Abstract
The present study was designed to highlight the ameliorative role of iron nanoparticles (FeNPs) against drought stress in spinach (Spinacia oleracea L.) plants. A pot experiment was performed in two-way completely randomize design with three replicates. For drought stress three levels were used by maintaining field capacity of the soil. This included control (100% field capacity), moderate drought stress (D1; 50% field capacity) and severe drought stress (D2; 25% field capacity). FeNPs synthesized by green method using rice straw were applied along with precursor FeCl3, used as Fe source for the synthesis of FeNPs, through foliar spray (40 mg L− 1 for both). Growth parameters, efficiency of photosynthetic machinery, gas exchange attributes, total soluble proteins, and inorganic ions (Ca2+, K+ & Fe2+) were significantly reduced at both D1 and D2 stress levels, compared to control plants. Fe supplements in the form of FeCl3 and FeNPs improved these attributes in both control and drought conditions. Malondialdehyde, H2O2, relative membrane permeability (stress indicators) and the activities of antioxidants were increased in response to drought stress. Fe supplements further improved the antioxidant defense activities and efficiently lowered the effects of stress indicators. These effects of FeCl3 and FeNPs resulted in improved growth of S. oleracea plants in control and drought conditions. Results showed that FeNPs had more prominent effects on growth of S. oleracea plants compared to FeCl3. These findings suggest that FeNPs could be a helpful tool for lessening the harmful consequences of drought stress and this can be used for abiotic stress alleviation in other crops as well.
Similar content being viewed by others
Introduction
Food security is now seriously threatened by global warming, which has also made economic growth extremely difficult in many nations1. The impact of global warming has a negative impact on crop development and yield. These modifications cause an increase in temperature, flooding, salinity, and other abiotic pressures that have direct effects on the existence of flora and animals around the world2. Drought causes billions of dollars losses in agricultural yield every year. Consequently, reduced agricultural output is a major concern. Crop that can endure drought have been quite slow to evolve, and there are still few economically viable strong drought-tolerant plants3.
Through developing proteins, and metabolites, enzymes, and by modifying gene expression, plants can adjust to stressful environments4. Plants prevent water loss by closing their stomata, which limits transpiration and carbon dioxide intake for photosynthesis. Plants may change their root development patterns for the purpose of checking for accessible water in the deeper layers of soil, increasing their ability to access moisture. Plants store osmolytes such as proline and carbohydrates to maintain cellular water potential and osmotic balance, hence preventing cellular dehydration5. Optimal water availability is a key element that enhances plant development and growth. On the other hand, plants exposed to dry environments show slower development6. Water deficit reduces the quality of morphological, physiological, and biochemical features, hinders crop development and productivity7. Drought causes massive damage and poses a severe threat to the ecosystem all over the planet.
When there is insufficient water for plants, they must give preference to essential physiological processes such water intake, development, and overgrowth. Drought-stressed plants therefore frequently exhibit decreased photosynthetic efficiency, growth, and yield8. Drought also caused oxidative stress due to overproduction of reactive oxygen species (ROS). This damaged biomolecules and lipid membranes, which makes them hazardous to plant cells9. Water deficit causes leaf degradation, closure of stomata and disintegration of leaf chlorophyll. These effects diminish the plant’s capacity to absorb light, and the interruption of electron mobility reduces photosynthesis and hinders the generation and the development of energy sources8. Drought stress reduces plant development due to impairments in cell elongation, loss of the turgor, oxidative rupture, and metabolic disbalance10.
Plants have developed antioxidant defense mechanisms to withstand drought stress and guard against oxidative stress. Certain plants store osmolytes, like proline, glycine betaine, and soluble sugars, to defend themselves and lessen the effects of drought-prone stress. According to recent research, certain phytohormones like brassinolides and volatile chemicals like terpenes, which are produced in plant organs, improve the plant’s defensive mechanism to lessen the impact of water deficit on crop11. Despite, plants have evolved various strategies to combat drought stress. These are not enough to overcome losses in crop productivity.
Nanotechnology is an important part of the present agricultural technological revolution, allowing for effective, productive, and sustainable agriculture systems whereas improving food security12. Nanotechnology, in the form of nanoparticles (NPs), has provided the finest insight on plant nutrition. Exogenous distribution of nano-fertilizers has proven to be effective since they offer nourishment to crops in a steady and specified fashion as opposed to conservative fertilization13. According to recent research on plants, nanoparticles participate in several biochemical and physiological processes that impact the development of plants in addition to the way they react to environmental stress14. Nanoparticles exhibit tiny size (less than 100 nm in at least one dimension), which result in enormous surfaces and surface charges, gives them additional characteristics. Thus, NPs are more reactive than comparable substances at bulk scale15. Nanoparticles provide vital nutrients to plants at the nanoscale, increasing plant productivity without harming ecosystems16. Nanoparticles have been proven to be particularly effective in increasing water use efficiency, reducing plant diseases, and resisting various abiotic stressors17. Nanomaterials can be manufactured using a various process, comprising sol-gel, chemical reduction, co-precipitation, hydrothermal synthesis, and so on. The chemicals used in these processes are deemed detrimental to the environment. Thus, owing to its convenience of use, affordability, and environmental friendliness, green synthesis of nanomaterials has been growing increasingly important in recent years18.
Application of Iron nanoparticle (FeNPs) could be an efficient strategy in enhancing iron uptake by plant roots and increasing their stability under severe drought3. The impacts of iron in reduction of stress caused by drought are associated to its capacity to reduce oxidative stress and enhanced activity of photosynthesis, and absorption of water and mineral nutrients19. FeNPs are significantly smaller than conventional iron oxide or iron molecules. They may create more associations with other molecules, which would enhance the quantity of iron that is accessible to plant organs20. Based on reports, the use of Fe3O2-NPs stimulates plant growth and improves adaptation to drought stress21. Use of FeNPs by soil application (100 mg Kg− 1) augmented growth and physiology of wheat plants in both control and drought stress22. Similarly, another study reported that up to 10 µM of FeNPs solution made in half strength Hoagland’s solution increased chlorophyll content and fluorescence in grape plants subjected to polyethylene glycol (PEG) induced drought stress. It also stimulated antioxidant protection by increasing activities of antioxidative enzymes and reducing the production of malondialdehyde and H2O223. Sreelakshmi et al.23 reported that green synthesized FeNPs from marine algae when applied to drought stressed S. italica plants, FeNPs served as nano nutrient and alleviated drought stress. Seedling treated with FeNPs had increased levels of chlorophylls and soluble sugar content. This suggested that enhanced iron uptake was used for the synthesis of photoassimilates which were used to overcome drought stress. Noor et al.24 investigated that use of FeNPs synthesized from ginger and cumin seeds extract to enhance growth and drought tolerance of wheat plants. They found that among all tested concentrations 0.6 mM of ginger FeNPs and 1.2 mM of cumin seeds FeNPs showed more prominent results and effectively enhanced wheat germination, biomass and drought tolerance. It is also observed that seed priming with 10 mg L− 1 of FeNPs improved plant height, total chlorophylls, anatomical structures and nutrient distribution in S. bicolor subjected to drought conditions. It also reduced oxidative damage by reducing ROS production, biomolecule degradation and inducing osmolytes accumulation19.
Spinach (Spinacia oleracea L.) is the well-known leafy vegetable plant with lowest growth cycle and annual plant in the Chenopodiaceae family. Because it contains essential minerals and vitamins, S. oleracea has a high nutritional value. Spinach is an excellent provider of calcium, iron, potassium, vitamin C, phosphorus, and salt among other minerals. Several bioactive chemicals identified in S. oleracea have an affinity to anti-obesity, diabetic, hypolipidemic, and cancer therapy qualities8. As the time progressed on, climatic changes have reduced water available for irrigation during crop cultivation, leading to the emergence of water deficit. It is commonly known that crops in severe stress can lose up to 50% of their yield each year. Since S. oleracea has a high-water content, drought stress can seriously harm it. It can also increase hydrogen peroxide and malondialdehyde while negatively affecting growth, nutrient uptake, stomatal activity, protein, biochemical contents, and saturated conductivity of water25.
This study was aimed to investigate the functional, structural and metabolic characteristics and yield of S. oleracea plants exposed to drought stress; to evaluate the potential of FeNPs in mitigation of drought stress; to investigate the potential use of rice straw as a renewable source for FeNPs synthesis, hence promoting environment friendly approach for synthesis of NPs. This study explored nanotechnology in agricultural systems and the potential influence of FeNPs in mitigating the drought stress. The study will contribute to the potential use of green synthesized FeNPs to mitigate the drought and other abiotic stresses in crop plants which is essential for improving food security in water deficit region.
Materials and methods
Green synthesis of Fe-NPs
The sample of rice straw (RS) was taken from the nearby field and washed 5 to 6 times with distilled water. After washing, the RS sample was dried in oven at 70 °C until completely dry. The RS dried sample was crushed to make a powder. 20 g of this powder was added in a beaker containing 200 mL of deionized water. This mixture was heated (90 °C) with continuous stirring using magnetic stirrer hot plate till the mixture’s volume reached half of it i.e. 100 mL. After cooling the resultant extract was filtered in a separate beaker. Ferric chloride hexahydrate (FeCl3.6H2O) was used as precursor for the synthesis of the FeNPs. 100 mL of rice straw extract was added dropwise in 0.1 M FeCl3.6H2O solution (2:1 V/V). This mixture was stirred continuously for 30 min, that resulted in yellowish solution turning brown, indicating the formation of FeNPs. This solution was incubated for an hour that resulted in precipitate formation, which were filtered through Whatman filter paper. The collected precipitates were then washed with ethanol and water several times. After washing, these precipitates were dried then dry powder was grounded to prepare the fine powder and subjected to characterization investigations (see Fig. 1)26.
Characterization of Fe-NPs
The Ultraviolet-Visible absorbance spectroscopy of the sample was performed with a range of 200–800 nm for confirmation of FeNPs. Its structural and surface morphological characteristics were assessed using scanning electron microscopy. To evaluate elemental composition of FeNPs energy-dispersive X-Ray (EDX) spectroscopy was used. The sample was measured in Fourier-transform infrared spectrophotometer ranging from 500 to 4000 cm for assessment of functional groups and chemical bonds. XRD patterns were recorded by an x-ray diffractometer for assessment of crystalline structure of the sample.
Experimental layout and research design
This experiment was conducted at the botanical garden, University of Education, Lahore. A two-way completely randomized design experiment was performed with three replicates. Plants were grown in plastic pots using loamy soil (Fig. 2A-C). Physicochemical properties of soil are listed in Table 1. The soil was sieved to remove any debris material and then filled in pots (5 Kg). Certified seeds of Desi Palak (Spinacia oleracea L.) were procured from Punjab Seeds Corporation, Lahore. Healthy and same size seeds were taken and surface-sterilized prior to sowing. For this, seeds were treated using a 5% sodium hypochlorite solution, then washed several times with 95% ethanol. After that seeds were rinsed three times in deionized water. Finally, 10 seeds were sown at equal distance in each pot. After germination, 5 seedlings were maintained in each pot. Two weeks after germination S. oleracea plants were subjected to drought conditions and subsequent foliar spray of FeCl3 and FeNPs (40 mg L− 1 for both). The concentration for FeNPs used in this experiment was selected based on previous studies on FeNPs27,28. For drought three levels were used based on the field capacity of the soil. This included control (100% field capacity), moderate drought stress (D1; 50% field capacity) and severe drought stress (D2; 25% field capacity). If the control plants require 100 mL of water to maintain 100% field capacity, then D1 and D2 stressed plants were given 50 and 25 mL of water only. It took 14 days to acquire desirable drought conditions. For assessment of field capacity of the soil used in this experiment, first we dried the soil in oven at 90 °C. Following that noted the initial dry weight (DW) and then final saturated weight (SW) of the soil sample. To find the field capacity the following formula was used.
Comparative analysis of S. oleracea plants grown under the effects of various treatments. (A) from right to left; control, D1 (50% field capacity) and D2 (25% field capacity), (B) from right to left; FeNPs (40 mg L− 1), D1 + FeNPs, D2 + FeNPs, (C) from right to left; FeCl3 (40 mg L− 1), D1 + FeCl3, D2 + FeCl3.
On the onset of desired drought levels S. oleracea plants were treated with foliar spray of FeCl3 and FeNPs (Table 2). An equal amount of distilled water was sprayed on control plants. Foliar spray was done 3 times in total during the whole experiment at one week interval. Spray was done using manual hand-sprayer in a way that whole plants get fully wet. Drought stressed pots were covered before foliar spray to prevent moisturization of soil by sprayed liquid. The total duration of the experiment was 2 months. On the completion of the experiment plants from each treatment were uprooted. The roots and shoots were separated and washed to remove soil and wrapped into blotting paper. After harvesting, growth indices and morphological characteristics were measured followed by subsequent physiological and biochemical analyses.
Determination of physiological attributes
Estimation of chlorophyll and carotenoid content
Arnon’s method was used for the identification of chlorophyll a, chlorophyll b, and total chlorophyll level in S. oleraceae leaves29. Fresh and healthy leaf samples were obtained from each pot. Leaves weighing 0.5 g were crushed with pestle and mortar. Crushing was performed in a mortar by adding 10 mL of 80% acetone solution. After that mixture was filtered through filter paper and then stored in the fridge at 4 °C for 24 h. Using UV/VIS spectrophotometer (METASH MODEL) absorbance was measured at 3 wavelengths of 480, 645 and 663 nm after 24 h.
For chlorophyll a and b determination the below formulas were used
Where V is the Volume, W is the weight and OD is Optical density.
Carotenoid contents were calculated using below formula as described by Lichtenthaler30.
SPAD determination
The chlorophyll content of S. oleracea leaf plant was determined by using a meter (SPAD) and reading of chlorophyll were given SPAD value. The measurement was performed on mature leaves. Chlorophyll measurements were taken at various points on the leaves, midway between the central vein and the leaf margin. By taking the average of these numbers the SPAD values of each leaf were determined.
Determination of photosynthetic efficiency and chlorophyll fluorescence
An OS30p+ Chlorophyll fluorometer (Opti-sciences, Inc. Hudson, NH 03051, USA) was used to measure the efficiency of photosynthetic machinery and chlorophyll fluorescence. The highest photochemical effectiveness in photosystem II (Fv/Fm) and OJIP parameters were evaluated using fluorometer after 15 min of dark adaptation.
Gas exchange attributes
An Infra-Red Gas Analyzer (IRGA) LCpro SD (ADC Bio scientific Ltd Hoddesdons, UK) was used for the determination of the Gas exchange attributes. Measurements were taken during 10:00–12:00 am. Fully matured and young leaf was placed in an airtight leaf chamber, after 3 min of acclimatation to chamber climate the values for the photosynthetic rate, transpiration rate, intercellular carbon dioxide and stomatal conductance were measured. The reference values for leaf chamber climate were as follows: ambient CO2 (Cref) at 420–460 µmol mol− 1, photosynthetically active radiation (PAR) or Qleaf at 915 µmol m− 2 s− 1, leaf chamber temperature (Tch) at 24–27 °C, leaf temperature at 25–27 °C, molar gas flow rate of the leaf chamber (U) at 201 µmol s− 1, ambient pressure (P) at 999 kPa, and leaf area at 6.25 cm2.
Estimation of oxidative stress markers
Determination of relative membrane permeability
The RMP determination was done as described by Zhang et al.31. From each replicate, uniformly sized leaves as well as mature leaves were taken. Chopped leaves were put into tubes with 20 milliliters of distilled water. After vertexing for 5 s the mixture, To determine the electrical conductivity (EC0), an EC meter was used. The tubes were stored in a refrigerator at 4 °C and EC1 was noted after 24 h. To determine EC2 values, the same samples were autoclaved for 20 min at 120 °C. This formula was used to get RMP% .
Hydrogen peroxide estimation
It was described employing the previously indicated technique by Velikova et al.32. Healthy leaves weighing (0.5 g) were taken from each replicate and were collected homogenized uniformized with 5 mL of trichloroacetic acid (0.1% ) in a pestle and mortar. The mixture was then transferred into conical tubes and centrifuged at 12,000 g for 15 min and 4 °C. The supernatant was isolated from the residues and 0.5 mL of the supernatant was shifted into a test tube. The contents of the test tubes were 0.5 mL Potassium phosphate buffer (pH 7), 1 mL potassium iodide. After vertexing the mixture absorbance was measured at 390 nm with a UV/VIS spectrophotometer.
Malondialdehyde
This technique determined by Cakmak et al.33 with little modification was used to find the lipid peroxidation. Fresh leaves having weight of 0.5 g were plucked from every replicate for sample preparation. Leaves were mixed in 1.0% trichloroacetic acid (3 mL) at 4 °C. Following the homogenate’s transfer to conical tubes, it was centrifuged for 15 min at 20,000 g. After centrifugation, 0.5 mL of filtrate was transferred into test tubes along with 3 mL of a solution of 0.5% thio-barbituric acid prepared in 20% trichloroacetic acid. The samples were kept in conical flask then heated at 95 oC for 50 min in a water bath. The reaction was stopped immediately by chilling in an ice water bath. Following another 10 min centrifugation at 10,000 g for all samples. With the help of UV/VIS spectrophotometer, the measurement was taken at absorbance of 532 and 600 nm. MDA level (nmol) was calculated for every sample using the following formula.
Estimation of biochemical attributes
Total soluble protein
The content of Protein was calculated by using the method of Bradford34. For extract preparation fresh leaf samples from each pot were taken, weighed (0.5 g), and mixed with 10 mL of 50 mM pre-cooled phosphate buffer (pH 7.8). The obtained extracts were centrifuged at 6000 g for 20 min at 4 °C. Supernatant was removed from residue and placed in a deep freezer. For the preparation of Bradford solution by dissolving 0.1 g of Coomassie Brilliant Blue in 50 mL of 95% ethanol. In the previous mixture, 100 mL of phosphoric acid (85% ) was mixed with distilled water to bring the volume up to 1 L. In 0.1 mL stored leaf extracts, Bradford solution (5 mL) was added then the mixture was vortexed. At defined wavelength of 595 nm Optical density was measured using UV/VIS spectrophotometer.
Determination leaf proline
Proline was calculated using the Bates et al.35 technique. In the procedure, 0.5 g of leaves was homogenized in 3% of 10 mL sulfuric acid. By using Whatman filter paper the homogenate was filtered. After mixing 1.25 g of ninhydrin into Glacial acetic acid (30 mL) and O-phosphoric acid (6 M, 20 mL), acid ninhydrin reagent was made. In a glass tube, 2 mL of homogenate filtrate was dissolved into galacial acetic acid (2 mL) and ninhydrin acid, then sample solution was incubated at 100 °C for 60 °C in oven. After this reaction mixture was kept in ice bath. The sample mixture was centrifuged where 2 mL of supernatant was collected. Further, 4 mL of toluene is added in reaction mixture for 1–2 min while passing a constant stream of air. From the upper aqueous phase, the chromophore was withdrawn. Absorbance was obtained at 520 nm with the help of UV spectrophotometer by using blank (toluene).
Determination of phenolics
The samples of powder dried leaf weighing 0.5 g were placed in centrifuge tubes. The samples were dissolved in 10 mL of 80% aqueous acetone for 1 min then mixture was centrifuged at 4000 g for 15 min at 4 °C. The dried solid residue was taken, and the supernatant was removed. Following that, 10 mL of methanol was mixed with the dried solid residue. The Folin-Ciocalteu procedure was used to determine the total amount of phenolics in given samples. 1 mL Folin Ciocalteu reagent and 0.8 mL sodium carbonate (7.5% ) and 2 mL of prepared samples from each replicate were collected in the tubes, then vigorously mixed and left for half an hour. Using UV/VIS spectrophotometer absorbances at 765 nm were obtained36.
Determination of antioxidant enzymes activities
Estimation catalase and peroxidase activity
Based on studies by Chance et al.37 with slight adjustments, the activities of Catalase (CAT, EC 1.11.1.6) and Peroxidase (POD, EC 1.11.1.7) were evaluated. There was a catalase reaction solution created. The solution of Catalase reaction was prepared by 1.9 mL of 5.9 mM H2O2, 1 mL of 50 mM phosphate buffer (pH 7.0) and 0.1 mL of enzyme extract (as indicated in total soluble protein) total 3 mL. Absorbance was examined at wavelength of 240 nm for every 30 s in the time interval of 120 s using UV/VIS spectrophotometer.
Likewise, for determination of POD activity, a 2 mL combination comprising of reaction solution was prepared by 0.6 mL of 20 mM guaiacol, 7 mL of 50 mM phosphate buffer (pH 5.0),0.6 mL of 40 mM H2O2 and 0.1 mL of Enzyme extract (discussed in total soluble protein). Using UV/VIS spectrophotometer absorbance was examined at 470 nm wavelength for every 30 s in a 150 s span.
Determination of ascorbate peroxidase activity
The ascorbate peroxidase (APX, EC 1.11.1.11) reaction mixture 3 mL was made, which contains 2.70 mL of 50 mM phosphate buffer, 0.10 mL of 7.50 mM ascorbic acid, 0.10 mL of 300 mM hydrogen peroxide, and 0.10 mL enzyme extract for each replicate. With the help of UV/VIS spectrophotometer, the measurement of APX reaction mixture was discovered at the absorbance of 290 nm wavelength for every 30 s after at interval of 1 min38.
Measurement of superoxide dismutase activity
For determination of superoxide dismutase (SOD, EC 1.15.1.1) reaction solution was prepared as 0.3 mL of 130 mM methionine, 0.3 mL of 50 µM nitro blue tetrazolium, 0.3 mL of 100 µM EDTA-Na2 and 0.3 mL of 20 µM riboflavin. Enzyme solution having 0.05 mL was mixed to reaction solution and then exposed to 4000 lx light for 30 min. Using UV/VIS spectrophotometer samples were monitored at 560 nm absorbance39.
Determination of nutritional ions content
After the drying samples of shoot and root were taken out, they were carefully grounded with a pestle and mortar. Samples weighing 0.1 g of the shoot and root were then digested using H2SO4. Samples were collected in test tubes and 2 mL of H2SO4 was added to each tube. On next day, these samples were heated on hot plate and hydrogen peroxide was added drop wise until the mixture lost its color. Following that, deionized water was added. The final capacity was increased to 50 mL. Using flame photometer K+ and Ca2+ ions were estimated. Atomic absorption spectrophotometer (AAS) (Hitachi Polarized Zeeman AAS, Z-8200, Japan) was used for detection of Fe content in shoot of plants.
Statistical analysis
Two-way analysis of variance (ANOVA) was performed along with least significant difference (LSD) mean compare test (p < 0.05) on R-software (RStudio Desktop - Posit, Version: 2024.09.0 + 375). Data presented in the figures is the mean value of three replicates ± standard errors. Pearson’s correlation and principal component analysis (PCA) were also performed on R-software using 5% confidence level (p < 0.05).
Results
Characterization of Fe-NPs
SEM
Our results of SEM confirmed the spherical form of Fe nanoparticles (Fig. 3a). When exhibiting Scanning Electron Microscopy (SEM) data pertaining to nanoparticles in a graphical or visual format, it is customary to integrate both imagery and quantitative assessment. SEM can achieve nanometer-scale resolution, which is ideal for imaging nanoparticles typically ranging from 1 to 100 nanometers in size. nanoparticles are generally distributed onto a conductive substrate, such as a silicon wafer or a carbon-coated grid. In the case of non-conductive specimens, a thin layer of conductive material (e.g., gold or platinum) may be deposited to mitigate charging effects under the influence of the electron beam. Drying: It is imperative that the specimen is entirely desiccated, particularly when dealing with biological or liquid-based samples, in order to avert distortion during the imaging process’s examination was done to assess the Fe-NPs surface morphology.
EDX
Our results confirmed the presence of Fe. Energy-dispersive X-ray spectroscopy (EDX) represents a sophisticated analytical methodology employed for the characterization of the elemental composition inherent in nanoparticles. The application of EDX to such samples yields comprehensive insights regarding the presence and spatial distribution of elements contained within the nanoparticles. Through the detection of characteristic X-rays that are emitted from the sample upon irradiation with an electron beam, EDX facilitates the presence and the identification of other elements that may constitute the nanoparticle framework or exist as contaminants. This capability is instrumental in evaluating the purity and overall composition of the nanoparticles, which is essential for their designated applications.
Moreover, EDX provides the means for quantitative analysis by estimating the concentrations of various elements within the nanoparticles, thereby offering valuable insights into their stoichiometric relationships. EDX evaluated the element constitution of Fe-NPs. The EDX confirmed the presence of iron. EDX measured the weight% of Fe (41.84%) and oxide (36.35%), Si (0.38% ), Cl (8.51% ), C (12.93% ). Cl presence was because of precursor salt. From the EDX spectrum the presence of Fe elements was determined from the Fe K peak. Bibi et al.40 recently studied that used pomegranate (Punica granatum) seed extract to manufacture Fe-NPs showed that the weight% of Fe and O in the sample was 58.5% and 17%, respectively (Fig. 3b).
FTIR
Our results confirmed the presence of active compounds significantly attributed to efficient synthesis, stabilization, and capping of Fe-NPs. FTIR analysis identifies functional groups such as = C–H, C = O, N–O, C–O, and C–N, which play a key role in the stabilization and capping of Fe-NPs. The FTIR spectrum of the synthesized nanoparticles is shown in Fig. 3c. Broad absorption band between 3400 and 3200 cm⁻¹ indicates the stretching vibrations of –OH groups. The bands between 1400 and 1000 cm⁻¹ can be attributed to methylene groups from proteins in the solution and C-N stretching vibrations of amines. Bands in the 1600–1500 cm⁻¹ range correspond to amide I and amide II regions, which arise from carbonyl stretching in proteins. Specifically, the C = O stretching frequency is observed at 1627 cm⁻¹. The aromatic C = C stretching frequency at 1397 cm⁻¹ describe the presence of ricin oleic acid in the sample. The peak at 1064 cm⁻¹ is due to C–O vibrations. Furthermore, a band at 653 cm⁻¹ is attributed to Fe vibrations, as reported in the literature. These active compounds significantly contribute to the efficient synthesis, stabilization, and capping of Fe-NPs.
UV
Ultraviolet-visible (UV-Vis) spectroscopy represents an essential methodology for the characterization of nanoparticles, as it elucidates critical information regarding their dimensions, morphology, surface characteristics, and state of aggregation. Nanoparticles display distinctive optical phenomena, such as surface plasmon resonance (SPR), which is intricately influenced by their elemental composition, size, and the characteristics of the surrounding medium. In the case of metallic nanoparticles, including gold and silver, the UV-Vis spectral profile typically reveals a prominent absorption peak attributable to SPR, which experiences a shift in response to variations in particle size or agglomeration. For semiconductor nanoparticles, exemplified by quantum dots, the threshold of absorption is indicative of their bandgap energy, exhibiting a shift towards shorter wavelengths (a blue shift) as particle size diminishes due to the effects of quantum confinement. Consequently, UV-Vis spectroscopy is regarded as an invaluable instrument for investigating the optical and electronic properties of nanoparticles. For UV visible spectral analysis, the peak range was between 200 and 300 nm however highest peak was formed at 250 nm showing the formation of Fe-NPs. Plant extract-derived Fe-NPs displayed an absorbance peak that ranged from 240 to 360 nm (Fig. 3d)41.
XRD
X-ray diffraction (XRD) constitutes a fundamental methodology for the examination of the structural characteristics of nanoparticles. When employed in the context of nanoparticles, XRD yields critical information regarding their crystallographic architecture, encompassing aspects such as dimensions, morphology, and the spatial organization of the crystalline domains within the substance. As X-rays engage with the orderly lattice of atoms intrinsic to the nanoparticles, they generate a diffraction pattern that is emblematic of the material’s crystalline structure. Through the meticulous analysis of this pattern, scholars can ascertain the distinct phases present, calculate lattice parameters, and evaluate the extent of crystallinity. The size of the crystalline domains (crystallites) within nanoparticles can be estimated using the Scherrer equation.
Information about the crystalline structure of the Fe-NPs can be gained from the X-ray diffraction pattern displayed in Fig. 3e. The pattern features distinct, well-defined broad peaks that indicate the presence of a nanocrystalline phase. The primary diffraction peaks appear at 20 values of 30.2°, 35.6°, 43.3°, 53.6°, 57.0°, and 62.9°, which correspond to the (220), (311), (400), (422), (511), and (440) planes, respectively. These peaks confirm the crystalline structure of the Fe-NPs and correspond with the Joint Committee on Powder Diffraction Standards (JCPDS) database, specifically file No. 39-1346. Using the Scherrer formula, the synthesized Fe-NPs average crystallite size was 18.68 nm. This X-ray diffraction analysis provides crucial information about the crystallinity and structure of the synthesized Fe-NPs.
Effects of Fe supplements (FeCl3 and FeNPs) on growth and biomass related attributes of S. oleracea plants under drought stress
Shoot fresh weight (SFW)
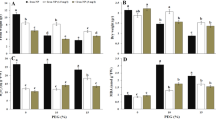
Moderate and severe drought stress had significant effect on SFW and reduced it by 19 and 52%, respectively. Application of Fe supplement in the form of FeCl3 had negative effect on SFW in moderate stress level but it improved SFW by 18% in severe drought conditions. On the other hand, FeNPs application was effective in improving SFW at both moderate and severe stress levels and increased SFW by 12 and 21%, respectively, compared to non-treated plants under moderate and severe stress (Fig. 4A).
Effect of foliar application of Fe (FeCl3) and FeNPs (40 mg L− 1 for both) on (A) shoot fresh weight, (B) shoot dry weight, (C) root fresh weight and (D) root dry weight of S. oleracea under drought stress. In graph bars represented mean values of three replicates ± standard errors in the form of error bars. Bars sharing similar small letters (a, b, c etc.) are not significantly different at p < 0.05.
Shoot dry weight (SDW)
SDW of S. oleracea was decrease by 26 and 60% under D1 and D2 (moderate and severe drought), respectively, compared to the control plant. Among the Fe supplements tested in this study, FeNPs were proved to more effective in improving SDW under both levels of drought. As its foliar application increased SDW by 24 and 52% in plants subjected to D1 and D2, respectively, in comparison to stress inly plants (Fig. 4B).
Root fresh weight (RFW)
D1 and D2 reduced the RFW of S. oleracea by 17% and 26%, respectively, compared to the control plant. Fe in the form of FeCl3 had very less or non-significant effect on RFW in drought stressed plants. Whereas FeNPs showed significant effects on RFW under all conditions (control and drought). It improved RFW by nearly 10 and 21%, respectively, in D1 and D2 treated plants, compared with stress only plants (Fig. 4C).
Root dry weight
D1 and D2 drought stress had significantly affected RDW and reduced it by 24 and 39%, respectively. Application of FeCl3 foliar spray had negative effect on RDW in control plants but it improved RDW in D1 and D2 stressed plants by 12 and 14%, respectively. On the other hand, FeNPs application was effective in improving RDW in all conditions. It effectively enhanced RDW by 21 and 52%, respectively at D1 and D2 stress levels compared to non-treated plants (Fig. 4D).
Shoot length (SL)
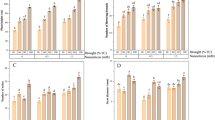
D1 and D2 reduced the SL of S. oleracea by 8 and 33%, respectively, compared to the control plant. In addition, treatment with foliar application of FeCl3 and FeNPs enhanced the SL by 12 and 28%, respectively, at D1 stress level. Whereas these supplements increased SL by 22 and 42%, respectively, at D2 stress level (Fig. 5A).
Effect of foliar application of Fe (FeCl3) and FeNPs (40 mg L− 1 for both) on (A) shoot length, (B) root length, (C) number of leaves and (D) leaf area of S. oleracea under drought stress. In graph bars represented mean values of three replicates ± standard errors in the form of error bars. Bars sharing similar small letters (a, b, c etc.) are not significantly different at p < 0.05.
Root length (RL)
RL was slightly increased under severe drougth stress i.e. D2 stress level but this increment was non-significant as depicted in the Fig. 5B. Fe foliar sprays in the form of FeCl3 and FeNPs had varying effects on RL in control and stressed conditions. For examlple, it improved RL in control and moderate i.e. D1 stress level, while it decreased RL (20%) under severe stress conditions (Fig. 5B).
Number of leaves
Drought stresses i.e. D1 and D2 reduced the number of leaves in S. oleracea plants by 32 and 44%, respectively, compared to the control plant. Foliar application of FeCl3 and FeNPs enhanced the number of leaves by 18% and 12%, respectively under D1. Whereas these Fe supplements increased the number of leaves by 21 and 36%, respectively, under D2 compared with stress only plants (Fig. 5C).
Leaf area
D1 and D2 drought stress had significant negative effects on leaf area and reduced it by 33 and 42%, respectively. Application of FeCl3 foliar spray had negative effect on leaf area in control plants but it improved leaf area in D1 and D2 stressed plants by 23 and 16%, respectively. On the other hand, FeNPs application was effective in improving leaf area in all conditions. It effectively enhanced leaf area by 27 and 16% at D1 and D2 stress levels, respectively, compared to non-treated plants (Fig. 5D).
Effects of Fe supplements (FeCl3 and FeNPs) on photosynthetic pigments and SPAD value of S. olerace a plants under drought stress
Chlorophyll a, b and carotenoids pigments
Drought stress either moderate or severe i.e. D1 and D2 had significantly negative effects on photosynthetic pigments synthesis in S. oleracea plants. Maximum reduction (46%) among the tested pigments was observed for chlorophyll b at D2 stress level, compared to control group. Fe foliar spray in the form of FeCl3 had non-significant effects on pigmentation level at both moderate and severe stress levels. Whereas FeNPs foliar application significantly incremented total chlorophyll and carotenoids in both D1 and D2 stressed plants. Among the studied pigments, maximum increase (62%) was observed for chlorophyll b at D2 stress level with foliar spray of FeNPs, compared to stress only plants (Fig. 6A-C).
Effect of foliar application of Fe (FeCl3) and FeNPs (40 mg L− 1 for both) on (A) chlorophyll a, (B) chlorophyll b, (C) carotenoid and (D) SPAD value of S. oleracea under drought stress. In graph bars represented mean values of three replicates ± standard errors in the form of error bars. Bars sharing similar small letters (a, b, c etc.) are not significantly different at p < 0.05.
SPAD value
D1 and D2 drought stress had significant negative effects on SPAD value of S. oleracea plants and reduced it by 30 and 53%, respectively, compared with control group. Application of FeCl3 foliar spray improved SPAD value in D1 and D2 stressed plants by 11 and 39%, respectively, compared to non-treated plants. Although it had negative effect on SPAD value in control plants. On the other hand, FeNPs application was more effective in improving SPAD value in drought conditions. It effectively enhanced SPAD value by 18 and 64% at D1 and D2 stress levels, respectively, compared to non-treated plants (Fig. 6D).
Effects of Fe supplements (FeCl3 and FeNPs) on photosynthetic machinery of S. oleracea plants under drought stress
Chlorophyll fluorescence and photosystems (PS) performance metrices shown in the Fig. 7 depicted that D2 stress had severe effects on maximum efficiency of PSII (Fv/Fm), performance index (PI) and related attributes of S. oleracea plants. While FeCl3 and FeNPs foliar spray reverse the adverse effects of drought. Fe supplements increased Fv/Fm i.e. photosynthetic efficiency of the S. oleracea plants. These supplements also improved electron transport (ETo/RC) i.e. energy transfer in S. oleracea plants at moderate and severe drought stress levels. Fe supplements also improved energy trapping efficiency of PSII (TRo/ABS) contributing to improved efficiency of photosynthetic machinery under drought conditions (Fig. 7).
Effects of Fe supplements (FeCl3 and FeNPs) on photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (gs) and intercellular CO2 (Ci) of S. oleracea plants under drought stress
Drought stress (D1 & D2) drastically reduced photosynthetic rate (30 & 50%), compared to the control plants (Fig. 8A). Transpiration rate as well as stomatal conductance were also reduced up to a significant level under both D1 and D2 stress levels (Fig. 8B & C). For example, at D2 stress level these attributes were reduced by 66 and 58%, respectively. This reduction is also evident from increased level of intercellular CO2 (34%) at D2 stress level, compared with control plants (Fig. 8D). Foliar spray of FeCl3 as Fe source was not much effective in reversing these effects of drought. While FeNPs application significantly improved Pn, Tr and gs in S. oleracea plants exposed to drought conditions compared to non-treated plants. It also reduced Ci level of the plants in drought conditions. FeNPs effects were more prominent under severe stress conditions. For instance, FeNPs treatment increased Pn, Tr and gs by 46, 71 and 66%, respectively and it reduced Ci by 40% at D2 stress level, compared with non-treated plants (Fig. 8A-D).
Effect of foliar application of Fe (FeCl3) and FeNPs (40 mg L− 1 for both) on (A) net photosynthesis rate, (B) transpiration rate, (C) stomatal conductance and (D) intercellular CO2 of S. oleracea under drought stress. In graph bars represented mean values of three replicates ± standard errors in the form of error bars. Bars sharing similar small letters (a, b, c etc.) are not significantly different at p < 0.05.
Effects of Fe supplements (FeCl3 and FeNPs) on oxidative stress markers and total soluble proteins in S. oleracea plants under drought stress
Malondialdehyde (MDA)
Significant increase by 35 and 64% in MDA content was observed in S. oleracea plants under the D1 and D2 stress levels, respectively, compared to the control plants. Fe supplements either FeCl3 or FeNPs had very less or negligible change in MDA contents in control group. Whereas their effects became prominent under drought stress (D1 & D2). Figure 9A showed that Fe applied as FeCl3 foliar spray reduced MDA contents by 24 and 21%, respectively, at D1 and D2 stress levels. Similarly, FeNPs foliar spray reduced MDA in S. oleracea plants by 40 and 57%, respectively, at D1 and D2 stress levels, compared with stress only plants.
Effect of foliar application of Fe (FeCl3) and FeNPs (40 mg L− 1 for both) on (A) malondialdehyde, (B) hydrogen peroxide, (C) relative membrane permeability and (D) total soluble proteins of S. oleracea under drought stress. In graph bars represented mean values of three replicates ± standard errors in the form of error bars. Bars sharing similar small letters (a, b, c etc.) are not significantly different at p < 0.05.
Hydrogen peroxide (H2O2)
D1 and D2 drought stress had significantly varying effects on H2O2 contents in S. oleracea plants as it was reduced by 31% at D1 and increased by 25% at D2 stress levels, compared to control group. Application of FeCl3 foliar spray reduced H2O2 contents in D1 and D2 stressed plants by 30 and 57%, respectively, compared to non-treated plants. On the other hand, FeNPs application was also effective in reducing H2O2 contents in all conditions. It effectively reduced H2O2 contents by 36% and 54% at D1 and D2 stress levels, respectively, compared to non-treated plants (Fig. 9B).
Relative membrane permeability (RMP)
S. oleracea plants exposed to severe drought stress (D2) significantly lose their selective permeability (41%) compared to control plants. Figure 9C revealed that FeCl3 and FeNPs foliar spray reversed the effect of drought stress on RMP at both levels (D1 and D2). But their response was more prominent at severe stress level (D2). These supplements reduced RMP by 23 and 32%, respectively, at D2 stress level in comparison to stress only plants.
Total soluble protein
Both D1 and D2 drought stress levels had significant negative effects on TSP contents of S. oleracea plants and reduced it by 32 and 68%, respectively, compared with control group. Although application of Fe foliar spray had negative effect on TSP contents in control plants. It improved TSP contents in D1 and D2 stressed plants by 10 and 21%, respectively, compared to non-treated plants. On the other hand, FeNPs application was more effective in improving TSP contents in all conditions. It effectively enhanced TSP contents by 15 and 45% at D1 and D2 stress levels, respectively, compared to non-treated plants (Fig. 9D).
Effects of Fe supplements (FeCl3 and FeNPs) on enzymatic antioxidants of S. oleracea plants under drought stress
Drought stress either moderate or severe (D1 & D2) had significantly impacted antioxidative enzymes activities in S. oleracea plants. It increased activities of antioxidant enzymes including CAT, POD, APX and SOD, compared to control group. This increment was more prominent at D2 stress level. For instance, activity of these enzymes was increased by 20, 27, 25 and 36%, respectively, at D2 stress level. Furthermore, foliar spray of FeCl3 as Fe source didn’t have much prominent effect on antioxidant enzymes activities compared to FeNPs in both control and drought stress conditions. FeNPs incremented CAT, POD and SOD activities but it decremented APX activity in both control and stressed plants. Among these antioxidants, maximum increase in activity was observed for SOD in control (48%), POD at D1 stress level (32%) and CAT at D2 stress level (32%) along with FeNPs foliar spray, compared to their respective controls. While maximum decrease in activity of APX was observed with FeNPs application at D2 stress level, compared to non-treated plants (Fig. 10A-D).
Effect of foliar application of Fe (FeCl3) and FeNPs (40 mg L− 1 for both) on (A) catalase, (B) peroxidase, (C) ascorbate peroxidase and (D) superoxide dismutase enzymes activity of S. oleracea under drought stress. In graph bars represented mean values of three replicates ± standard errors in the form of error bars. Bars sharing similar small letters (a, b, c etc.) are not significantly different at p < 0.05.
Effects of Fe supplements (FeCl3 and FeNPs) on non-enzymatic antioxidants of S. oleracea plants under drought stress
Both drought levels D1 and D2 significantly enhanced production of non-enzymatic antioxidants like total phenolics and proline. For instance, D1 increased total phenolics and proline accumulation by 14 and 47% while D2 by 26 and 96% respectively, compared to control plants. Although FeCl3 application had significant effect on total phenolics and proline in control plants (without drought) but its effect under drought conditions was less significant. In contrast, FeNPs had clearly significant effects on these attributes under both control and stressed conditions. It incremented total phenolics and proline by 54 and 125% in control, 24 and 34% at D1 stress level and 13 and 11% at D2 stress level, respectively, compared to stress only plants (Fig. 11A & B).
Effect of foliar application of Fe (FeCl3) and FeNPs (40 mg L− 1 for both) on (A) total phenolics, (B) proline and (C) Fe2+ ions in shoot of S. oleracea under drought stress. In graph bars represented mean values of three replicates ± standard errors in the form of error bars. Bars sharing similar small letters (a, b, c etc.) are not significantly different at p < 0.05.
Effects of Fe supplements (FeCl3 and FeNPs) on Fe content of S. oleracea plants under drought stress
Fe contents of the S. oleracea plants were reduced significantly under both moderate (21%) and severe (31%) stress levels, compared with control group. As shown in Fig. 11C, Fe supplements either in the form of FeCl3 or FeNPs significantly improved Fe content of the S. oleracea plants in control as well as stress conditions. Maximum increase in Fe content (29%) of the S. oleracea plants was observed at D2 stress level with foliar spray of FeNPs, compared to stress only plants.
Effects of Fe supplements (FeCl3 and FeNPs) on nutritional ions in S. oleracea plants under drought stress
Moderate drought stress (D1) had little or no effect on Ca2+ ion contents in the root and shoot of S. oleracea plants. While severe drought (D2) significantly reduced Ca2+ ions in both root (19%) and shoot (36%) of the tested plants, compared to control plants. Foliar spray of FeCl3 and FeNPs was effective in reversing the effects of drought on Ca2+ ionic contents of the S. oleracea plants. Maximum increase in Ca2+ ions in root as well as shoot of plants were observed in D2 stressed plants with foliar spray of FeNPs, compared to stress only plants (Fig. 12A & B).
Effect of foliar application of Fe (FeCl3) and FeNPs (40 mg L− 1 for both) on (A) root Ca2+, (B) shoot Ca2+, (A) root K+ and (B) shoot K+ in S. oleracea under drought stress. In graph bars represented mean values of three replicates ± standard errors in the form of error bars. Bars sharing similar small letters (a, b, c etc.) are not significantly different at p < 0.05.
K+ ions in root and shoot of S. oleracea plants were greatly reduced under both drought stress levels i.e. D1 (19 & 18%, respectively) and D2 (33 and 24%, respectively), compared with control plants. Foliar spray of Fe supplements enhanced K+ ions in root as well as shoot of the plants under stress conditions. Maximum increase in K+ ions in root (74%) and shoot (17%) of S. oleracea plants was observed at D2 stress level with foliar spray of FeNPs, compared with stress only plants (Fig. 12C & D).
Pearson’s correlation and principal component analysis
Pearson’s correlation test, shown in Fig. 13, revealed that oxidative stress markers viz. lipid peroxidation (in term of increased MDA contents), H2O2, and electrolytic leakage are in negative correlation with growth and photosynthesis related attributes of S. oleracea plants. This showed that S. oleracea plants given drought stress faced severe oxidative damage to cells. Indicated via increased lipid peroxidation and membrane damage. This in turn, hampered growth and photosynthetic efficiency of S. oleracea plants. On the other hand, various physiological attributes and examined ionic contents (Ca, K and especially Fe) in S. oleracea plants are in positive correlation with growth and photosynthesis related parameters. This is conceivable that FeNPs treatment and increased Fe level in the shoot triggered physiological and metabolic changes to overcome oxidative damage and increased growth under stress conditions. For example, increased activities of enzymatic antioxidants scavenged reduced oxidative species. Fe is an essential component of various metabolic processes. It is co-factor of various enzymes, involved in synthesis of chlorophylls and an electron carrier during respiration and photosynthesis. Thus, it is estimated that FeNPs treatments improved physiological and biochemical attributes through metabolic changes which improved growth of S. oleracea plants in drought conditions. PCA shown in Fig. 14A & B, also validates the findings of Pearson’s correlation. It confirmed that all studied attributes can be divided into two groups. These groups are aligned in PC1 (55.14%) and PC2 (17.21%). Attributes that fall in PC1 are in positive relation with each other while these are in negative relation with attributes of PC2. Same is the case with PC2. Dots along with numerics (1, 2, 3…, 9) represents treatments applied in this study. It is shown that the D1 and D2 stress levels (represented by numerics 2 & 3) are well separated from all other treatments applied in this experiment, revealing clear cut effects of drought stress on all of the explained variables (parameters studied) in the PCA biplot.
Pearson’s correlation for all studied parameters of S. oleracea plants treated with foliar spray of FeCl3) and FeNPs (40 mg L− 1 for both) in control and drought conditions. (Various abbreviations used are as follows; RL root length, RFW root fresh weight, RDW root dry weight, SL shoot length, SFW shoot fresh weight, SDW shoot dry weight, nL number of leaves, LA leaf area, Chl chlorophyll, Pn net photosynthesis rate, Tr transpiration rate, gs stomatal conductance, Ci intercellular CO2, Fv/Fm maximum quantum efficiency of PSII, Fv/Fo maximum efficiency of the water-splitting complex on the donor side of PSII, PI performance index, ABS/RC absorption per reaction center, TRo/RC trapped energy flux per reaction center, DIo/CS dissipated energy flux per cross section, ETo/RC electron transport flux per reaction center, TRo/ABS trapping efficiency of PSII, MDA malondialdehyde, H2O2 hydrogen peroxide, RMP relative membrane permeability, TSP total soluble proteins, CAT catalase, POD peroxidase, APX ascorbate peroxidase, SOD superoxide dismutase, Fe Fe2+ in shoot, K potassium, Ca calcium.
Principle component analysis for all studied parameters of S. oleracea plants treated with foliar spray of FeCl3) and FeNPs (40 mg L− 1 for both) in control and drought conditions. (where; A) bar graph represented percentage contribution of all variables in total of 8 principal components. B) PCA biplot. Various abbreviations used are same as given in Fig. 17. Dots with numbers 1–9 represented different treatments applied in this experiment. Where; 1 = control, 2 = D1, 3 = D2, 4 = FeCl3 (40 mg L− 1), 5 = 2 + 4, 6 = 3 + 4, 7 = FeNPs (40 mg L− 1), 8 = 2 + 7 & 9 = 3 + 7).
Discussion
Drought stress, which decreases agricultural quality and quantity, has been worse in recent years because of climate change. Serious measures must be implemented to boost crop plants tolerance to acute drought conditions that are predicted to arise because of global warming42. Drought affects agriculture when plants fail to get enough moisture to develop regularly and complete their life cycles. A continual decrease in precipitation and an increase in evapotranspiration increase the severity of the drought43.
Metal oxide nanoparticles (MONPs) contribute to signaling networks, defense mechanisms, metabolism, and regulatory processes during drought stress. During drought-induced oxidative stress MONPs may invade chloroplasts and interact with the reaction center of the photosystem II, boosting electron transmission, oxygen evolution, and light absorption in chloroplasts. Application of iron nanoparticles could be an efficient strategy for enhancing the uptake of iron and improving plant stability under stress caused by drought3.
Our finding revealed that water deficit decreased the growth of S. oleracea plants compared with control plants in terms of shoot length, root and shoot weight (fresh and dry), no of leaves and leaf area. Similar results were found by Ndou et al.19 in Sorghum plant. They observed that drought stress stunted the growth of sorghum plants, resulting in lower plant height, fresh and dry weight. Water restriction causes osmotic stress, affecting nutrient absorption and transport. This leads to decreased turgidity and cell expansion, resulting in reduced growth. Our results demonstrated that FeNPs improved the shoot length, and biomass (fresh and dry) of S. oleracea plants in drought conditions. Likewise, Dhoke et al.44 discovered that the application of ZnO-NPs increased fresh and dry biomass in Vigna radiata plants under water deficiency stress. Tawfik et al.45 additionally demonstrated that applying Fe-NPs topically enhanced the plant’s dry weight, stem diameter, leaf area, and overall biomass. The foliar application of 40 ppm FeNPs shown the highest increase in plant. It is observed that FeNPs not only reduced the oxidative damage cause by drought but also serve as nano nutrients for the plants resulting in efficient distribution of nutrients within the plants. This improved physiological responses in plants under stress conditions leading to improves growth and biomass23. In another study, Tawfik et al.45 showed that plant height increased considerably with 60 ppm concentration of FeNPs as compared to in control plant. Similarly, Shankramma et al.46 observed that Fe3O4 NPs improved tomato plant growth metrics.
Our finding showed that length of roots has increased in drought stress plants. Our findings are in direct opposition to study by Hasnain et al.6 as they observed a significant loss in length of shoot and root in Brassica rapa plants under stress caused by drought. Deeper root systems with higher root densities are thought to be an excellent stress-reduction strategy, because they not only permit better extraction of soil water but also help the plant preserve optimal growth and development under drought stress circumstances47. Similarly, According to Miyahara et al.48 some crop species experience an increase in the number of lateral and fine roots during drought stress.
Our recent findings revealed that physiological characteristics such as chl a, chl b, carotenoids, SPAD, chlorophyll florescence, efficiency of photosystems and energy conversion rate of S. oleracea plants were decreased in drought stress. Similar to findings of Jabeen et al.49 studied that chl b content declined in S. oleraceae plants when they were subjected to varied levels of water deficits. Formerly, Yasmeen et al.50 in wheat and Akram et al.51 in radish found that water scarcity reduced the concentration of chl b, which they attributed to water stress-induced damage to the antioxidant response system. One of the most effective pigments for light harvesting is chl b. A decrease in chl b content caused by drought may have been more pronounced than decrease in chl a due to the adverse effects of water scarcity on the PS-I, light harvesting/capturing system, energy transfer, and antenna complex accessories52. In this study, Pn, Tr and gs of S. oleracea plants were also reduced due to increased intercellular CO2 levels. It is believed that plants under water deficit conditions close their stomata to prevent loss of water. As result, plants face the consequences of reduced Pn, Tr and gs. Souza et al.53 described that reduced CO2 assimilation rates during drought were mostly due to stomatal closure, which lowered accessible internal CO2 and limited water loss via transpiration. Hessini et al.54 also found that Spartina alterniflora plants displayed noticeably reduced stomatal conductance under water deficit conditions. Drought stress decreased gas exchange in the plants by lowering transpiration and photosynthetic rates in addition to having an impact on stomatal closure55.
Rizwan et al.56 demonstrated that use of FeNPs increased photosynthetic pigments in Cd-stressed wheat. SPAD levels are correlated to the level of chlorophyll in the leaf57. Bisht et al.58 also stated that photosynthetic pigments (Chl a, b and carotenoid) were enhanced in both studied varieties of Trigonella foenum-graecum under controlled and stressful conditions most effectively at a concentration of 50 mg/L NPs. It was found that FeNPs application improved Fe uptake in plants which is cofactor for various enzymes involve in chlorophyll formation. Furthermore, FeNPs applications effectively reduced ROS generation and protect photosynthetic machinery from oxidative stress. This ultimately leads to improved chl formation, fluorescence, efficiency of photosystems and energy conversion rate in treated plants59. In this study gas exchange related attributes (Pn, Tr and gs) were also improved with foliar application of FeNPs under drought conditions.
It is observed that drought stress increased the relative membrane permeability of S. oleracea plant. Also, the H2O2 and MDA content were enhanced under both drought stress levels (D1 and D2). In the same way, when maize seedlings were subjected to water deficit conditions in contrast to regular irrigation, H2O2 and MDA content were increased17. The MDA level indicates membrane oxidative damage caused by lipid peroxidation. In drought stress, the concentration of ROS production increased in many species resulting in lipid peroxidation, fatty acid saturation, and overall membrane degradation60. Comparable findings reported by Mirzaee et al.61 that salinity has been shown to modify the lipid matrix of the plasma membrane. Additionally, saline stress caused a significant enhance in the levels of MDA and H₂O₂ in various plant species. The excessive production of ROS disrupts photosynthesis, mineral absorption, and assimilation, affecting membrane integrity and cellular function62.
In this study, FeNPs foliar spray reduced the level of H2O2 and MDA and minimized the negative effect of drought in the form of oxidative damage to the membrane. Ndou et al. observed that Sorghum bicolor seeds priming with 10 mg L− 1 of FeNPs reduced ROS generation and prevent biomolecules degradation and improved membrane stability under drought stress. FeNPs application also stimulated antioxidant defense through increasing the concentration and activities of enzymatic and non-enzymatic antioxidants59. Similar results were observed in this study, where foliar application of FeNPs improved activity of CAT, POD and SOD enzymes. CAT is a key antioxidant that performs a vital part in plants’ defense against abiotic stressors. This enzyme is responsible for the conversion of H2O2 to O2 and H2O. Recent study indicated that FeONPs and SiNPs improved the CAT activity in P. vulgaris seedlings subjected to Cd stress63. The increase in SOD activity under drought, clearly demonstrates SOD’s interaction in defensive system accountable for mitigating oxidative damage17. In another study, Noor et al.24 demonstrated that FeNPs application triggered gene expression for the synthesis of POD enzymes to improve drought tolerance in wheat plants. Also, the total phenolics and proline content were enhanced with treatment of FeNPs in drought stressed S. oleracea plants. Phenolics are non-enzymatic antioxidants that can effectively scavenge uncontrolled ROS generation during stress64. Jabeen et al.49 found total phenolics were incremented in S. oleracea plants when exposed to drought. Proline is an essential organic solute that accumulated and increased in plants during drought situations65. Proline plays a vital role in eliminating ROS. In addition, in drought conditions, the plants developed an osmotic adjustment strategy for the functioning of physiological machinery by producing suitable cellular solutes66. The formation of organic substances like proline is a sign of a stress tolerance response that protects cell balance and lessens the detrimental effects of drought-induced stress67. It has been reported that proline, when present in copious amounts as an osmolyte, protects the structures of cells and enzymes68.
Our results displayed that total soluble proteins were decreased under drought stress. While foliar application of Fe supplements improved TSP in S. oleracea plants under drought conditions. Generally, it is observed that plant can generate stress-induced proteins, which build up in respond to elevated temperature, cold, moisture retention, drought, and salt stress52. Fe is required as a cofactor for the functioning of various enzymes involved in protein synthesis. This is predicted that FeNPs enhanced Fe availability in plants can stimulate synthesis of stress responsive proteins in drought conditions leading to increased level of TSP in S. oleracea plants4.
Results of this study showed that inorganic ions including Fe2+ Ca2+ and K+ S. oleracea plants were reduced under water deficit conditions. This is evident from the fact that under water scarcity, water uptake by plants is significantly reduced which also limited nutrients uptake. Supplementation of iron nanoparticles itself act as nano nutrients, and also improved nutrient uptake and their distribution within plants. Increase in Fe2+ concentration is also directly linked to exogenous application of Fe supplements23. Furthermore, Ahmed et al.69, found that with increasing concentration of Fe3O4 treatment N, P and K contents were steadily enhanced.
Conclusion
Spinach plants exposed to high drought experienced cell damage which decreased their quantity of chlorophyll and antioxidant enzymes, therefore decreasing the growth parameters. This study investigated the impact of drought stress (25% and 50% Field capacity) on S. oleracea plants. Many developmental parameters in S. oleracea plant were decreased in response of drought stress which reduced the plant growth, root, shoot weight, shoot length, water content, chlorophyll content, total soluble protein while increasing the oxidative stress and membrane damage like Malondialdehyde (MDA), Hydrogen per oxide (H2O2),Relative membrane permeability (RMP). When Fe-NPs synthesized from rice straw were given externally, drought stress mitigated, by improving the all physiological and biochemical parameter when compared to the control group. However, Iron application did not produce meaningful results in plants in contrast to the Fe-NPs which positively influenced physiological and morphological parameter of S. oleracea.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ali, J. et al. Biochemical response of okra (Abelmoschus esculentus L.) to Selenium (Se) under drought stress. Sustainability 15, 5694 (2023).
Khan, I. et al. Silicon nanoparticles improved the osmolyte production, antioxidant defense system, and phytohormone regulation in Elymus sibiricus (L.) under drought and salt stress. Environ. Sci. Pollut. Res. 1–15 (2024).
Alabdallah, N. M. et al. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 10, 1730 (2021).
Akpinar, A. & Cansev, A. Physiological and molecular responses of roots differ from those of leaves in spinach plants subjected to short-term drought stress. South. Afr. J. Bot. 151, 9–17 (2022).
Zaib, M. et al. Drought stress and plants production: a review with future prospects. Int. J. Sci. Res. Eng. Dev. 6, 1278–1293 (2023).
Hasnain, Z., Zafar, S., Usman, S., Zhang, L. & Elansary, H. O. Elucidating role of melatonin foliar spray in ameliorating adverse effects of drought stress on growth and physio-biochemical attributes of Brassica rapa plants. Sci. Hortic. 321, 112336 (2023).
Abdelaal, K. et al. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology (Basel). 10, 520 (2021).
Alotaibi, N. M. et al. Zn-quantum dot biochar regulates antioxidants and nutrient uptake to improve rapeseed growth and yield in drought stress. Plant. Stress. 11, 100286 (2024).
Khan, M. T. et al. Influence of zinc oxide nanoparticles to regulate the antioxidants enzymes, some osmolytes and agronomic attributes in Coriandrum sativum L. grown under water stress. Agronomy 11, 2004 (2021).
Neysanian, M., Iranbakhsh, A., Ahmadvand, R., Ardebili, Z. O. & Ebadi, M. Selenium nanoparticles conferred drought tolerance in tomato plants by altering the transcription pattern of microRNA-172 (miR-172), bZIP, and CRTISO genes, upregulating the antioxidant system, and stimulating secondary metabolism. Protoplasma 1–13 (2024).
Mihaljević, I. et al. Comparative study of drought stress effects on traditional and modern apple cultivars. Plants 10, 561 (2021).
Chen, L. et al. Comparative study of the effectiveness of nano-sized iron-containing particles as a foliar top-dressing of peanut in rainy conditions. Agric. Water Manag. 286, 108392 (2023).
Faizan, M. et al. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 161, 122–130 (2021).
Van Nguyen, D. et al. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant. Growth Regul. 1–12 (2021).
Semida, W. M. et al. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L). Plants 10, 421 (2021).
Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 15, 15–22 (2017).
Sharf-Eldin, A. A. et al. Response of maize seedlings to silicon dioxide nanoparticles (SiO2NPs) under drought stress. Plants 12, 2592 (2023).
Bhuiyan, M. S. H. et al. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 6, (2020).
Ndou, N. et al. Green Synthesis of Iron Oxide (Hematite) nanoparticles and their influence on Sorghum bicolor Growth under Drought stress. Plants 12, 1425 (2023).
Feng, Y. et al. Effects of iron oxide nanoparticles (Fe3O4) on growth, photosynthesis, antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum) Plants. Plants 11, 1894 (2022).
Linh, T. M. et al. Metal-based nanoparticles enhance drought tolerance in soybean. J Nanomater 1–13 (2020). (2020).
Adrees, M. et al. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 238, 124681 (2020).
Sreelakshmi, B. et al. Drought stress amelioration in plants using green synthesised iron oxide nanoparticles. Mater. Today Proc. 41, 723–727 (2021).
Noor, R. et al. Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress. Chemosphere 292, 133201 (2022).
Kausar, A. et al. Modulation of growth and biochemical responses in spinach (Spinacia oleracea L.) through foliar application of some amino acids under drought conditions. South. Afr. J. Bot. 158, 243–253 (2023).
Areeshi, M. Y. Rice straw mediated green synthesis and characterization of iron oxide nanoparticles and its application to improve thermal stability of endoglucanase enzyme. Int. J. Food Microbiol. 374, 109722 (2022).
Bidi, H., Fallah, H., Niknejad, Y. & Tari, D. B. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol. Biochem. 163, 348–357 (2021).
Manzoor, N. et al. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 769, 145221 (2021).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase Beta vulgaris Plant. Physiol. 24, 1 (1949).
Lichtenthaler, H. K. [34] chlorophylls and carotenoids: pigments of photosynthetic biomembranes. in Methods in Enzymology vol. 148 350–382 (Academic, (1987).
Yang, G., Rhodes, D. & Joly, R. J. Effects of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine-deficient and glycinebetaine-containing maize lines. Funct. Plant Biol. 23, 437–443 (1996).
Velikova, V., Yordanov, I. & Edreva, A. J. P. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 151, 59–66 (2000).
Cakmak, I. & Horst, W. J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 83, 463–468 (1991).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Bates, L. S., Waldren, R. P. A. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207 (1973).
Lamuela-Raventós, R. M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. Meas. Antioxid. Activity Capacity: Recent. Trends Appl. 107–115 (2018).
Chance, B. & Maehly, A. C. [136] Assay of Catalases and Peroxidases. (1955).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880 (1981).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971).
Bibi, I. et al. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 8, 6115–6124 (2019).
Aksu Demirezen, D., Yılmaz, Ş., Demirezen Yılmaz, D. & Yıldız, Y. Ş. Green synthesis of iron oxide nanoparticles using Ceratonia siliqua L. aqueous extract: improvement of colloidal stability by optimizing synthesis parameters, and evaluation of antibacterial activity against Gram-positive and Gram-negative bacteria. Int. J. Mater. Res. 113, 849–861 (2022).
Ozturk, M. et al. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 172, 1321–1335 (2021).
Al-Khayri, J. M. et al. The role of nanoparticles in response of plants to abiotic stress at physiological, biochemical, and molecular levels. Plants 12, 292 (2023).
Dhoke, S. K., Mahajan, P., Kamble, R. & Khanna, A. Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanatechnol. Dev. 3, e1–e1 (2013).
Tawfik, M. M., Mohamed, M. H., Sadak, M. S. & Thalooth, A. T. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull. Natl. Res. Cent. 45, 1–9 (2021).
Shankramma, K., Yallappa, S., Shivanna, M. B. & Manjanna, J. Fe 2 O 3 magnetic nanoparticles to enhance S. Lycopersicum (tomato) plant growth and their biomineralization. Appl. Nanosci. 6, 983–990 (2016).
Lopes, M. S., Araus, J. L., Van Heerden, P. D. & Foyer, C. H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 62, 3135–3153 (2011).
Miyahara, M., Takenaka, C., Tomioka, R. & Ohta, T. Root responses of siberian larch to different soil water conditions. Hydrol. Res. Lett. 5, 93–97 (2011).
Jabeen, M., Akram, N. A., Ashraf, M. & Aziz, A. Assessment of biochemical changes in spinach (Spinacea Oleracea L.) subjected to varying water regimes. Sains Malays. 48, 533–541 (2019).
Yasmeen, A. et al. Improving Drought Resistance in Wheat (Triticum aestivum) by exogenous application of growth enhancers. Int. J. Agric. Biol. 15, (2013).
Akram, N. A., Waseem, M., Ameen, R. & Ashraf, M. Trehalose pretreatment induces drought tolerance in radish (Raphanus sativus L.) plants: some key physio-biochemical traits. Acta Physiol. Plant. 38, 1–10 (2016).
Ashraf, M. H. P. J. C. & Harris, P. J. Photosynthesis under stressful environments: an overview. Photosynthetica 51, 163–190 (2013).
Souza, R. P., Machado, E. C., Silva, J. A. B., Lagôa, A. M. M. A. & Silveira, J. A. G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 51, 45–56 (2004).
Hessini, K. et al. Biomass production, photosynthesis, and leaf water relations of Spartina alterniflora under moderate water stress. J. Plant. Res. 121, 311–318 (2008).
Toscano, S., Scuderi, D., Giuffrida, F. & Romano, D. Responses of mediterranean ornamental shrubs to drought stress and recovery. Sci. Hortic. 178, 145–153 (2014).
Rizwan, M. et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214, 269–277 (2019).
Ling, Q., Huang, W. & Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth Res. 107, 209–214 (2011).
Bisht, S., Sharma, V. & Kumari, N. Biosynthesized magnetite nanoparticles from Polyalthia longifolia leaves improve photosynthetic performance and yield of Trigonella foenum-graecum under drought stress. Plant. Stress. 5, 100090 (2022).
Bidabadi, S. S., Sabbatini, P. & VanderWeide, J. Iron oxide (Fe2O3) nanoparticles alleviate PEG-simulated drought stress in grape (Vitis vinifera L.) plants by regulating leaf antioxidants. Sci. Hortic. 312, 111847 (2023).
Yildirim, E., Ekinci, M. & Turan, M. Impact of biochar in mitigating the negative effect of drought stress on cabbage seedlings. J. Soil. Sci. Plant. Nutr. 21, 2297–2309 (2021).
Mirzaee, M., Moieni, A. & Ghanati, F. Effects of Drought stress on the lipid peroxidation and antioxidant enzyme activities in two Canola (Brassica Napus L.) cultivars. (2013).
Zhang, H., Zhao, Y. & Zhu, J. K. Thriving under stress: how plants balance growth and the stress response. Dev. Cell. 55, 529–543 (2020).
Koleva, L. et al. Iron oxide and silicon nanoparticles modulate mineral nutrient homeostasis and metabolism in cadmium-stressed Phaseolus vulgaris. Front. Plant. Sci. 13, 806781 (2022).
Frary, A. et al. Salt tolerance in Solanum pennellii: antioxidant response and related QTL. BMC Plant. Biol. 10, 1–16 (2010).
Liang, X., Zhang, L., Natarajan, S. K. & Becker, D. F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 19, 998–1011 (2013).
Kohli, S. K. et al. Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8, 641 (2019).
Ghosh, S., Saha, J. & Biswas, A. K. Interactive influence of arsenate and selenate on growth and nitrogen metabolism in wheat (Triticum aestivum L.) seedlings. Acta Physiol. Plant. 35, 1873–1885 (2013).
Askary, M., Talebi, S. M., Amini, F. & Bangan, A. D. B. effects of iron nanoparticles on Mentha Piperita L. under salinity stress. Biologija 63, (2017).
Ahmed, M. A., Abdel-Fattah, G. H. & Shahin, S. M. The role of magnetic iron in enhancing the ability of Acalypha wilkesiana Müll. Arg Transplants Tolerate soil. Salinity J. Plant. Prod. 7, 379–384 (2016).
Acknowledgements
Authors are thankful to Researchers Supporting Project number (RSP2025R393), King Saud University, Riyadh, Saudi Arabia.
Funding
Researchers Supporting Project number (RSP2025R393), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
JN; Experimentation and Methodology, AAS; Conceptualization, Supervision and Validation, SU; Statistical analysis, writing-original draft preparation, SA; writing-revised draft preparation, MKG; Resource acquisition and Investigation, and SS & SJ; Data curation and Formal analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
We declare that the manuscript reporting studies do not involve any human participants, human data or human tissues. So, it is not applicable.
Our experiment follows with the relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Naseem, J., Shah, A.A., Usman, S. et al. Green synthesized FeNPs ameliorate drought stress in Spinacia oleracea L. through improved photosynthetic capacity, redox balance, and antioxidant defense. Sci Rep 15, 1782 (2025). https://doi.org/10.1038/s41598-024-84061-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84061-4
Keywords
This article is cited by
-
Evaluating the Toxicity Profile of Green-Synthesized Ferric Nanoparticles Using Madhuca indica in a Zebrafish Model
Biomedical Materials & Devices (2025)