Abstract
This study aimed to quantify fundus microvascular alterations in patients requiring revascularization for coronary heart disease (CHD) using swept-source optical coherence tomography angiography (SS-OCTA) and to investigate the correlation between these alterations and the severity of coronary artery lesions. SS-OCTA was employed to assess the fundus neurovascular parameters of all participants, while the Gensini score was utilized to gauge the severity of coronary artery lesions in observation group. A total of 98 participants (49 CHD patients and 49 controls) were included. Analysis of the SS-OCTA parameters revealed that the vascular density (VD) of the superficial vascular plexus (SVP), the superficial vascular complex (SVC), the intermediate capillary plexus (ICP) in the parafoveal region, the mean ICP in the macula, deep capillary plexus (DCP) and deep vascular complex (DVC) in each macular region were significantly reduced in the observation group compared to the control group (P < 0.05). Multivariate logistic regression indicated that lower VD values in the SVP, SVC, ICP, DCP and DVC across macular regions were significantly associated with an increased likelihood of severe CHD (OR < 1, P < 0.05). ROC curve analysis revealed that the maximum area under the curve for overall DCP VD in the macula was 0.707, with a cutoff value of 19.64, sensitivity of 65.30%, and specificity of 73.50%. In CHD group, Pearson correlation analysis demonstrated a negative correlation between the Gensini score and mean DCP VD (r = − 0.491, P < 0.001). Retinal VD in patients requiring revascularization for CHD is significantly lower compared to healthy controls. SS-OCTA-based retinal microvascular damage assessment is a valuable tool for risk stratification and early intervention in CHD.
Similar content being viewed by others
Introduction
Coronary heart disease (CHD) arises from atherosclerosis-induced narrowing of the coronary arteries, leading to inadequate myocardial blood supply, making early detection and management of suspected coronary angina pectoris imperative1. In recent years, the critical importance of microvascular structure and dysfunction in ischemic heart disease has been increasingly recognized. Specifically, abnormalities in the coronary microvasculature have been significantly associated with long-term adverse cardiovascular events and the progressive decline of cardiac function Early identification of these abnormalities is crucial in preventing severe cardiovascular events. However, alterations in the coronary arteries often manifest insidiously, making them difficult to detect. Retinal microvessels, being the only microvessels in the human body that can be observed directly, have demonstrated morphological changes that are closely correlated with the severity of coronary artery disease and the associated risk of mortality2,3. Early screening for microvascular pathology provides valuable insights for timely diagnosis, disease prognosis, and therapeutic strategies. While previous studies predominantly utilized fundus color photography, advancements in retinal imaging technologies have made it possible to detect early and subtle changes in retinal microvasculature with greater precision. Swept-source optical coherence tomography angiography (SS-OCTA), a novel noninvasive diagnostic imaging modality4, operates on the principle of generating depth-resolved, high-resolution images of retinal and choroidal vasculature. This highly sensitive and reproducible technique facilitates the assessment and quantification of vascular density or blood flow across different anatomical layers of the fundus without the need for dye injection5. Despite its potential, research investigating the correlation between retinal and choroidal blood flow measured by SS-OCTA and coronary artery disease remains limited. This paper aims to explore the relationship between fundus microvascular damage detected via SS-OCTA and the severity of coronary obstructive lesions in CHD, thereby providing a theoretical foundation for personalized and precise clinical diagnosis and treatment.
Information and methods
Study participants
Forty-nine patients diagnosed with coronary artery disease requiring revascularization were prospectively enrolled in the observation group between January 2024 and March 2024 at the Department of Cardiology of Xuzhou First People’s Hospital and Jiawang District People’s Hospital of Xuzhou City. Concurrently, 49 healthy individuals from the same period who underwent routine medical checkups were selected as the control group. All participants in the observation group underwent comprehensive ophthalmologic examinations and SS-OCTA assessments prior to coronary angiography. Following revascularization, the angiographic data of the patients with coronary artery disease were collected, and the severity of coronary artery obstruction was quantified using the Gensini score. This study received ethical approval from the Ethics Committee of Xuzhou First People’s Hospital (approval number: xyy11[2023]057) and the Ethics Review Committee of the People’s Hospital of Jiawang District, Xuzhou City (approval number: 2023KY-066-01).
Inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Observation group: coronary angiography indicating a lumen diameter reduction of ≥ 50% in at least one major coronary artery, consistent with the Chinese Guidelines for the Diagnosis and Management of Patients with Chronic Coronary Syndrome6; patients requiring coronary revascularization7, including stent implantation, pharmacological balloon dilatation, or coronary artery bypass grafting; (3) Control group: general population without coronary artery disease.
Exclusion criteria were as follows: (1) Severe heart failure (New York Heart Association Class III–IV), cardiomyopathy, or severe heart valve disease; (2) Hemodynamic instability; (3) Presence of malignant tumors, renal failure, hematological disorders, or other severe systemic diseases; (4) History of coronary interventions or conditions that may confound the assessment of coronary artery disease severity; (5) Participants who had taken medications that affect vascular diameter, such as nitrates and angiotensin-converting enzyme inhibitors, in the last 3 months; (6) Previous macular degeneration, diabetic retinopathy, hypertensive retinopathy, or other significant fundus or intraocular diseases aside from cataracts; (7) Inability to cooperate with ophthalmologic examination or refractive media opacities precluding clear fundus imaging; (8) Refractive spherical lens power beyond − 6.00D to 6.00D and/or astigmatism beyond − 3.00D to 3.00D; (9) History of intraocular surgery other than cataract surgery.
Data collection
Patient data included basic demographic information (age, gender) and medical history (ocular diseases, diabetes, hypertension, smoking habits).
Criteria for evaluating the severity of coronary obstruction
The Gensini score, used to evaluate the complexity of coronary artery lesions, applies quantitative coronary angiography (QCA) to quantify lesion severity8,9. Stenosis in coronary vessels (left main, anterior descending, circumflex, right coronary) is graded by percentage: ≤ 25%, 26–50%, 51–75%, 76–90%, 91–99%, and complete occlusion (100%), corresponding to scores of 1, 2, 4, 8, 16, and 32, respectively. Each coronary segment is assigned a specific weight: left main × 5; anterior descending (proximal × 2.5, mid × 1.5, distal × 1); circumflex (proximal × 2.5, distal × 1, obtuse marginal × 1); diagonal branches (D1 × 1.5, D2 × 0.5); right coronary (proximal, mid, distal ×1); posterior descending × 1; and left ventricular posterior × 0.5. The final Gensini score is calculated by multiplying the stenosis score by the segment’s weighting coefficient, then summing all affected vessels’ scores to determine the patient’s overall coronary artery stenosis severity. The methodology employed in the calculation of the Gensini score is predicated on the guideline of Gensini G. G in 19839.
General ocular examination and measurement of fundus microvascular alteration indices
All participants underwent comprehensive ophthalmological evaluations, including assessments of both eyes, though data from only one eye were included for analysis. For individuals born in even-numbered years, data from the right eye were utilized, while for those born in odd-numbered years, data from the left eye were used10. In cases where data from the selected eye were unavailable, the other eye’s data were substituted. The examinations included refraction tests, uncorrected visual acuity and BCVA measurements, intraocular pressure (IOP) assessments, slit-lamp evaluations, fundoscopy, and 45° dilated fundus color photography focused on the posterior pole of each eye. These tests ensured that only participants meeting the inclusion criteria were enrolled. Additionally, each participant underwent an SS-OCTA (VGS; Intalight Imaging, Henan, China) examination, with only images of sufficient quality (≥ 7/10) being analyzed.
The SS laser employed had a central wavelength of 1050 nm, scanning at a rate of 100,000 A-scans per second, with an axial optical resolution of 3.8 μm and a posterior segment scanning depth of 3 mm. High-definition scans of both the optic disc (4.5 × 4.5 mm2) and macular (6 × 6 mm2) regions were performed. The optic disc region was divided into the papillary area (a 2 mm-diameter circle centered on the optic disc) and the peripapillary area (a 2–4 mm concentric ring around the optic disc). The macula was divided into the foveal area (a 1 mm-diameter circle at the center), the parafoveal area (a concentric ring from 1 to 3 mm), and the perifoveal area (from 3 to 6 mm). Previously, retinal vasculature was divided into SVC and DVC in OCTA due to projection artifact removal limits, but in the new OCTA segmentation nomenclature proposed by Zhang et al.11, retinal vasculature is divided into four layers: RPCP, SVP, ICP, and DCP corresponding to human anatomy. In each participant, the layer segmentation settings stay consistent as default, The specific Settings of each layer are shown in Table 1.
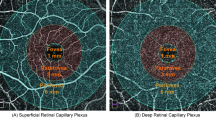
Following scanning, image processing software generated the following data: (1) optic disc vascular parameters including the vascular density (VD) of the radial peripapillary capillary plexus (RPCP), superficial vascular plexus (SVP), intermediate capillary plexus (ICP), deep capillary plexus (DCP); (2) retinal nerve fiber layer (RNFL) thickness; (3) ganglion cell-inner plexiform layer (GC-IPL) thickness; (4) macular retinal thickness (µm); and (5) macular vascular parameters such as choroidal vascular index (CVI), the VD of the RPCP, SVP, superficial vascular complex (SVC), ICP, DCP, and VD of the deep vascular complex (DVC) in the macula. SS-OCTA images from the control group are presented in Fig. 1.
Retinal layers and vascular density analysis examined by SS-OCTA, with corresponding regions depicted in the thickness map of the control group. (A) Radial peripapillary capillary plexus (RPCP); (B) Superficial vascular plexus (SVP); (C) Intermediate capillary plexus (ICP); (D) Deep capillary plexus (DCP); (E) Superficial vascular complex (SVC); (F) Deep vascular complex (DVC); (G) Choroid; (H) RPCP of the optic disc; (I) SVP of the optic disc; (J) ICP of the optic disc; (K) DCP of the optic disc. Vascular density was measured using ETDRS zonation in the macular region and Garway-Heath zonation in the optic disc region.
Statistical analysis
Data analysis was conducted using SPSS software (IBM SPSS, Version 25.0, IBM Corporation, Armonk, New York). Categorical variables were presented as percentages and compared using the chi-square test. Continuous data following a normal distribution were analyzed with t-tests and expressed as mean ± standard deviation, while non-normally distributed data were analyzed using the Mann-Whitney U test and expressed as median [quartile]. A multifactorial logistic regression model (stepwise forward regression) was applied to examine the correlation between retinal vascular parameters and severe CHD. The predictive ability of these parameters for severe CHD was evaluated via receiver operating characteristic (ROC) curve analysis. Additionally, Pearson’s correlation analysis was used to assess the relationship between Gensini scores and DCP VD in the macula, with P < 0.05 indicating statistical significance.
Results
General information on the study participants in both groups
This study recruited 98 participants, comprising 49 patients with coronary artery disease and 49 healthy controls. The results indicated no statistically significant differences between the two groups in terms of gender, eye type, smoking status, hypertension, diabetes mellitus, age, intraocular pressure, or BCVA (P > 0.05), as detailed in Table 2.
Comparison of SS-OCTA parameters between the two groups of study participants
Analysis of the SS-OCTA parameters revealed that the observation group exhibited significantly lower VD in several areas compared to the control group. These included the VD of SVP and SVC across all macular regions, ICP in the parafoveal area, mean ICP of the macula, DCP in all macular regions, and the VD of the DVC in all macular regions. The differences between the groups were statistically significant (P < 0.05), as shown in Table 3.
Correlation between retinal vascular parameters in the macula and the development of severe coronary heart disease
A multivariate logistic analysis (stepwise forward regression) was conducted using the presence of severe CHD as the dependent variable (coded as 1 for the CHD group and 0 for the control group). Statistically significant SS-OCTA parameters, specifically the VD of SVP, SVC, ICP, DCP, and DVC in each macular region, were incorporated as independent variables. The analysis was adjusted for gender, ocular side, smoking status, hypertension, diabetes mellitus, age, BCVA, and intraocular pressure. The results indicated that lower VD in the SVP, SVC, ICP, DCP, and DVC across macular regions were significantly associated with an increased likelihood of severe CHD (OR < 1, P < 0.05), as detailed in Table 4.
ROC curve analysis to assess the predictive power of macular retinal vascular parameters for the development of severe coronary artery disease
ROC curve analysis of the vascular densities of SVP, SVC, ICP, DCP, and DVC across macular regions in participants with and without coronary artery disease revealed that the mean DCP VD had the highest area under the curve (AUC) at 0.707. The cutoff value for this parameter was 19.64, with a sensitivity of 65.30% and a specificity of 73.50%. The metrics with the largest AUC for SVP, SVC, ICP, DCP, and DVC vessel densities are presented in Table 5 and illustrated in Fig. 2.
ROC curves of retinal vascular parameters in the macula for predicting the development of severe coronary artery disease. SVP superficial vascular plexus, SVC superficial vascular complex, ICP intermediate capillary plexus, DCP deep capillary plexus, DVC deep vascular complex, ROC receiver operating characteristic.
Correlation analysis between retinal vascular parameters and Gensini score in patients with severe coronary artery disease
Pearson correlation analysis between the Gensini score and mean DCP VD in the macula was conducted in the coronary artery disease group. The results demonstrated a significant negative correlation between Gensini score and mean DCP VD (r = − 0.491, P < 0.001), as depicted in Fig. 3. Figure 4 shows the SS-OCTA images of DCP in three different groups: the control participants and observation group with lower and higher Gensini scores.
Discussion
CHD remains a leading cause of cardiovascular mortality, with its incidence rising due to population aging and increasing prevalence of hypertension, hyperlipidemia, obesity, and diabetes12. The progression of coronary atherosclerosis, characterized by lumen narrowing or occlusion, leads to myocardial ischemia, which heightens the risk of acute myocardial infarction. Prolonged ischemia irreversibly damages myocardial cells, severely impairing heart function and potentially resulting in heart failure, malignant arrhythmias, or sudden death13. Additionally, patients with atrial fibrillation are more susceptible to mural thrombi, which can embolize cerebral vessels, causing ischemic stroke14. Since CHD evolves slowly, often taking 10 to 20 years from plaque formation to the onset of ischemic symptoms, early detection is challenging due to the absence of initial symptoms15. Therefore, developing novel strategies for early diagnosis and screening is crucial for reducing the risk of severe cardiovascular events.
Severe coronary artery disease is largely due to microvascular damage16. Coronary microvascular injury and endothelial dysfunction precipitate myocardial ischemia, significantly increasing the risk of adverse cardiovascular events such as ischemic episodes and heart failure17,18. Research has shown that retinal and coronary microvessels share structural and functional similarities, making the retinal vasculature an effective indicator for predicting CHD progression18,19,20. SS-OCTA offers rapid and precise quantification of retinal and choroidal microvascular parameters21.
In this study, a comparison of retinal microvascular parameters obtained through SS-OCTA revealed that patients with severe coronary artery disease exhibited significantly reduced vascular densities. The VD of SVP and SVC in all macular regions, ICP of the parafoveal area, mean ICP of the macula, and DCP and DVC in all macular regions were all lower compared to healthy controls. The reduced VD in SVP, SVC, ICP, DCP, and DVC across macular regions was inversely associated with the development of severe coronary artery disease. These findings suggest a clear correlation between retinal microvascular alterations and changes in coronary vasculature.
Due to the exceptionally high oxygen consumption of retinal neural tissue and blood flow to the choroid is relatively high rendering the retina and choroid particularly vulnerable to systemic diseases such as coronary artery disease, hypertension, diabetes mellitus, and progressive neurodegenerative diseases22. A meta-analysis by Rusu et al.23 demonstrated a significant reduction in retinal nerve fiber layer (RNFL) thickness in patients with coronary artery disease, along with a markedly lower macular vascular density, particularly in the superficial plexus, compared to controls. Wu et al.4 utilized OCTA to compare fundus retinal vessel density between patients with myocardial infarction and healthy controls. and found that in the macular area, the superficial and deep retinal microvessel density decreased significantly. These findings align with the present study, reinforcing the association between coronary artery disease and decreased retinal vascular density. OCTA was also used to assess the retinal microvasculature in rare diseases, Fabry disease (FD) is an X-linked gene disease24, Rinaldi et al.25 used optical coherence tomography angiography (OCTA) to assess the effects of Fabry disease (FD) on the retinal microvasculature, and found that the density of the retinal microvasculature was reduced. This study used color Doppler imaging in addition to OCTA to observe retinal vessel density in Fabry patients, providing additional information about ocular hemodynamics and tissue perfusion. We refer to the methodology of that study to observe retinal vessel density with OCTA and to study the relationship between retinal microvessels and coronary artery stenosis. To explore noninvasive methods to determine the severity of coronary artery disease.
Analysis using the ROC curve revealed that the AUC for mean DCP VD in the macula was 0.707, with a sensitivity of 65.30% and specificity of 73.50%, indicating its effectiveness as a predictor of the onset and progression of severe coronary artery disease. Pearson correlation analysis further showed a negative correlation between the Gensini score and mean DCP VD in the macula. Retinal microvascular alterations is known precursors to symptomatic atherosclerosis21, supporting the predictive value of retinal microvascular changes for long-term adverse cardiovascular events. The retina’s unique capacity for direct, noninvasive in vivo assessment of microcirculation has been previously validated in studies using digital fundus photographs, which have shown correlations between changes in retinal vascular caliber and conditions such as hypertension and diabetes mellitus. Early, subtle alterations in the retinal vascular system also reflect the status of cerebral and coronary circulation26. SS-OCTA allows for a more detailed and nuanced assessment of retinal microvascular function compared to traditional fundus photography, offering deeper insights into the relationship between retinal and coronary microvasculature.
Research applying SS-OCTA to explore the relationship between retinal microvessels and coronary vessels remains limited. Traditional methods, such as fundus contrast imaging, have predominantly been employed to investigate the diameters of retinal arteries and veins, as well as vascular morphology in the context of coronary artery disease27,28,29. They have proposed the use of retinal vessels as markers for coronary artery disease, e.g. the narrower the small retinal arteries, the greater the risk of coronary artery disease. The presence of arteriovenous traces in the retina suggests a greater likelihood of coronary artery stenosis, and the smaller the arteriole to venule ratio, the correspondingly greater the risk of coronary artery disease. Compared with fundus photographs, SS-OCTA allows for finer characterization of retinal microvascular alterations and the discovery of new retinal vascular biomarkers, which can lead to a more in-depth understanding of the relationship between the retinal microvascular system and the coronary vasculature system. SS-OCTA is inexpensive, about one-tenth of the cost of coronary angiography, and its operation is simple, and the examination can be completed within 1 min for each eye. Currently, OCTA has been widely used in the diagnosis of ophthalmic diseases. SS-OCTA is non-invasive and does not require injection of contrast agent to avoid the risk of allergy. Most patients with coronary artery disease can undergo SS-OCTA, except for a very small number of patients with severe refractive media clouding.
This study introduces a new generation of SS-OCTA, which features enhanced scanning speed, greater imaging depth, improved tissue penetration, and an AI-powered stratification method. These advancements enable retinal vascular stratification that closely aligns with histological structures and provide clear choroidal imaging. In contrast, conventional spectral-domain OCTA (SD-OCTA) is limited to capturing data from the superficial and deep retinal vascular networks, corresponding to the SVC and DVC of SS-OCTA. SS-OCTA further subdivides the SVC into RPCP and SVP, and the DVC into ICP and DCP. Previous studies have indicated that microaneurysms tend to originate in the inner nuclear layer and its boundary region30,31, that is, the deeper capillary plexus, which may be related to hypoxia responses30 and subsequent VEGF production. Scarinci et al.32 shows that macular photoreceptor disruption on SD-OCT in patients with diabetic retinopathy corresponds to areas of capillary non-perfusion at the level of DCP. The research purport that it is the ischemia at the level of the DVP that results in outer retina and photoreceptor disruption. Coscas et al.33 used OCTA to assess macular perfusion and edema and found that DCP appears to be more severely affected than the SVP in RVO. And other studies have shown that DCP tended to decrease with progressive stages of hypertensive retinopathy34. These studies suggest that ischemia reperfusion and neovascularization of DCP play an important role in many retinal vascular diseases. Anatomically, the DCP contains only uniformly sized retinal capillaries, with no larger vessels connecting these capillary plexuses, and each capillary unit is surrounded by continuous endothelial cells and peripheral pericytes35. It is mainly influenced by the function of the vascular endothelium. Given that endothelial dysfunction plays a crucial role in the progression of coronary stenosis36,37,38, Therefore, decreased DCP vessel density is associated with coronary stenosis progression. However, the SVP is a network of large and small vessels directly connected to the retinal arterioles and supplying all other vascular plexuses. The perfusion autoregulatory function of the SVP is mainly mediated by smooth muscle cells in the retinal arterioles and small arterioles. Therefore, SVP is mainly affected by retinal arterioles and less by vascular endothelial cells, so that deep capillaries are more prone to ischemia and circulatory abnormalities than superficial capillaries39.
In this study, the area under the ROC curve for mean DCP VD in the macula was the largest compared to DVC VD across various macular regions, and a negative correlation was observed between the Gensini score and mean DCP VD in the macula. This suggests that DCP VD may serve as a more reliable predictor of coronary artery disease severity than DVC VD. The findings affirm the superiority of the new generation SS-OCTA in providing accurate data for disease diagnosis, treatment, and research. Currently, There are fewer studies of predictive modeling40, which are based on retinal vascular vessel diameters in fundus photographs to explore the relationship between changes in the retinal microvascular system and the development of coronary artery disease. We detected new biomarkers with SS-OCTA, which opens up the possibility of expanding the sample size in the future and incorporating the biomarkers detected by SS-OCTA into the predictive model to improve the accuracy of the model.
However, the study is limited by a small sample size, the lack of multicenter studies to validate the findings, and insufficient clinical follow-up. and the clarity of the refractive media may also affect OCTA measurement. The findings of this study still need to be strengthened and validated with further prospective data in large samples before clinical application in routine populations. Whether retinal microvascular characteristics can be applied in practice as an independent predictor of severe coronary artery disease remains to be confirmed.
In conclusion, SS-OCTA assessment of retinal vasculature provides valuable insights into the severity of coronary artery disease, facilitates the monitoring of disease progression, and may inform decisions regarding future invasive testing.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Komilovich, E. B. Coronary heart disease, angina treatment. J. New Cent. Innov. 46 (1), 95–104 (2024).
Al-Mohaissen, M. A. Echocardiographic assessment of primary microvascular angina and primary coronary microvascular dysfunction. Trends Cardiovasc. Med. 33 (6), 369–383 (2023).
Ullrich, H. et al. Coronary venous pressure and microvascular hemodynamics in patients with microvascular angina: a randomized clinical trial. JAMA Cardiol. 8 (10), 979–983 (2023).
Wu, J. Y. et al. Ocular microvascular alteration in patients with myocardial infarction—a new OCTA study. Sci. Rep. 14 (1), 4552 (2024).
Lu, B. et al. Computational retinal microvascular biomarkers from an OCTA image in clinical investigation. Biomedicines 12 (4), 868 (2024).
Chinese Society of Cardiovascular Disease & Chinese Journal of Cardiovascular Disease Editorial Committee. Guidelines for the diagnosis and management of patients with chronic coronary syndrome in China. Chin. J. Cardiovasc. Dis. 52 (06), 589–614 (2024).
Interventional Cardiology Group of the Cardiovascular Disease Branch of the Chinese Medical Association. Chinese guidelines for percutaneous coronary intervention. Chin. J. Cardiovasc. Dis. 44 (5), 382–400 (2016).
Wang, K. Y., Zheng, Y. Y., Wu, T. T., Ma, Y. T. & Xie, X. Predictive value of Gensini score in the long-term outcomes of patients with coronary artery disease who underwent PCI. Front. Cardiovasc. Med. 8, 778615 (2022).
Gensini, G. G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 51, 606 (1983).
Zhong, P. et al. Retinal microvasculature impairments in patients with coronary artery disease: An optical coherence tomography angiography study. Acta Ophthalmol. 100 (2), 225–233 (2022).
Zhang, M. et al. Projection-resolved optical coherence tomographic angiography. Biomed. Opt. Express 7 (3), 816–828 (2016).
Schef, K. W. et al. Prevalence of angina pectoris and association with coronary atherosclerosis in a general population. Heart 109 (19), 1450–1459 (2023).
Stone, P. H., Libby, P. & Boden, W. E. Fundamental pathobiology of coronary atherosclerosis and clinical implications for chronic ischemic heart disease management—the plaque hypothesis: a narrative review. JAMA Cardiol. 8 (2), 192–201 (2023).
Masenga, S. K. & Kirabo, A. Hypertensive heart disease: risk factors, complications and mechanisms. Front. Cardiovasc. Med. 10, 1205475 (2023).
Yue, J., Kazi, S., Nguyen, T. & Chow, C. K. Comparing secondary prevention for patients with coronary heart disease and stroke attending Australian general practices: a cross-sectional study using nationwide electronic database. BMJ Qual. Saf. 33 (8), 499–510 (2024).
Khabibovna, Y. S. & Alisherovna, K. M. Stress testing in patients with coronary heart disease. J. New. Cent. Innov. 45 (3), 28–33 (2024).
Matuszewska, U. et al. Microvascular angina—an abstruse path to diagnose and to treat–a review of literature. J. Educ. Health Sport 51, 198–216 (2024).
Sucato, V. et al. Biomarkers of coronary microvascular dysfunction in patients with microvascular angina: a narrative review. Angiology 73 (5), 395–406 (2022).
Niida, T. et al. Layered plaque is associated with high levels of vascular inflammation and vulnerability in patients with stable angina pectoris. J. Thromb. Thrombol. 1, 1–8 (2024).
Ren, Y. et al. Impaired retinal microcirculation in patients with non-obstructive coronary artery disease. Microvasc. Res. 148, 104533 (2023).
Rusu, A. C. et al. Retinal structural and vascular changes in patients with coronary artery disease: A systematic review and meta-analysis. Life 14 (4), 448 (2024).
Kur, J., Newman, E. A. & Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 31 (5), 377–406 (2012).
Ay, İ. E. et al. Is it useful to do OCTA in coronary artery disease patients to improve SYNTAX-based cardiac revascularization decision? J. Photodiagn. Photodyn. Ther. 42, 103540 (2023).
Desnick, R. J., Dean, K. J., Grabowski, G. A., Bishop, D. F. & Sweeley, C. C. Enzyme therapy XVII: metabolic and immunologic evaluation of alpha-galactosidase A replacement in Fabry disease. Birth Defects Original Article Ser. 16 (1), 393–413 (1980).
Rinaldi, M. et al. Resistive index of central retinal artery, aortic arterial stiffness and OCTA correlated parameters in the early stage of fabry disease. Sci. Rep. 14 (1), 24047 (2024).
Wu, Y. et al. Retinal vascular parameters and risk of heart failure. MedRxiv 1, 1 (2023).
Gao, Z. et al. The eye-image features of patients with coronary heart disease assed: A prospective, observational study of traditional Chinese medicine combined with modern medicine. MedRxiv 1, 1 (2023).
Zhang, W. et al. Retinal microvascular changes and risk for coronary heart disease: A systematic review and meta-analysis. J. Retina 10, 1097 (2022).
Liang, C., Gu, C. & Wang, N. Retinal vascular caliber in coronary heart disease and its risk factors. J. Ophthal. Res. 66 (1), 151–163 (2023).
Moore, J., Bagley, S., Ireland, G., McLeod, D. & Boulton, M. E. Three dimensional analysis of microaneurysms in the human diabetic retina. J. Anat. 194 (1), 89–100 (1999).
Hasegawa, N., Nozaki, M., Takase, N., Yoshida, M. & Ogura, Y. New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 57 (9), OCT348–OCT355 (2016).
Scarinci, F., Nesper, P. L. & Fawzi, A. A. Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am. J. Ophthalmol. 168, 129–138 (2016).
Coscas, F. et al. Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am. J. Ophthalmol. 161 (1), 160–171 (2016).
Liu, Y. et al. Morphological changes in and quantitative analysis of macular retinal microvasculature by optical coherence tomography angiography in hypertensive retinopathy. Hypertens. Res. 44 (3), 325–336 (2021).
Gardiner, T. A., Archer, D. B., Curtis, T. M. & Stitt, A. W. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation 14 (1), 25–38 (2007).
Juonala, M. et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation 110 (18), 2918–2923 (2004).
Al-Fiadh, A. H. et al. Retinal microvascular structure and function in patients with risk factors of atherosclerosis and coronary artery disease. Atherosclerosis 233 (2), 478–484 (2014).
Al-Fiadh, A. H. et al. Usefulness of retinal microvascular endothelial dysfunction as a predictor of coronary artery disease. Am. J. Cardiol. 115 (5), 609–613 (2015).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68 (3), 409–427 (2010).
Wong, Y. L. et al. Association between deep learning measured retinal vessel calibre and incident myocardial infarction in a retrospective cohort from the UK Biobank. BMJ Open 14 (3), e079311 (2024).
Funding
This study was supported by Xuzhou Medical Key Talents Project (No. XWRCHT20220048), Xuzhou Key R & D Program (No. KC22099) and Natural Science Foundation of Jiangsu Province (BK20241765).
Author information
Authors and Affiliations
Contributions
H.L generalized the idea of the new research, and revised the manuscript. H.X and D.Z analyzed and interpreted the patient data, and drafted the manuscript. J.Z recommend patients for enrollment. Y.N contributed to the acquisition and analysis of data. T.L and M.L interpreted the results and edited the photos. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study received ethical approval from the Ethics Committee of Xuzhou First People’s Hospital (approval number: xyy11[2023]-057) and the Ethics Review Committee of the People’s Hospital of Jiawang District, Xuzhou City (approval number: 2023KY-066-01). Informed consent was obtained from all subjects. All methods were con-ducted in accordance with the tenets of the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zong, D., Xi, H., Ni, Y. et al. SS-OCTA assessment of fundus microvascular changes and their correlation with coronary lesion severity in severe coronary heart disease. Sci Rep 14, 31931 (2024). https://doi.org/10.1038/s41598-024-83467-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83467-4
Keywords
This article is cited by
-
Coronary artery and retinal vascularization by optical coherence tomography angiography: are eyes the window to the heart?
Graefe's Archive for Clinical and Experimental Ophthalmology (2025)