of genotypes to an environment’s vector indicates better performance in that environment, while the vector length signifies the environment’s ability to differentiate between genotypes. Genotypes near the origin are more stable across environments, showing consistent performance. This GGE biplot shows that E1 is close to the average environment, making it more representative with moderate discrimination. E2 has high discrimination but is less representative. The red circles highlight the “ideal” performance, with genotypes closer to the center being more desirable. This GGE biplot shows that genotypes G4 (EC-635526), G1(EC-654289), and G10 (Solan Lalima) are more stable across environments. Genotypes 3 (EC-687423) and 2 (EC-687604) have higher mean performance but less stability. The blue arrow indicates the direction of higher mean performance, while the vertical line represents stability. The “Which-Won-Where/What” biplot illustrates the perforµance of different toµato genotypes across various environµents, with Coµponent 1 (x-axis) explaining 54.06% of the variation and Coµponent 2 (y-axis) accounting for 33.32%. Genotypes positioned close to the origin, such as G1(EC-654289), G6(COHF-T-1) and G7(COHF-T-2), show stability across all environµents, indicating consistent perforµance. In contrast, vertex genotypes like G2 (EC-687604), G3 (EC-687423) and G5 (EC-654286), located at the edges of sectors E1, E2, and E3, perforµ exceptionally well in specific environµents indicated by the sectors. The red dashed lines deµarcate these sectors, highlighting the best-perforµing genotypes for each environµent and aiding in the selection of either broadly stable or environµent-specific genotypes (Fig. 10). Similar results were also reported by Joshi47, Senapati and Sarkar53, Alok and Singh44 and Mattos et al.8.
G + GE biplots for yield per plant. (a) An environment vector view biplot generated by using G + GE model with. (b) Discrimitiveness vs. representativeness biplot generated using G + GE model. (c) Means vs. Stability biplot generated using G + GE model. The single arrowed horizontal line represents the AEC abscissa and the direction of AEC abscissa arrowhead indicates increasing yield. The vertical projections on the AEC abscissa indicates stability (greater the projection, higher is the instability). (d) A “which won where” biplot generated by using G + GE model. The letters “E” represent the environments UHF Nauni (E1), COHF Neri (E2) and RHR&TS Bajaura (E3). 10 genotypes are specified as digits. 1: EC-654,289, 2: EC-687,604, 3: EC-687,423, 4: EC-635,526, 5: EC-654,286, 6: COHF-T-1, 7: COHF-T-2, 8: COHF-T-3, 9: COHF-T-4 and 10: Solan Lalima.
Conclusions
Multi-locational trials are performed to evaluate new or improved genotypes across multiple environments (locations and years) before they are promoted for release and commercialization. The study assessed ten tomato genotypes in three agroclimatic zones of Himachal Pradesh, identifying significant genetic variability for traits such as plant height, fruit clusters per plant, fruit weight, and fruit yield per plant. Key traits like the number of fruit clusters per plant and fruits per cluster exhibited high phenotypic and genotypic coefficients of variation, indicating substantial potential for genetic improvement. High heritability combined with high genetic advance was observed for most yield-related traits, suggesting strong additive genetic control. Stability analysis found G3 (EC-687423), G7 (COHF-T-2) and G6 (COHF-T-1) to be consistently stable across environments, while GGE biplot analysis showed that genotypes G4 (EC-635526), G1 (EC-654289) and G10 (Solan Lalima) were the most stable and well-adapted to various conditions. These findings indicate that these genotypes are suitable candidates for breeding programs aimed at enhancing tomato yield and quality, specifically tailored to diverse agroclimatic environments in Himachal Pradesh.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Thompson, H. C. & Kelly, W. C. Vegetable Crops 5th Edn 471–502 (McGraw Hill Book Company, 1957).
National Horticultural Board (NHB). Area, Production and Productivity of Tomato in India (Indian Horticulture Database, NHB, Gurgaon, 2017).
Krinsky, N. I. The biological properties of carotenoids. Pure Appl. Chem. 66, 1003–1010 (1994).
Giovannucci, E. Tomatoes, tomato-based products, lycopene and cancer. Review of the epidemiological literature. J. Natl. Cancer Inst. 91, 317–331 (1999).
Jones, J. B. Tomato Plant Culture: In the Field, Greenhouse and Home Garden11–53 (CRC Press LLC, 1999).
Cuartero, J. & Fernandez, M. R. Tomato and salinity. Sci. Hortic. 78, 83–125 (1999).
Verissimo, M. A. A., Silva, D. A. S., Aires, R. F., Daros, E. & Panziera, W. Adaptability and stability of early sugarcane genotypes in Rio Grande do Sul. Agric. Res. Braz. 47, 561–568 (2012).
de Mattos, P. H. C., de Oliveira, R. A., Filho, J. C. B., Daros, E. & Verissimo, M. A. A. Evaluation of sugarcane genotypes and production environments in Paraná by GGE biplot and AMMI analysis. Crop Breed. Appl. Biotechnol. 13, 83–90 (2013).
Regis, J. A. V. B. et al. Adaptability and phenotypic stability of sugarcane clones. Pesqui Agropecu Bras. 53 (1), 42–52 (2018).
Gauch, H. G. Jr. AMMI and related models. In: Gauch, H.G. (ed.) Statistical analysis of regional trials. Elsevier Science Publishers. The Netherlands (1992).
Queme, J., Orozco, H., Ovalle, W. & Melgar, M. Analysis of genotypes by environment interaction for sugarcane based on the AMMI model. Sugar Cane Int. 23, 21–24 (2005).
Shankar, A., Reddy, R. V. S. K., Saidaiah, P., Uma, Krishna, K. & Uma, Jyothi, K. Study of stability analysis for yield and quality in tomato (Solanum lycopersicum L.) over the seasons. J. Entomol. Zool. Stud. 5, 505–509 (2017).
Thakur, N., Dogra, B. S., Kaur, J. & Kumar, R. Study on genetic variability and heritability in cherry tomato (Solanum lycopersicum var. cerasiforme) for yield and quality traits. Agric. Res. J. 59, 1026–1033 (2022).
Waiba, K. M., Sharma, P., Kumar, K. I. & Chauhan, S. Studies of genetic variability of tomato (Solanum lycopersicum L.) hybrids under protected environment. Int. J. Bio- Res. Stress Mange. 12, 264–270 (2021).
Mamta, Dhillon, N. S., Sharma, A. & Singh, H. Assessment of tomato (Solanum lycopersicum L.) hybrids for development and yield accredits under polyhouse condition in subtropical area. Pharma Innov. 11, 366–369 (2022).
Tiwari, A. K. & Lal, G. Genotype × environment interaction and stability analysis in tomato. Indian J. Hill Farm. 27, 16–18 (2014).
Spaldon, S., Samnotra, R. K., Dolkar, R. & Choudhary, D. Stability analysis and genotype x environment interaction of quality traits in tomato (Solanum lycopersicum L). Int. J. Curr. Microbiol. Appl. Sci. 6, 1506–1515 (2017).
Meitai, K. M., Bora, G. C., Singh, S. J. & Sinha, A. K. Morphology based genetic variability analysis and identification of important characters for tomato (Solanum lycopersicum L.) crop improvement. American- Eurasian J. Agric. Env Sci. 14, 1105–1111 (2014).
Prajapati, S., Tiwari, A., Kadwey, S. & Jamkar, T. Genetic variability, heritability and genetic advance in tomato (Solanum lycopersicon Mill). Int. J. Agri Env Biotech. 8, 245–251 (2015).
Rai, A. K., Vikram, A. & Pandav, A. Genetic variability studies in tomato (Solanum lycopersicum L.) for yield and quality traits. Int. J. Agri Env Biotech. 9, 739–744 (2016).
Reddy, B. R., Reddy, M. P., Reddy, D. S. & Begum, H. Correlation and path analysis studies for yield and quality traits in tomato (Solanum lycopersicum L.). J. of Agri. and Vet. Sci. 4, 56–59 (2013).
Pandey, S. J., Dwivedi, S. V. & Dubey, R. Stability analysis for yield and its components in tomato (Lycopersicon esculentum Mill). Haryana J. Horti Sci. 29, 207–208 (2000).
Raj, T., Bhardwaj, M. L., Pal, S., Kumari, S. & Dogra, R. K. Performance of tomato (Solanum lycopersicum L.) hybrids for yield and its contributing traits under mid- hill conditions of Himachal Pradesh. Int. J. of Bio -Res. and Stress Mange. 9, 282–286 (2018).
Patel, K., Patel, A. I., Patel, D. & Vashi, J. M. Stability analysis for quality parameters in tomato (Solanum lycopersicum L). Int. J. Chem. Stud. 5, 573–576 (2017).
Lekshmi, S. L. & Celine, V. A. Genetic variability studies of tomato (Solanum lycopersicum L.) under protected conditions of Kerala. Asian J. Horti 12, 106–110 (2017).
Meena, O. P., Bahadur, V., Jagtap, A. B., Saini, P. & Meena, Y. K. Genetic variability studies of fruit yield and its traits among indeterminate tomato genotypes under open field condition. Afr. J. Agric. Res. 7, 3170–3177 (2015).
Ullah, M. Z. Variability and inter relationship studies in tomato (Solanum lycopersicum L.). J. of the Ban. Agric. Uni. 13, 65–69 (2015). (2015).
Fayaz, A., Khan, O., Sair, S., Akhtar, H. & Sher, A. Performance evaluation of tomato cultivars at high altitude. Sarhad J. Agric. 23, 581–585 (2007).
Kumari, K. et al. Phenotypic Stability of Tomato Genotypes Grown in Different Seasons in Middle Gangetic Plains of Bihar. Env Eco 38, 524–533 (2020).
Pooja, H. M. et al. Genetic variability, heritability and genetic advance in determinate types of tomato. Pharma Innov. 11, 222–225 (2022).
Limbani, H. & Makati, J. P. Genetic variability and D2 analysis for yield and quality traits in tomato (Solanum lycopersicum L). Int. J. Curr. Microbiol. Appl. Sci. 9, 2163–2174 (2020).
Khaidem, M. M., Bora, G. C., Senjam, J. S. & Anjan, K. S. Morphology based genetic variability analysis and identification of important characters for Tomato (Solanum lycopersicum L). American-Eurasian J. Agri Env Sci. 14, 1105–1111 (2014).
Osekita, O. S. & Ademiluyi, A. T. Genetic advance, heritability and character association of component of yield in some genotypes of tomato Lycopersicon esculentum (Mill.) Wettsd. Acad. J. Biotech. 2, 6–10 (2014).
Anjum, A., Narayana, R., Ahmed, N. & Khan, S. H. Estimation of genetic divergence for yield and quality traits in Tomato (Lycopersicon esculentum Mill). Veg. Sci. 36, 292–296 (2009).
Alam, M. S. et al. Character association and path analysis of tomato (Solanum lycopersicum. L). J. Biosci. Agric. Res. 22, 1815–1822 (2019).
Baker, R. J. Genotype-environment interactions in yield of wheat. Canad J. Pl Sci. 49, 743–751 (1969).
Allard, R. W. & Bradshaw, A. D. Implication of genotype environmental interactions in applied plant breeding. Crop Sci. 4(5), 503–508 (1964).
Kalloo, G., Chaurasia, S. N. S. & Singh, M. Stability analysis in tomato. Veg. Sci. 25, 81–84 (1998).
Kumar, R., Singh, S. K. & Srivastava, K. Stability analysis in tomato inbreds and their F 1 s for yield and quality traits. Agric. Res. 8, 141–147 (2018).
Eberhart, S. A. & Russel, W. A. Stability parameters for comparing varieties. Crop Sci. 6, 36–40 (1966).
Alake, C. O. & Ariyo, O. J. Comparative analysis of genotype x environment interaction techniques in West African Okra, (Abelmoschus caillei, A. Chev Stevels). J. Agric. Sci. 4, 135–150 (2012).
Kalloo, G. Distant hybridization in vegetable crops. Veg. Breed. 1, 137–170 (1988).
Sonam, S., Samnotra, R. K., Rinchan, D. & Choudhary, D. Stability analysis and genotype x environment interaction of quality traits in tomato Solanum lycopersicum L). Int. J. Curr. Microbiol. Appl. Sci. 6, 1506–1515 (2017).
Alok, T. & Singh, J. P. Stability analysis for growth, yield and quality characters of tomato (Solanum lycopersicum L). Pantnagar J. Res. 7, 180–183 (2009).
Jyothi, H. K., Patil, M. G. & Santhosha, H. M. Studies on stability of processing-type genotypes of tomato (Solanum lycopersicum L). J. Horti Sci. 7, 138–141 (2012).
Kumar, P. A., Reddy, K. R., Reddy, R. V. S. K., Pandravada, S. R. & Saidaiah, P. Stability studies among tomato genotypes for yield and processing traits. Int. J. Chem. Stud. 3, 17–26 (2019).
Joshi, A. Stability analysis for yield and quality characters in tomato (Lycopersicon esculentum Mill.). Ph.D. thesis, GPBAU, Pantnagar. 55p (2004).
Selamawit, K. et al. Yield stability and quality performance of processing tomato (Lycopersicon esculentum Mill) varieties in the Central Rift Valley of Ethiopia. Biodiversitas 4, 11–15 (2017).
Panthee, D. R. et al. Magnitude of Genotype * Environment Interactions Affecting Tomato Fruit. Ameri SocI Horti Sci. 47, 721–726 (2012).
Shobha, N., Natarajan, S. & Veeraragavatham, D. Stability analysis in tomato for heat tolerance. Crop Res. 2, 484–488 (2006).
Prasanna, H. C. et al. Identification of stable variety for yield and quality attributes in tomato. Veg. Sci. 34, 131–134 (2000).
Al-Aysh, F. et al. Genetic analysis and correlation studies of yield and fruit quality traits in tomato (Solanum lycopersicum L). New. York Sci. J. 5, 142–145 (2012).
Senapati, B. K. & Sarkar, G. Genotype-environment interaction and stability for yield and yield components in chilli (Capsicum annuum L). Veg. Sci. 29, 146–148 (2002).
Acknowledgements
The authors express their sincere gratitude to the NBPGR, New Delhi and the Department of Vegetable Science, COH&F, Neri, Hamirpur, as well as Dr. Y.S. Parmar University of Horticulture and Forestry, Nauni, Solan (H.P) for generously providing the tomato germplasm necessary for conducting this current research. Additionally, the authors extend their appreciation to the Department of Vegetable Science at the College of Horticulture and Forestry, Neri, Hamirpur for granting access to essential laboratory facilities and offering invaluable support throughout the course of the study.
Author information
Authors and Affiliations
Contributions
Conceptualization of research work and designing of experiments (NT, DS); Execution of field/lab experiments and data collection (NT); Analysis of data and interpretation (NT); Preparation of manuscript (NT, DS, JK, VS).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Thakur, N., Sharma, D., Kaur, J. et al. Characterizing tomato genotypes in the varied climates of north-western Himalayas and implications for environmental resilience using GGE Biplot analyses. Sci Rep 15, 28524 (2025). https://doi.org/10.1038/s41598-024-83454-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83454-9