Abstract
Prostate cancer ranks among the most prevalent malignancies affecting males globally. This study sought to document the incidence, deaths and disability-adjusted life-years (DALYs) that were due to prostate cancer in the Middle East and North Africa (MENA) region, and its 21 countries, from 1990 to 2021. We analysed publicly accessible data from the Global Burden of Disease 2021 study to present findings on the incidence, deaths, and disability-adjusted life years (DALYs) associated with prostate cancer. These metrics were reported as counts and age-standardised rates, each accompanied by 95% uncertainty intervals. In 2021, the age-standardised incidence of prostate cancer in the region was 27.4 per 100,000 population, representing a 125.1% increase from 1990. The age-standardised mortality rate was 10.1, and the age-standardised DALY rate was 173.6, neither of which showed significant increases since 1990. Qatar recorded the highest age-standardised DALY rate in 2021, while Algeria had the lowest. The largest number of incident cases, deaths, and DALYs in 2021 were found in the 65–69, 80–84, and 70–74 age groups, respectively. Additionally, from 1990 to 2021, the burden of prostate cancer largely increased with the socio-demographic index (SDI) at the regional level. The burden of prostate cancer in the MENA region rose markedly between 1990 and 2021. Further studies into the underlying causes of this increasing incidence are essential to devise effective strategies for the early detection and prevention of the disease.
Similar content being viewed by others
Introduction
Prostate cancer (PC) is a malignancy of the prostate tissue, characterised by a wide range of histopathological features, and is typically graded using the Gleason system1. The disease can be screened and diagnosed through multiple methods, such as digital rectal examination (DRE), ultrasonography, prostate-specific antigen (PSA) testing, magnetic resonance imaging (MRI), and tissue biopsy2,3. Although PC is a sex-specific malignancy, it is the fourth most commonly diagnosed cancer globally and is the eighth leading cause of cancer-related death when considering men and women combined4. According to GLOBOCAN estimates for 2020, PC represents 14.1% of all new cancer cases in males, is second only to lung cancer, and accounts for 6.8% of cancer-related deaths among males4.
The Global Burden of Disease (GBD) study in 2019 reported that, from 1990 to 2019, the global incidence of PC rose by approximately 169%, while deaths and disability-adjusted life years (DALYs) increased by 109% and 98%, respectively5. Importantly, as the incidence and DALYs of the disease rise, so does the economic burden. An American study reported that the 10-year treatment costs for PC range from US$46,000 in low-risk cases to nearly US$189,000 in high-risk patients6. The high incidence and mortality rates of PC make it a worldwide concern, especially as populations age.
It is well-established that age plays a significant role in the development of PC3. The probability of developing PC in individuals aged 70 is nearly nine times higher than in those aged 607. It is estimated that every man has a 12.5% chance of being diagnosed with PC in his lifetime7. Diet al.so plays a crucial role; as pro-inflammatory diets have been linked to a higher incidence of PC. Fatty acids and simple carbohydrates are among the contributing factors7. Conversely, lycopene, a compound found in tomatoes, has long been recognized as a protective agent against PC7. Obesity, smoking, alcohol intake, and certain autoimmune and inflammatory diseases, such as inflammatory bowel disease, Sjogren’s disease, and prostatitis, have also been identified as associated risk factors7,8. Moreover, genetic composition and family history of PC, being among the most important risk factors, have been thoroughly investigated9,10. Some genotypes and mutations, such as BRCA1 and BRCA2, have been associated with a heightened risk of developing PC11. It is also well-established that PC is more prevalent among individuals of African descent and results in higher mortality rates in Sub-Saharan Africa. This disparity may be influenced by healthcare inequalities, limited access to optimal treatments, and dietary factors7,8. This link to genetics and race necessitates further exploration of the underlying causes and epidemiological patterns of this disease.
In the Middle East and North Africa (MENA) region, PC has also imposed a significant burden. In 2019, PC had an age-standardised rate (ASR) of 23.7, 11.7, and 186.8 for incidence, deaths, and DALYs per 100,000 population, respectively12. Both the incidence and DALYs associated with PC increased from 1990 to 2019, while the death rate remained unchanged12. Furthermore, this region exhibited significant socio-demographic disparities alongside the disease burden, making MENA one of the most heterogeneous super-regions globally13. As previous data suggest, the mortality-to-incidence ratio (MIR) increases as the Human Development Index (HDI) and income levels decrease, leading to a significantly higher MIR in regions with lower HDI and income levels compared to MENA’s incidence rate14. Furthermore, the occurrence of coronavirus disease 2019 (COVID-19) has drastically affected the diagnosis, treatment, and registration of PC, particularly in low-to-middle income countries. This underscores the necessity for an updated assessment of the burden of PC in the post-COVID-19 era. In response to the growing burden of PC in the MENA region, this study aimed to deliver a current analysis of incidence, deaths, and DALYs from 1990 to 2021, utilizing data from the GBD 2021 study.
Methods
Overview
The Global Burden of Diseases, Injuries, and Risk Factors (GBD) project is an extensive initiative aimed at estimating the burden of 371 diseases and injuries across 204 countries and territories. The GBD 2021 study is the latest iteration, updating estimates for 2021 and extending previous estimates (1990–2021) using additional data sources and improved estimation methods15. An earlier article provides a comprehensive explanation of the methodology and main features of GBD 202115. The GBD 2021 study includes estimates for 34 cancer groups, including PC15. The data sources utilised for the cancer estimates were acquired from several sources, including vital registration (VR), verbal autopsy (VA), and cancer registry (CR) data. These sources can be accessed via the following link: Global Burden of Disease Study 2021 (GBD 2021) Sources Tool. Pathology-based and hospital-based cancer data were not included in the analysis. Redundant cancer registry data were excluded from either the final incidence data input or the mortality-to-incidence ratio (MIR) model input if a more comprehensive source was available for the same region. Registries providing national coverage were preferred over those with subnational coverage, except in cases where the GBD study provides subnational estimates. When preparing the incidence input, the most standardised sources were prioritised. For the MIR model input, only those sources reporting both incidence and mortality were included. Estimates were calculated with 95% uncertainty intervals (UIs). Rates were standardised to the GBD reference population and expressed per 100,000 individuals. Uncertainty intervals were propagated in accordance with previous GBD iterations. Each step in the estimation process consisted of 1,000 iterations, and the final estimate was the mean across these iterations. The UIs were established as the 25th and 975th values from a sequence of 1,000 numerically ordered iterations.
Estimation framework
PC included all cancers coded as C61-C61.9, D07.5, D29.1, and D40.0 in the International Classification of Diseases (ICD) 1015. Six sequelae were identified for PC, each with their own disability weights (DWs) (Table S1)15. The sources of data utilized to model the non-fatal and fatal burden of PC are available through the GBD Data Input Sources Tool15.
Mortality estimation
Cancer incidence data are generally more accessible than mortality data. Mortality-to-incidence ratios (MIR) were produced using linear-step mixed-effect models, focusing on locations with concurrent incidence and mortality data for the same year. The model was adjusted for sex, age, and the Healthcare Access and Quality (HAQ) Index, enabling the GBD to capture data that accurately represented the population and reflected the efficacy of healthcare systems. Following this, spatiotemporal Gaussian processes regression (ST-GPR) was employed to refine the estimates across both space and time. ST-GPR involves regression techniques designed to analyse heterogeneous and incomplete data by employing statistical smoothing across time, age, and location15. Data points were identified as outliers if they disproportionately influenced the model. For example, a data point was considered an outlier if it showed a single-year, single age group aberration in model predictions that deviated from the trend suggested by adjacent data points.
Initially, mortality estimates were calculated by multiplying the corresponding incidence estimates by the MIR. These estimated mortality figures, along with death data sourced from vital registration systems and verbal autopsies, were fed into the Cause of Death Ensemble model (CODEm)15. This method assesses the predictive validity of different models to find the best fit using the currently available data and covariates. The cause of death corrected (CoDCorrect) algorithm, which rescales the cause-specific mortalities, was utilised to align the overall number of single-cause deaths estimated for each sex-age-location-year group with the all-cause mortality estimates15. These mortality estimates were subsequently smoothed using a Bayesian noise-reduction algorithm to address instances of zero counts. This technique was also applied to the CoD data inputs. A comprehensive description of these methods is provided in a separate publication16.
Incidence, prevalence, and disability estimation
The final estimates were obtained by dividing the mortality estimates made with CODEm by the MIR. The 10-year prevalence of PC was estimated by modelling survival for each country using MIRs, categorising them into the five different sequalae (Table S1). Sequela-specific years lived with disability (YLDs) were determined by multiplying the DWs by the sequelae-specific prevalence. Additionally, surgery-related YLDs, resulting from prostatectomy-related impotence and incontinence, were modelled for PC, in addition to sequelae-specific YLDs (Table S1). To estimate disability due to PC surgery, the percentage of PC patients that underwent prostatectomy, obtained from hospital records, was used to provide input to the model in Disease Model-Bayesian meta-regression (DisMod-MR 2.1) to model the rate across all locations, stratified by age, sex, and year15. The years of life lost (YLLs) were modelled by multiplying the estimated number of deaths at each age by the standard life expectancy at that age. DALYs were estimated by adding the YLDs and YLLs together. The current research investigated the relationships that the SDI from each country had with the incidence, DALYs, and mortality attributed to PC using smoothing splines models15. The SDI is a comprehensive index that contains the lag-distributed per capita income, mean years of education for those aged more than 15 years, and fertility rates among women under 25 years of age. SDI ranges from 0 (lowest mean income and education; highest fertility) to 1 (highest mean income and education; lowest fertility)15. The figures for the age-standardised incidence, prevalence, and DALYs were created using R software, version 3.5.2.
Results
The middle East and North Africa region
In 2021, there were 55.9 thousand incident cases (95% UI: 39.2 to 67.9) of PC in the region, with an age-standardised incidence of 27.4 per 100,000 (95% UI: 19.3 to 33.4), which was 125.1% higher than in 1990 (95% UI: 84.6 to 178%) (Table 1 and Table S2). In the MENA region, prostate cancer caused more than 16.7 thousand deaths (95% UI: 11.8–20.2) in 2021, with an age-standardised mortality rate of 10.1 per 100,000 population (7.1 to 12.2), representing no statistically significant difference since 1990 (11.9% [95% UI: -9.3 to 42.5]) (Table 1 and Table S3). Similarly, the percentage change in the age-standardised rate (ASR) of DALYs due to prostate cancer did not statistically change from 1990 to 2021 (16.2% [95% UI: -4.2 to 43.7]), with 327.8 thousand DALYs (95% UI: 230.7 to 392.3) of prostate cancer in 2021, and an ASR of 173.6 (95% UI: 123.1 to 209.4) per 100,000 population (Table 1 and Table S4).
Country level
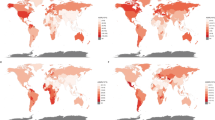
In 2021, the number of new prostate cancer cases in MENA countries ranged from 135 to 21.9 thousand, with age-standardised incidence rates ranging from 6.7 to 85.3 per 100,000. Qatar [85.3 (95% UI: 53.2 to 130.2)], Lebanon [63.6 (95% UI: 42.5 to 87)], and Bahrain [61.8 (95% UI: 42.5 to 85.5)] had the highest age-standardised incidence rates of prostate cancer in MENA. Conversely, in 2021 the lowest rates were observed in Algeria [6.7 (95% UI: 4.3 to 9.4)], Afghanistan [10.7 (95% UI: 7.0 to 14.9)], and Morocco [12.3 (95% UI: 6.4 to 17.6)]. Between 1990 and 2021, the countries with the largest increases in age-standardised prostate cancer incidence were Kuwait [233.3% (95% UI: 130 to 369.1)], Egypt [209.7% (95% UI: 76.2 to 388.5)], and Lebanon [157.5% (95% UI: 80.9 to 273.5)]. Conversely, Afghanistan [42.9% (95% UI: 4.1 to 104.4)], Palestine [56.3% (95% UI: 13.8 to 113.4)], and Yemen [68.2% (95% UI: 10.5 to 177.1)] had the lowest statistically significant changes in the age-standardised incidence (Table S2).
In 2021, the number of deaths attributed to prostate cancer in MENA ranged from 24 to 5,766, with the ASR ranging from 2.9 to 20.4 per 100,000. Qatar [20.4 (95% UI: 13.9 to 29.4)], Palestine [19.7 (95% UI: 15.1 to 28.1)], and Bahrain [19.7 (95% UI: 14.5 to 26.1)] had the highest age-standardised death rates in 2021. In contrast, Algeria [2.9 (95% UI: 1.7 to 4.0)], Oman [4.5 (95% UI: 2.9 to 6.4)], and Saudi Arabia [4.7 (95% UI: 3.1 to 8.9)] had the lowest ASRs in 2021. Furthermore, the age-standardised mortality rate of PC increased only in Kuwait [88.3% (95% UI: 42.6 to 141.4)] and Egypt [84.9% (95% UI: 8.2 to 194.6)] over the measurement period. The remaining MENA countries showed no statistically significant changes over that same period (Table S3).
The DALYs due to prostate cancer in 2021 ranged from 584 to 111,275, while the age-standardised DALYs were between 46.2 and 355 per 100,000 population. Qatar [355 (95% UI: 229.7 to 526.7)], Bahrain [331.4 (95% UI: 238.6 to 441.8)], and Palestine [318.2 (95% UI: 248 to 453.7)] had the highest age-standardised DALYs in 2021. Conversely, Algeria [46.2 (95% UI: 29.1 to 63.3)], Oman [80.9 (95% UI: 54.4 to 109.6)], and Saudi Arabia [87.2 (95% UI: 57.1 to 163.5)] had the lowest rates. Similar to changes of the age-standardised death rates across the measurement period, increases in the age-standardise DALYs were observed only in Kuwait [92.2% (95% UI: 43.0 to 154.6)] and Egypt [83.5% (95% UI: 8.1 to 182.1)]. Between 1990 and 2021, the other 19 countries showed no statistically significant changes in ASRs (Table S4).
Sex and age patterns
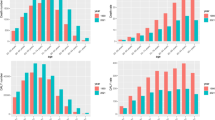
In 2021, the number of new prostate cancer cases in the region rose with age, peaking among those aged 65 to 69, before declining. The incidence rate per 100,000 population also climbed with age, but dropped after the 85–89 age group (Fig. 1A). In addition, the number of deaths rose with age, reaching its maximum in the 80–84 age group, and then declined. Death rates peaked in the 90–94 age group before declining with further age (Fig. 1B). The total DALYs of prostate cancer in 2021 were highest in the 70–74 age group, decreasing thereafter. Similar to the death rates, the DALY rates of prostate cancer peaked in the 90–94 age group, and then decreased with increasing age (Fig. 1C).
Number of incidence cases and rate (A), number of deaths and death rate (B) and the number of DALYs and DALY rate (C) of prostate cancer per 100,000 population in the Middle East and North Africa region, by age in 2021; Dotted and dashed lines indicate 95% upper and lower uncertainty intervals, respectively. DALY = disability-adjusted-life-years. (Generated from data available from http://ghdx.healthdata.org/gbd-results-tool).
In both 1990 and 2021, the ratios of age-standardised DALY rates in MENA compared to the global average were below 1 across all age groups, indicating that the overall burden of PC in MENA was lower than the worldwide average. In 2021, males aged 20–24, 30–39, and 70–74 years exhibited DALY rates that were close to the global rate (ratio of MENA/global YLD rate = 0.9). In 2021, DALY rates were higher across all age groups than in 1990. Age groups 25–29, 40–44, and 50–54 were the only exceptions to this pattern, as they retained the same rates as in 1990 (Fig. 2).
Ratio of the Middle East and North Africa region to the global prostate cancer DALY rate by age, 1990 and 2021. DALY = disability-adjusted-life-years. (Generated from data available from http://ghdx.healthdata.org/gbd-results-tool).
Linking the prostate cancer burden to Socio-demographic index (SDI)
From 1990 to 2021, there was generally a positive correlation between the age-standardised DALY rate per 100,000 population and the socio-demographic index (SDI) at the regional level. Overall, the regional age-standardised DALY rate closely matched the expected values across different SDI levels. At the national level, Bahrain, Lebanon, Libya, Palestine, Qatar, and Turkey exhibited a higher-than-expected burden, whereas Egypt, Iraq, Jordan, Kuwait, Morocco, Algeria, Tunisia, Oman, Sudan, and Saudi Arabia showed lower-than-expected burdens (Fig. 3).
Age-standardised DALY rates of prostate cancer for the 21 MENA countries by SDI, 1990–2021; Expected values based on the Socio-demographic Index and disease rates in all locations are shown as the black line. Each point shows the observed age-standardised DALY rate for each country during 1990–2019. DALY = disability-adjusted-life-years. SDI = Socio-demographic Index (Generated from data available from http://ghdx.healthdata.org/gbd-results-tool).
Discussion
According to the analyzed results, the age-standardised rate (ASR) of prostate cancer incidence in the MENA region surged by approximately 125.1% from 1990 to 2021, reaching an ASR of 27.4 per 100,000 in 2021. In contrast, a previous GBD study in MENA from 1990 to 2019 reported a 77.4% increase, with an ASR of 23.7 per 100,000 in 2019, indicating a dramatic change over three years12. Conversely, there was a 6% increase in the death rate from 1990 to 2019, which further rose to 11.9% in 202112. It should be noted that the ASR of death decreased from 11.7 per 100,000 population in 2019 to 10.1 in 2021. Furthermore, the ASR of DALYs in 2019 was 186.8 per 100,000 population, which decreased to 173.6 in 2021. However, the percentage change of DALYs increased from 6% in 2019 to 16.2% in 202112. Although the changes in death rates and DALYs were not statistically significant over the investigated period, the upward trend suggests that this insignificance should be interpreted with caution.
The significant rise in incidence rates may be linked to several factors, including early detection through screenings and increased life expectancy, as it is well-established that ageing is a recognised risk factor for PC16. The growing number of cases in developing countries within the MENA region can be attributed to improved access to healthcare and enhanced documentation and reporting of cases17. Furthermore, according to Schumacher et al., the MENA region has experienced the largest increase in life expectancy, along with a marked rise in vital registrations in recent years, both of which have contributed to a higher documented burden of chronic diseases, such as PC15.
Awareness of PC has greatly increased in recent years, resulting in wider access to PSA testing and medical screenings, which, in turn, have led to the identification of more cases. This enhanced healthcare seeking behaviour is largely driven by a general increase in awareness about prostate cancer, highlighting that improved understanding of its warning signs and the importance of routine examinations can further enhance case detection18. Furthermore, the increase in incidence rates in regions where PSA testing is not commonly practiced may indicate a shift towards a more westernised lifestyle, characterised by factors such as obesity, tobacco smoking, lack of exercise, and specific dietary habits17. The decrease in the ASRs of deaths and DALYs from 2019 to 2021 can be attributed to several factors. The overall management strategies for PC have been optimised in recent years, especially with the introduction of novel surgical methods, radiotherapy, and targeted therapies19,20. These treatments have been specifically designed for more advanced and metastatic PCs, potentially lowering death rates21. Furthermore, heightened general awareness of routine screening and examination among the regional population may have contributed to improved case discovery, thereby facilitating more efficient disease management. Additionally, there may be a time lag between the discovery of PC cases and mortality, as many cases are identified in the elderly population, where deaths frequently occur due to other causes. Moreover, the disability attributed to PC in the elderly is often obscured by the presence of other chronic diseases, potentially affecting the accuracy of the reported DALYs. It is also important to note that data variability, along with differences in registration and sampling of cross-sectional studies, might have affected the final results, potentially leading to an underestimated burden in 2021. On the other hand, the impact of Coronavirus Disease 2019 (COVID-19) on healthcare systems should not be overlooked. According to a study on the impact of COVID-19 in MENA, the pandemic affected the quality of care for chronic diseases, especially cancer patients22. With the implementation of quarantines and the focus of healthcare providers on COVID-19, many cancer patients were neglected22,23. A study conducted in England revealed a 33% decrease in the number of newly diagnosed PC cases, with most of these patients being in the advanced stages of the disease. Additionally, treatments and diagnostic procedures were limited to reduce the risk of COVID-19 transmission24. Given that PC most frequently occurs in older individuals, the implementation of quarantines may have had a confounding effect on the disease’s burden, potentially leading to undetected cases and unrecorded deaths between 2019 and 2021. According to Wang et al., the MENA region experienced excess mortality of 1.7 million due to COVID-19 in 2020 and 2021, with particularly severe impacts on vulnerable populations, such as the elderly25. As a result, it can be concluded that the increasing trend in PC’s incidence, prevalence, and mortality was significantly affected by the pandemic, suggesting that the regional burden of PC might be significantly higher than current estimates indicate23.
As expected, and consistent with previous studies, the highest incidence rate in the MENA region in 2021 was observed among the 85–89 age group, while the largest DALY and mortality rates were observed in the 90–94 age group12. This highlights the need for earlier screening tests to detect early-stage cancers and to enhance end-of-life care for older adults. Focusing on these areas could help reduce DALYs, as many end-stage and inoperable patients succumb to causes other than PC26.
At the national level, Kuwait and Egypt were the only countries that showed a significant increase in their respective ASR of incidence, deaths, and DALYs from 1990 to 2021. This significant change was also observed in the GBD 2019 study12. Analysing the risk factors associated with PC reveals that these countries have higher tobacco usage and a lower age of smoking onset compared to other MENA countries27,28. Furthermore, obesity is another prevalent risk factor among their populations29. Beyond these known risk factors, the reasons behind the significant increase in the burden of PC in Kuwait and Egypt require more comprehensive studies to be fully understood.
Our analysis identified significant variations in the burden of prostate cancer across MENA countries. This heterogeneity in findings, if not considered accurate, may be attributed to wide variations in healthcare systems12. Counties in the MENA region vary dramatically in their economic and social status, as reported in the results. The linear correlation between the DALYs of PC and the SDI underscores the importance of economic and healthcare factors in the burden of PC. Since PC is more prevalent in older age groups, countries with higher life expectancy are likely to experience higher PC incidence, which can lead to increased deaths and DALYs due to the disease2,30.
Countries such as Bahrain, Qatar, and the United Arab Emirates have experienced significant positive changes in their economies and socio-demographic factors, which, in turn, have impacted their life expectancy over recent decades31,32. This change may have contributed to their higher-than-expected PC burden. In contrast, countries such as Afghanistan, which are on the lower end of the SDI spectrum, exhibit a lower burden of PC. This low burden might be due to the inefficiency of healthcare systems in diagnosing PC cases, which can be attributed to economic imbalances and ongoing conflict33.
The MENA region experienced a lower overall burden of PC compared to the global average. This observation may be attributed to the generally lower life expectancy and the younger population in the MENA region compared to higher-income regions34. Conversely, more efficient healthcare systems in higher-income regions are often more successful in screening and managing the disease, resulting in a higher rate of newly diagnosed patients35.
Finally, PC can be diagnosed and treated through many methods36. There have been many novel markers and routes of screening suggested in modern medicine, which might have the potential to be implemented worldwide36,37. Routine screenings via DRE and PSA can be utilized in the patients with a positive family history, and specific screening protocols can be developed according to the national and regional burden of this disease. Optimizing screening methods to a region-specific level can both lower healthcare costs and control the DALYs and death rates of PC38,39. As populations age, the incidence of cancers, particularly PC, tends to increase. The MENA region, despite having a relatively younger population compared to global standards, is also susceptible to this trend. This necessitates that healthcare providers, policymakers, and healthcare systems develop and implement up-to-date preventive and treatment strategies tailored to their national and regional needs.
Limitations
Although this study offers the most recent and complete analysis of the burden of prostate cancer (PC) in the MENA region, it does have several limitations. The available data in the MENA region are highly heterogeneous, making it challenging to interpret the results at a regional level. Additionally, this study focused on PC across its entire spectrum due to the scarcity of available data, which did not allow for the division of PC into low-to-high risk groups or the inclusion of metastatic cases. Future studies are needed to further investigate this disease in multiple subgroups, while also accounting for different histopathological aspects.
Another limitation is the reliability of the available data. The countries in MENA implement different policies for registering and providing their data, which may result in missed recordings. Some data sources and registries in the MENA region are classified and not accessible to the public, which may lead to incomplete estimations for this region. Furthermore, inconsistency in healthcare reporting systems, along with differences in healthcare access and quality of care, can introduce biases to the available data. Although this issue has been addressed as much as possible by using country-level covariates in the modelling process, it remains a concern.
In addition, cancer registry data are susceptible to various biases. A substantial number of poorly categorised cancer cases might need reallocation to other cancer types, which can introduce bias. Furthermore, changes in coding systems may lead to inaccuracies in disease estimation, while misidentifying metastatic sites as primary cancers can result in overestimated cancer incidence. Given that cancer registries are largely situated in urban areas, their representativeness of the broader non-urban population is often limited. The quality of the vital registration system also affects the accuracy of mortality data reported by some cancer registries, with incomplete or low-quality systems potentially resulting in biased, lower mortality-to-incidence ratios. Therefore, these findings should be interpreted with caution, considering these potential data inconsistencies.
Conclusions
From 1990 to 2021, the burden of prostate cancer in the MENA region has risen substantially, underscoring its importance as a public health issue. The notable disparities in incidence rates and age-specific trends suggest that focused screening and awareness campaigns could be helpful in mitigating this issue. Future research should focus on investigating the underlying reasons for the rising incidence rates and develop robust strategies for prevention and early detection.
Data availability
The data used for these analyses are all publicly available at http://ghdx.healthdata.org/gbd-results-tool.
References
DeMarzo, A. M., Nelson, W. G., Isaacs, W. B. & Epstein, J. I. Pathological and molecular aspects of prostate cancer. Lancet 361 (9361), 955–964 (2003).
Litwin, M. S. & Tan, H-J. The diagnosis and treatment of prostate Cancer: A review. JAMA 317 (24), 2532–2542 (2017).
Sekhoacha, M. et al. Prostate Cancer Review: Genetics, diagnosis, treatment options, and alternative approaches. Molecules 27 (17), 5730 (2022).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 71(3), 209–9. (2021).
Zhang, W. et al. Global burden of prostate cancer and association with socioeconomic status, 1990–2019: A systematic analysis from the global burden of disease study. J. Epidemiol. Global Health. 13 (3), 407–421 (2023).
Gustavsen, G., Gullet, L., Cole, D., Lewine, N. & Bishoff, J. T. Economic burden of illness associated with localized prostate cancer in the United States. Future Oncol. 16 (1), 4265–4277 (2019).
Bergengren, O. et al. 2022 update on prostate Cancer epidemiology and risk Factors—A systematic review. Eur. Urol. 84 (2), 191–206 (2023).
Barsouk, A. et al. Epidemiology, staging and management of prostate Cancer. Med. Sci. 8 (3), 28 (2020).
Cui, H. et al. Risk factors for prostate cancer: An umbrella review of prospective observational studies and mendelian randomization analyses. PLoS Med. 21 (3), e1004362 (2024).
Darst, B. F. et al. Combined effect of a polygenic risk score and rare genetic variants on prostate Cancer risk. Eur. Urol. 80 (2), 134–138 (2021).
Trapani, D., Siqueira, M., Sengar, M. & Lombe, D. C. Prostate cancer: Burden, Epidemiology and Priority Interventions, p. 112–117 (Routledge, 2023).
Abbasi-Kangevari, M. et al. The burden of prostate cancer in North Africa and Middle East, 1990–2019: Findings from the global burden of disease study. Front. Oncol. ;12. (2022).
Luo, L-S. et al. Spatial and temporal patterns of prostate cancer burden and their association with Socio-Demographic Index in Asia, 1990–2019. Prostate 82 (2), 193–202 (2022).
Kearney, G. et al. Burden of prostate cancer in the Middle East: A comparative analysis based on global cancer observatory data. Cancer Med. 12 (23), 21419–21425 (2023).
Schumacher, A. E., Kyu, H. H., Aali, A., Abbafati, C., Abbas, J. & Abbasgholizadeh, R., et al. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. The Lancet. 2024;403(10440), 1989–2056.
Global incidence. Prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the global burden of Disease Study 2021. Lancet 403 (10440), 2133–2161 (2024).
Abbasi-Kangevari, M. et al. The burden of prostate cancer in North Africa and Middle East, 1990–2019: Findings from the global burden of disease study. Front. Oncol. 12, 961086 (2022).
Sayan, M. et al. Prostate Cancer awareness in the Middle East: A cross-sectional International Study. JCO Global Oncol. (10), e2400171. (2024).
He, M. et al. Unleashing novel horizons in advanced prostate cancer treatment: investigating the potential of prostate specific membrane antigen-targeted nanomedicine-based combination therapy. Front. Immunol. 14. (2023).
Achard, V., Panje, C. M., Engeler, D., Zilli, T. & Putora, P. M. Localized and locally advanced prostate Cancer: Treat. Opt. Oncology 99 (7), 413–421 (2021).
Swami, U., McFarland, T. R., Nussenzveig, R. & Agarwal, N. Advanced prostate Cancer: Treatment advances and future directions. Trends Cancer. 6 (8), 702–715 (2020).
Al-Hussaini, M., Al-Ani, A., Hammouri, M., Al-Huneidy, L. & Mansour, A. Investigating the impact of COVID-19 on patients with cancer from areas of conflict within the MENA region treated at King Hussein Cancer Center. Front. Oncol. , 13. (2023).
Sayan, M. et al. Prostate cancer presentation and management in the Middle East. BMC Urol. 24 (1), 35 (2024).
Nossiter, J. et al. Impact of the COVID-19 pandemic on the diagnosis and treatment of men with prostate cancer. BJU Int. 130 (2), 262–270 (2022).
Wang H, Paulson KR, Pease SA, Watson S, Comfort H, Zheng P, et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–21. The Lancet. 399(10334), 1513-36 (2022).
Bjørnelv, G., Hagen, T. P., Forma, L. & Aas, E. Care pathways at end-of-life for cancer decedents: Registry based analyses of the living situation, healthcare utilization and costs for all cancer decedents in Norway in 2009–2013 during their last 6 months of life. BMC Health Serv. Res. 22 (1), 1221 (2022).
Nasser, A. M. A., Geng, Y. & Al-Wesabi, S. A. The prevalence of smoking (cigarette and waterpipe) among University students in some Arab countries: A systematic review. Asian Pac. J. cancer Prevent. APJCP. 21 (3), 583–591 (2020).
Alanazi, A. M. et al. The associations between cigarette smoking behavior and the use of heated tobacco products among Arab cigarette smokers: Findings from Saudi Arabia, Egypt, Kuwait, and Yemen. Journal of Ethnicity in Substance Abuse 1–14.
Nikoloski, Z. Obesity in Middle East. Metabolic Syndrome: A Comprehensive Textbook, p. 65–80 (Springer, 2024).
Huynh-Le, M-P. et al. Age dependence of modern clinical risk groups for localized prostate cancer—A population-based study. Cancer 126 (8), 1691–1699 (2020).
Wirayuda, A. A. B., Al-Mahrezi, A. & Chan, M. F. Factors impacting life expectancy in Bahrain: Evidence from 1971 to 2020 data. Int. J. Soc. Determinants Health Health Serv. 53 (1), 74–84 (2023).
Wirayuda, A. A. B. et al. Unlocking the secrets of longevity: Exploring the impact of socioeconomic factors and health resources on life expectancy in Oman and Qatar. INQUIRY J. Health Care Organ. Provis. Financ. 60, 00469580231212224. (2023).
Hameed, M. A., Rahman, M. M. & Khanam, R. The health consequences of civil wars: Evidence from Afghanistan. BMC Public. Health. 23 (1), 154 (2023).
Balkhi, B., Alshayban, D. & Alotaibi, N. M. Impact of healthcare expenditures on healthcare outcomes in the Middle East and North Africa (MENA) region: A cross-country comparison, 1995–2015. Front. Public. Health 8. (2021).
Jain, M. A., Leslie, S. W. & Sapra, A. Prostate cancer Screening (StatPearls Publishing, 2023).
Ilic, D., Neuberger, M. M., Djulbegovic, M. & Dahm, P. Screening for prostate cancer. Cochrane Database Syst. Rev. (1). (2013).
Ried, K., Tamanna, T., Matthews, S., Eng, P. & Sali, A. New screening test improves detection of prostate cancer using circulating tumor cells and prostate-specific markers. Front. Oncol. 10, 582 (2020).
Washington, S. L. et al. Regional variation in active surveillance for low-risk prostate cancer in the US. JAMA Netw. open. 3 (12), e2031349–e (2020).
Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 79 (2), 243–262 (2021).
Acknowledgements
We would like to thank the Institute for Health Metrics and Evaluation staff and its collaborators who prepared these publicly available data. We would also like to thank the Clinical Research Development Unit of Tabriz Valiasr Hospital, Tabriz University of Medical Sciences, Tabriz, Iran for their assistance in this research.
Funding
The Bill and Melinda Gates Foundation, who were not involved in any way in the preparation of this manuscript, funded the GBD study. The Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No. 43004455) also supported the present report.
Author information
Authors and Affiliations
Contributions
SS and AAK designed the study. SS analysed the data and performed the statistical analyses. AS, AF, MJMS and AAK drafted the initial manuscript. All authors reviewed the drafted manuscript for critical content and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval
The present study was reviewed and approved by Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethics code: IR.SBMU.RETECH.REC.1402.046). All methods were performed in accordance with the national guidelines and regulations.
Author note
This study is based on publicly available data and solely reflects the opinions of its authors and not that of the Institute for Health Metrics and Evaluation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Safiri, S., Shamekh, A., Hassanzadeh, K. et al. The burden of prostate cancer in the North Africa and Middle East Region from 1990 to 2021. Sci Rep 15, 1853 (2025). https://doi.org/10.1038/s41598-024-83840-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83840-3
Keywords
This article is cited by
-
Global, regional, and national burden and trends of prostate cancer in elderly from 1990 to 2021: results from global burden of disease 2021
World Journal of Surgical Oncology (2025)
-
Severe and enduring prostate cancer burden attributable to smoking among old men amid global decline and socioeconomic disparities
Scientific Reports (2025)