Abstract
To reduce greenhouse emissions and producing electricity with the smallest environmental impact, developing solar power technology is one of the most important milestones to achieve. Thus, to improve the efficiency of the concentrated solar power (CSP) plants, with lower environmental impact, is of great interest. This work reports the development of nanofluids, a colloidal suspension of nanomaterials in a fluid, based on an environment-friendly base fluid for improving the performance of the heat transfer process in CSP plants. The nanofluids contain Pt nanoparticles in a linear silicone-based heat transfer fluid, and their stability is guaranteed for several weeks. Their properties of interest, density, surface tension, viscosity, isobaric specific heat and thermal conductivity were characterized to determine the performance of the nanofluids in solar thermal technology. Improvements of about 6% and 24% in specific heat and thermal conductivity were found, without significant increases in viscosity. In addition, their effect on the performance of collectors and heat exchangers in CSP using these nanofluids was analysed, and an enhancement of about 40% is found. Specific heat enhancements are also discussed in view of the strength of the interactions between methyl siloxane groups and low Miller index surfaces of Pt, with data from density functional theory simulations.
Similar content being viewed by others
Introduction
One of the challenges faced by society today is to meet the relentless demand for energy with the smallest environmental impact for the planet and with net-zero greenhouse gas emissions1,2. To achieve this goal, developing thermal solar energy technology is one of the most important milestones to achieve, paying attention in concentrating solar power (CSP). It plays an extremely important role as technology that converts thermal energy into electricity3,4. There is a variety of CSP technology, although two of these are more highly developed, namely the heliostat tower technology and the parabolic trough collectors (PTC). This technology involves the use of a heat transfer fluid (HTF) with the capacity to store and transport the energy captured towards a heat exchanger that, in turn, produces the steam that actions a turbine connected to an electric generator. The efficiency of the CSP-PTC plants depends to a certain extent on several properties of the HTF such as its density, viscosity and surface tension, and also on its thermophysical properties, such as its specific heat capacity and thermal conductivity. Therefore, optimizing the thermal properties of the fluid is key to improve the efficiency of this kind of solar power plant.
At present, CSP-PTC plants use synthetic oils as the HTF. The one normally used is the eutectic mixture (26.5:73.5) of biphenyl and diphenyl oxide. It possesses interesting thermal and rheological properties, but presents several drawbacks, mainly the fact that it is not environment-friendly. The mixture is classified in its safety datasheet as hazardous because it causes skin and eye irritation, reproductive and specific target organ toxicity, and is a short-term (acute) and long-term (chronic) aquatic hazard. Consequently, it is necessary to reconsider the fluid used in these plants. In this sense, new fluids based on the siloxane group, known as linear silicone fluids, have been developed recently. These fluids are dimethylsiloxane polymers and are generally called polydimethylsiloxane (PDMS). They have diverse applications and, depending on the number of monomers they are composed of, different thermal and rheological properties. With respect to the conventional synthetic oil used in CSP-PTC plants, this linear silicone-based fluid is environment-friendly, classified as a non-hazardous substance. Comparing the properties of interest for this application, this linear silicone-based fluid has similar thermal conductivity and viscosity compared to the HTF currently in use. But it can work at a higher operating temperature, up to 425 °C, while the typical eutectic mixture of diphenyl oxide and biphenyl works up to 400 °C5,6. However, some properties, such as density and specific heat, are inferior to those of the conventional fluid, which is a drawback that must be overcome. Therefore, it is of interest to perform research to improve the performance of the PDMS-fluid for its use in CSP-PTC plants.
One of the strategies for improving the thermophysical properties of HTFs is to design nanofluids. They are colloidal suspensions of nanomaterials in a fluid7 that exhibit a noticeable enhancement of their thermal and physical properties even at moderate nanoparticle concentrations. At the early stages of the research in nanofluids, researchers showed that adding nanoparticles to conventional fluids remarkably enhanced the thermal conductivity, thermal diffusivity, viscosity, and convective heat transfer coefficients compared to the base fluids. But the addition of nanomaterials in a fluid alters the rheology of the fluid because the viscosity and density are affected. Hence, understanding the rheological properties of the nanofluids is of great importance for the further development of their practical applications. Variables, such as the size, shape, and surface properties of the nanoparticles, affect the viscosity and thermal properties of the nanofluids, resulting in a surprisingly efficient heat transport compared to standard solid–liquid suspensions. If prepared so that they are highly stable, they usually present enhanced thermal properties with respect to the fluid alone due to the presence of new heat transfer and storage mechanisms8,9. Nanofluids are usually prepared using metal10,11 or metal oxide nanoparticles12,13, and different fluids, such as water14,15 or ethylene glycol16,17. Nanofluids with applications in CSP-PTC have also been prepared using the conventional fluid based on the eutectic mixture described before and different materials, such as metals18, metal oxides19,20,21, or transition metal dichalcogenides22. However, nanofluids using PDMS as the base fluid for application in CSP are scarcely reported because this is a new fluid in this application. Among the few experimental studies published, Wan et al. reported nanofluids prepared using dimethyl silicone oil and carbon nanotubes23. Aslfattahi et al. reported nanofluids based on MXene (Ti3C2) and silicone oil24. These fluids, namely dimethylsiloxane-based polymers, vary depending on the number of monomers and present different properties such as viscosity.
Therefore, the objective of this work is to prepare nanofluids based on a dimethylsiloxane-based fluid, which has been designed to be applied in CSP-PTC plants. This fluid could substitute the conventional HTF used, which show problems due to its toxicity. But, for being used, it is necessary improving its performance, and we propose here the development of nanofluids for improving the fluid’s properties. In turn, this kind of nanofluids has not been studied previously, which shows the novelty of this work. In addition, the nanomaterial used were commercial Pt nanoparticles because of their good thermal conductivity, as typically is found for metals. Also, Pt nanoparticles have been used previously for preparing nanofluids with interesting results25. The nanofluids prepared were widely characterized to determine their physical stability and other properties of interest, such as density, viscosity, surface tension, isobaric specific heat and thermal conductivity. In addition, an analysis of the performance of these nanofluids in CSP-PTC plants will be shown. The enhancement in thermal properties of nanofluids, particularly in specific heat, is very dependent on the chemistry of the solid–liquid interfaces. Density functional theory (DFT) simulations were accomplished to survey Pt-PDMS interfaces, which is of interest for the discussion of the experimental findings presented in this work and for future comparisons as more nanofluids will be produced with this brand new dimethylsiloxane-based HTF.
Methodology
Nanofluids preparation
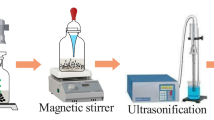
The nanofluids were prepared following a simple experimental procedure. All the chemicals were used as received. The base fluid was a commercial heat transfer fluid based on polydimethylsiloxane (PDMS, commercially named as Helisol 5A, supplied by Wacker, dynamic viscosity 4–6 mPa s) and platinum nanoparticles (Pt, nanopowder, size < 50 nm) were supplied by Sigma-Aldrich. Table 1 shows the basic properties of the base fluid and Pt nanoparticles. Two mass concentrations were prepared, 0.03% and 0.08%, without surfactant. The procedure to disperse the nanoparticles in the base fluid involved the following two steps. First, the weighted mass of Pt nanoparticles needed for both concentrations was added to 100 mL of the base fluid, and the resulting mixture was placed in an ultrasonic bath with a frequency of 37 MHz in continuous mode for ten minutes. In a second step, the previous dispersion was shaken using an ultra turrax machine (model T18 supplied by IKA) for ten minutes at 20,000 rpm. This methodology is able to supply high energy in all the volume, which is good for avoiding high local temperature increase that is typical when sonication method is used. To avoid the overheating of both the ultra turrax system and the nanofluids, the energy to disperse the nanoparticles was applied in a 5:10:5 min on:off:on routine. The nanofluids were then considered ready for characterization. Figure S1 in the Supplementary material summarizes the methodology used.
Nanofluids characterization
One of the main challenges in nanofluids is to obtain nanofluids with high physical stability for a prolonged time. For this reason, the stability of the nanofluids was analysed by means of UV–vis spectroscopy and the dynamic light scattering (DLS) technique to obtain the particle size of the agglomerates in suspension. Therefore, the change in the load of nanoparticles in the base fluid was analysed according to the extinction coefficient obtained from UV–vis spectroscopy by using a DH-2000-BAL light source and USB2000 + general purpose spectrometer, both supplied by OceanOptics. In addition, the particle size was analysed by measuring the solvodynamic diameter of the nanoparticles in suspension, estimated by DLS using a Zetasizer Nano ZS supplied by Malvern Instruments. Both the extinction coefficient and particle size were measured for 25 days, performing measurements 3 times per day. For particle size, each measurements involved the record of three dataset, for statistical relevance.
In addition, the nanofluids prepared were characterized to determine their properties of interest: surface tension, density, dynamic viscosity, isobaric specific heat and thermal conductivity. All these properties were measured after the nanofluids reached physical stability. Surface tension was measured by means of the pendant drop method using the OCA25 equipment supplied by Dataphysics. The dosage volume was 5 µL with a dosage rate of 0.1 µL s−1, using a Hamilton 250 µL syringe. Ten measurements were performed for each sample, at room temperature. Density, ρ, and isobaric specific heat, CP, are interesting properties because they define the sensible heat storage of an HTF according to \(\rho \cdot {C}_{P}\). Density was measured by using the excitation pulse technique (DM densitometer, Anton Paar). Five replicas were performed for each sample, at room temperature. Isobaric specific heat was measured by means of the temperature-modulated differential scanning calorimetry (TMDSC) technique, using a DSC 214 Polyma supplied by Netzsch. The temperature program was set as: (i) dynamic step from 20 to 100 °C at 10 °C min−1; (ii) isothermal step for 10 min; (iii) dynamic step from 100 to 20 °C at 10 °C min−1; (iv) isothermal step for 10 min; (v) temperature-modulated dynamic step from 20 to 205 °C at 1 °C min−1 with a modulation of ± 1 °C in amplitude and 120 s in periodicity. Steps i–iv were performed to purge the sample holder and thermally equilibrate the samples. Only step v is considered for determining the isobaric specific heat. The DSC analysis was carried out in triplicate for each sample, being these ones repetitive. Dynamic viscosity in the temperature range between 25 and 175 °C was measured using a concentric cylinder geometry and the shear rate ranged between 1 and 100 s−1, using a HR10 viscometer supplied by TA Instruments. Finally, the transient hot bridge (THB) technique and a hot point sensor (HPS) were used to measure thermal conductivity (THB-100, Linseis). The combination of the THB technique and HPS sensor leads to a decrease in the uncertainty of the measurements thanks to a reduction in the natural convection during the measurement. An input power of 30 mW was established, which is an adequate value for obtaining a good signal-to-noise ratio, using a measurement time of 20 s and a delay time between the replicas of 30 s to equilibrate the temperature. Ten replicas were performed for each sample and temperature, in the range between room temperature and 110 °C, controlled by a dry block heater, model DB 5.2, supplied by IKA.
DFT simulations of Pt-PDMS interfaces
We performed a DFT study of the adsorption of hexamethyldisiloxane (HMDS), on different Pt surfaces, using the VASP code26,27,28,29. Taking this small molecule as a simplified representation of PDMS is justified as the complex conformational profile of PDMS would preclude convergence within an affordable timeframe. The commercial Pt nanoparticles used in this work were synthesized by combustion chemical vapour deposition. Group 10 and 11 transition metals, with fcc lattice structures, are predicted to spontaneously grow into nanocrystals faceted by the low-index (111), (100), and (110) planes30. Therefore, Pt (111), Pt (100) and Pt (110) will be the surfaces of interest for this work. Surface models are represented by supercells in which the relevant surface terminations are limited by a vacuum slab of 20 Å that minimises unphysical interactions between periodic images in the surface normal direction. The HMDS molecule is initially placed on the surface terminations so that each Si atom is located at one of the high symmetry sites described in Fig. 1. A set of initial geometries were screened and submitted for structural relaxation. In these calculations we used the GGA-PBE functional31,32, including with Grimme’s DFT-D3 dispersion corrections with the Becke-Johnson damping function to correctly describe van der Waals interactions33,34. Valence wavefunctions were described by sets of planewaves with kinetic energies up to 400 eV. Interactions between valence electrons and ionic cores were described using PAW pseudopotentials35,36. Brillouin zone was sampled using a Monkhorst–Pack 3 × 3 × 1 k-point mesh37,38. A Methfessel-Paxton smearing of 0.2 eV was used to describe orbital occupancies39. Dipole moment corrections to the total energy were applied.
High-symmetry sites on (111), (100) and (110) surface terminations of fcc-structures transition metals. These visualizations were made with OVITO40.
Results and discussion
Nanofluids stability
The physical stability of the nanofluids with time was estimated by analysing changes in the load and size of the heat carriers. The load of Pt nanoparticles in the nanofluids prepared was analysed by the extinction coefficient, in which the extinction of the light due to absorption and scattering is considered. For this, UV–vis spectroscopy was used, and spectra were registered for 25 days. No changes were found in the shape of all the spectra registered, which means no chemical changes are observed in the nanofluids. Figure 2a shows the values of the spectral extinction at a wavelength of 500 nm recorded for 25 days for the two nanofluids prepared. The behaviour is similar for both nanofluids: a strong decrease in the spectral extinction observed during the first day, after which the values are practically constant for 24 days. This means dispersion process is performed successfully but after agglomeration and sedimentation occur quickly in the first few hours after nanofluid preparation. At zero time, there are differences in the spectral coefficient for both nanofluids, the nanofluid with the higher concentration of nanoparticles presenting the highest value, as expected. After the sedimentation process, the extinction values observed are similar for both nanofluids; that is, they tend to the same equilibrium situation, a similar number of nanoparticles therefore remaining in suspension.
In addition, the size of the agglomerated nanoparticles in suspension was analysed by the dynamic light scattering (DLS) technique. The particle size values obtained are shown in Fig. 2b. Again, the behaviour was similar for both nanofluids. During the first day, the values were higher, but these agglomerates settled and the values found after were lower. Thus, both nanofluids showed similar values for particle size, between 300 and 600 nm, with a certain deviation in the data due to the dynamic behaviour of the colloidal suspension. The deviation shows the particle size distribution generated in each measurement combined with the different measurements performed for each sample. The results from DLS are clearly in line with the spectral extinction analysis.
Nanofluids properties
Typically, the thermal performance of a heat transfer fluid can be analysed by the heat transfer coefficient, h, which is proportional to the Mouromtseff number, Mo, which depends on several of its properties, as given by
where k is thermal conductivity, ρ is density, CP is the isobaric specific heat, η the viscosity and σ is the surface tension. In addition, the exponents in Eq. (1) are empirical or theoretical constants depending on boundary conditions41. Typically, e is zero if no phase change occurs. Therefore, the effect of the surface tension will be significant if convective heat transfer process with phase change occurs. This is this way because during the boiling process, a portion of the liquid will vaporize and form bubbles, and surface tension plays an important role in the formation and detachment of bubbles42. This the case of this work, but surface tension was measured to confirm that he changes in the surface tension values are not significant. These five properties were characterized for the nanofluids prepared when they reached physical stability. Moreover, the base fluid used, that is the commercial PDMS, was also characterized in the same properties for comparison purposes.
Surface tension
Even though the HTF experiences no phase changes in CSP-PTC, this property was measured at room temperature. Table 2 shows the values measured for surface tension for the nanofluids and the base fluid. The standard deviation of surface tension measurements is better than ± 0.1%. The value measured for the base fluid was 18.89 mN m−1 at 25 °C. The manufacturer does not give values for surface tension in the technical datasheet, thus the measured value cannot be compared. The changes in surface tension for the nanofluids with respect to the base fluid are negligible, as they fall within the uncertainty of the measurement.
Density
Since many reports in the literature have shown that an increase in density leads to a more efficient heat transfer process43, the density of the base fluid and the Pt-based nanofluids was measured at 25 °C. The values obtained are also shown in Table 1. The standard deviation of density measurements is better than ± 0.01%. The density value obtained for the base fluid was 919.9 kg m−3 at 25 °C, which is in a good agreement with the value reported by the manufacturer (923.0 kg m-3), with a deviation of 0.3%. The values increased slightly for the nanofluids with respect to the base fluid, by 0.15% and 0.13% for the nanofluids with a Pt nominal mass concentration of 0.03% and 0.08%, respectively. The changes in the values between the nanofluids are also negligible as they fall within the uncertainty of the measurement. This result is in good agreement with the spectral extinction analysis, in which very similar values were found for both nanofluids. This means there is a similar number of heat carriers in suspension in the nanofluids after stabilisation, which leads to similar density values.
Dynamic viscosity
Viscosity is an important property for the application of the heat transfer fluids as it can limit their use in heat transfer processes. It is a key property because the incorporation of nanoparticles into a fluid typically leads to an increase in viscosity values, which is counterproductive for the application of nanofluids in heat transfer processes. Viscosity affects pumping power and can lead to pressure drops. Thus, viscosity was measured for the base fluid and for the Pt-based nanofluids at several temperatures in the 25–175 °C range. Figure 3 shows the shear flow plots of the base fluid and the nanofluids. As observed, for the shear rate range, the viscosity is constant, which means the base fluid and the Pt-based nanofluids show Newtonian behaviour. From these plots, the dynamic viscosity of the base fluid and the nanofluids was estimated. The standard deviation of viscosity measurements is better than ± 1.5%. Figure 4 shows the values obtained with respect to the temperature. At 25 °C, the value obtained for the base fluid was 4.73 mPa s, within the range supplied by the supplier (4–6 mPa s). As expected, the viscosity values for all the samples decreased with temperature, down to 0.96 mPa s for the base fluid at 175 °C. The most important result is that the viscosity values for the nanofluids prepared were close to those measured for the base fluid (see Fig. 4). A decrease of up to 2% is observed in the viscosity values for the nanofluids, but this difference is within the uncertainty of the technique. This result is of great interest because the changes in the viscosity of the nanofluids will not generate significant increases in pumping power, pressure drop or friction factor under conditions close to the application. In this sense, the friction factor was evaluated to analyse the performance of the nanofluids in comparison with the base fluid. It was estimated from44
where Re is the Reynolds number, which was calculated as
where Vav is the average fluid velocity in the pipe, which is defined using flow rates between 100 and 300 L/min. D is the inner pipe diameter, considered to be 0.066 m45. ρ and η are density and dynamic viscosity as was defined previously. Figure 5 shows the friction factor values obtained. As expected, the higher the temperature and the higher the flow rate, the lower the friction factor. No significant changes were observed for the nanofluids with respect to the base fluid, due to the slight changes in the values of density and viscosity, as it was previously explained. This is a promising result for the application of these nanofluids in CSP plants.
Isobaric specific heat
As discussed previously in Eq. (1), the higher the isobaric specific, the higher the heat transfer efficiency; thus, CP is a key property for evaluating the performance of heat transfer nanofluids. Therefore, this property was measured for both the nanofluids and the base fluid. The standard deviation of specific heat capacity measurements is better than ± 3.5%. The value measured for the base fluid was 1.474 J g−1 °C−1 at 25 °C, which is in a good agreement with the value reported by the supplier (1.483 J g−1 °C−1), with a deviation of 0.6%. As Fig. 6 shows, the changes in the isobaric specific heat for the nanofluids are minimal. The deviation in the measurements for all the nanofluids are within 1.8–2.4%. At room temperature, the CP values are slightly lower, but the trend is a little different with respect to the base fluid, and at 200 °C the values measured for the nanofluids are slightly higher. The maximum increase in the isobaric specific heat for the nanofluids at 200 °C is 5.8%. This implies that the nanofluids improve the thermal energy storage of the base fluid, but also affect the heat transfer process. Authors have reported previously increases in isobaric specific heat for nanofluids46,47,48, and the explanation of this phenomenon is of interest. Typically, it is related with the interaction of the fluid molecules with the nanomaterial, and its strength of the interactions between the components of the system46,47.
A parametrization of the Mouromtseff number, Mo, to analyse the convective heat transfer efficiency for heat transfer fluids in parabolic trough collectors was performed by Lenert et al.49. They reported the Mo ∝ CP1.6 relationship, which gives an idea of the impact of the enhancement of the isobaric specific heat. For the highest increase obtained for the Pt-based nanofluids, an enhancement factor of the Mo number of 1.09 was found. This means using these nanofluids can be of interest in CSP technology.
Thermal conductivity
Thermal conductivity is the most important property for evaluating the performance of heat transfer fluids. This property was measured for the Pt-based nanofluids prepared and the base fluid for comparison purposes. The standard deviation of thermal conductivity measurements is better than ± 3.5%. The value measured for the base fluid was 0.127 W m-1 K−1 at 25 °C, which is in a good agreement with the value reported by the supplier (0.126 W m−1 K−1), with a deviation of 0.8%. In the whole range of measurements, our values fit (by an average of 98.5%) the thermal conductivity values reported in the manufacturer’s datasheet. The values obtained are shown in Fig. 7a. The trend with temperature is different for the nanofluids, which has been reported previously for several nanofluids18. This could be due to the presence of a different heat conduction mechanism in nanofluids with respect to pure fluids, such as Brownian motion or clustering phenomenon50. Moreover, an increase in the thermal conductivity values for the nanofluids with respect to the base fluids is clearly observed. Figure 7b shows the ratio of the thermal conductivity of nanofluids with respect to the fluid, knf/kbf. Enhancements of up to 24% at about 110 °C were obtained for the Pt-based nanofluids. This increase is clearly higher than the uncertainty of the measurements, deviations up to 2.5% was fund at higher temperatures. The difference between both nanofluids was not significant, probably due to the similar final load of nanoparticles in the fluids after the stabilization period.
Nanofluids performance in CSP-PTC
The study of the performance of the nanofluids prepared in this work in CSP-PTC systems was developed using the analytic equations and solutions reported by Bellos and Tzivanidis51, which evaluate the outlet temperature (Tout) and the collector efficiency (Ψcoll) for a 1D surface-absorber PTC under turbulent flow and steady state conditions. Both, the outlet temperature and the collector efficiency were calculated by using:
All the details about these equations used, and variables and parameters in them are reported in the Supplementary Information. This model was validated by Bellos and Tzivanidis51 with the literature’s experimental results, supplied by Dudley et al.52 about the LS-2 PTC. Eight different cases were examined. The mean deviation in the outlet temperature is found to be 0.06%. This value is low and so it is proved that the model used in this work is valid for various operating conditions.
In addition, the performance of a heat exchanger performance (Ψhex) in which phase change for one of the fluids (water evaporation) occurs was also calculated using the number of transfer units (NTU) methodology53. Finally, the overall system performance is calculated by Ψsys = ΨcollΨhex. All the details about these calculations are included in the Supplementary Information.
The maximum outlet temperature for the linear silicone fluid its maximum permitted working temperature, 425 °C, according to the technical datasheet. Therefore, the first calculation is to estimate the collector length, for different flow rates, at which this maximum temperature is reached using the base fluid and the nanofluid. This calculation was performed using the values of the properties of interest measured (density, surface tension, viscosity, isobaric specific heat and thermal conductivity) for the base fluid and for the nanofluid labelled as Pt-0.03%, because the nominal amount of nanomaterial is lower and the properties are very similar to those obtained for the nanofluid with the highest nominal concentration of nanomaterial. Do note that the upper limit of the range of characterisation temperatures is limited by either the boiling point of the HTF (215 °C) or the maximum reliable working temperature for the apparatus, whichever is more restrictive. For temperatures beyond this limit, we extrapolate the data for nanofluid properties from fitting functions with the same mathematical shape than those observed for the properties of the HTF (e.g., a linear fit for specific heat capacity vs. temperature, or a negative exponential fit for dynamic viscosity vs. temperature) using the datasets obtained from characterisation as a source for the fitting. The flow rate values are those used for calculating the friction factor previously, that is in the 100–300 L min-1 range. Thus, Fig. 8 shows the estimated outlet temperature with respect to the collector length, estimated according to Equations S1-S7 in the Supplementary Information. The required collector length to achieve 425 °C increases with the flow rate, because under constant irradiation of the absorber, a higher flow rate implies a lower time of residence of the fluid in the collector and, therefore, a lower outlet temperature. The flow rate for a typical surface collector is about 150 L min−1 (high flow rates are typically required to prevent overheating and degradation of the absorber), and the collector length estimated is ~ 227 m and ~ 252 m for the base fluid and the nanofluid, respectively. This is due to the higher storable energy density (that is the product of the specific heat and density) for the nanofluid with respect to the base fluid, which leads to a longer collector for reaching the outlet temperature. However, this higher energy density for the nanofluid favours the steam generation in heat exchangers, as it is discussed below.
In addition, the overall performance of the system was estimated as it is shown above, considering the efficiency of the collector and that of the heat exchanger, using the same fluids and flow rate range. Figure 9 shows the values obtained for the efficiencies of the collector, the heat exchanger and the overall system. As it is observed, the efficiency of the collector does not show significant changes with respect to the flow rate or the use of the base fluid or the nanofluid. For the typical flow rate (150 L min-1), the increase in the efficiency of the collector for the use of the nanofluid is lower than 1%. In terms of the heat exchanger efficiency, we observe a decrease in its efficiency when the flow rate increases, as consequence of a decrease in the number of transfer units. On the other hand, the changes in the efficiency of the heat exchanger are significant when the nanofluid is used, increasing for all flow rates tested. For a flow rate of 150 L min−1, the heat exchanger efficiency is about 0.256 and 0.366 for the base fluid and the Pt-0.03% nanofluid respectively. This means an increase of about 43%, which is clearly significant. Therefore, the increase in the heat exchanger efficiency for the nanofluid leads to an increase in the overall system efficiency, calculated as Ψsys = ΨcollΨhex. The highest overall efficiency estimated was 29.2% for a flow rate of 100 L min-1. The flow rate for a typical surface collector is about 150 L min-1, and at this rate the overall efficiency is 18.6% and 26.8% for the base fluid and the Pt-0.03% nanofluid, an increase in efficiency of about 44%.
Interaction of PDMS with Pt surfaces
The chemistry of solid–liquid interfaces and its relationship with nanofluids stability and energy storage and transport capabilities is an uncharted territory we are just starting to understand. Some reports about how affects the interaction of the base fluid molecules with the nanomaterial used has been reported, such as the adsorption phenomenon of Cu and CuO nanoparticles in water show how this phenomenon help to explain the thermal conductivity values54. In previous works by our group46,47, we have been able to prove that the achievable enhancements in specific heat by dispersing a nanostructured solid in a liquid are determined by the chemistry of the interface that appears between them. Particularly, we found that the stronger the interactions between species at solid–liquid interfaces, the higher the specific heat enhancements. Probing the energetics of the Pt-PDMS interface using DFT helps to validate the experimental findings presented in previous sections of this paper, but more importantly, situates the chosen solid–liquid pair in a shared framework of solid–liquid affinity that is ultimately related to is thermal performance, thus allowing to compare it to previous and future results.
Figure 10 showcases the well-converged, non-redundant adsorption structures found for HMDS at Pt surfaces. It also lists, for each structure, the sites on which Si atoms are located and their adsorption energy. Adsorption energies were calculated by subtracting the free energy of the corresponding bare surface termination and the gas phase molecule to that of the slab, each of them relaxed at the same level-of-theory. The chemical nature of the species involved in these interfaces and the order of magnitude of the adsorption energies predicted by DFT indicate adsorption in these cases is mediated by van der Waals forces only. The relative stability of the presented adsorption structures of HMDS at different sites is hardly arguable in terms of a preferential directionality, as no chemical bonding occurs. The adsorption energy differences just respond to the preferential occupation of sites in which van der Waals forces are maximised with minimum distortions to molecular geometry.
Geometries and adsorption energies for the HMDS molecule at different sites on Pt (111), Pt (100) and Pt (110) surfaces. These visualizations were made with OVITO40.
The magnitude of adsorption energy of HMDS on Pt surfaces, within 1.2–1.4 eV, is lower than the values reported for the adsorption of diphenyl ether and biphenyl (their eutectic and azeotropic mixture, commercially available as Dowtherm A, is also used as HTF for CSP-PTC plants) on Pd surfaces, within 3.5–4.7 eV46. In the latter case, interactions between species at interfaces were ruled by chemical bonding. This explains why the specific heat enhancement in Pt-PDMS nanofluids is modest compared to Pd-Dowtherm A nanofluids. While the experimental findings on specific heat are still positive, there is still room for improvement in favour of the application. This work opens the search for materials with a stronger chemical affinity for PDMS so that greater enhancements in thermal properties can be achieved.
Conclusions
In this work, nanofluids based on a linear silicone heat transfer fluid and Pt nanoparticles were developed to analyse their use in concentrated solar power plants based on parabolic trough collector technology. The nanofluids prepared reached a similar equilibrium after a few days, after which they remained stable. With respect to properties of interest of the nanofluids, the changes in surface tension and density for the nanofluids with respect to the base fluid are quite small, and they are close to the uncertainty of the measurements. Moreover, a decrease of up to 2% in the viscosity values is observed for the nanofluids, but this result is within the uncertainty values of the technique used. In addition, the maximum increase in the isobaric specific heat for the nanofluids at 200 °C is 5.8%. This implies the nanofluids improve the thermal energy storage of the base fluid, but also affect the heat transfer process. Finally, enhancements in thermal conductivity of up to 24% at about 110 °C were obtained for the Pt-based nanofluids.
The values of the properties measured were applied in a model to evaluate the efficiency of the collector and the heat exchanger in a CSP-PTC plant, enabling its overall efficiency to be estimated. An enhancement of about 44% in the overall efficiency of CSP-PTCs plants was found by using the Pt nanofluids. This means the use of the nanofluids prepared in this work can be of interest in CSP technology, because of this enhancement is significant. Future research should be focused on the test of the nanofluids in prototypes closer to reality, in order to confirm the promising features observed in this work.
DFT simulations revealed that the adsorption of PDMS on Pt surfaces is mediated by van der Waals forces only, which explains why the specific heat enhancements in Pt-PDMS nanofluids are modest compared, for instance, to Pd-Dowtherm A nanofluids, in which adsorption is mediated by chemical bonding. We will continue to explore more nanostructured materials and their affinity with PDMS, aiming for the best thermal performance.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- C A :
-
Concentration factor (WL to πRro2L ratio)
- CP :
-
Isobaric specific heat (J Kg−1 °C−1)
- D:
-
Inner pipe diameter (m)
- h:
-
Heat transfer coefficient (W m−2 K−1)
- k:
-
Thermal conductivity (W m−1 °C−1)
- f:
-
Friction factor
- G s :
-
Solar irradiation (W m−2)
- L:
-
PTC array total length (m)
- Mo:
-
Mouromtseff number (W m−2 K−1)
- Re:
-
Reynolds number
- T:
-
Temperature (°C)
- V:
-
Flow rate (m3 s−1)
- Vav :
-
Average fluid velocity (m s−1)
- W :
-
Concentrator mirror aperture width (m)
- \(\uprho\) :
-
Density (kg m−3)
- η:
-
Dynamic viscosity (Pa s)
- λ:
-
Wavelength (m)
- σ:
-
Surface tension (N m)
- ψ:
-
Efficiency (–)
- ṽ:
-
Flow rate (l s−1)
- amb:
-
Ambient
- bf:
-
Base fluid
- coll:
-
Collector
- hex:
-
Heat exchanger
- in:
-
Inlet
- nf:
-
Nanofluid
- out:
-
Outlet
- sys:
-
Overall CSP-PTC system
- CSP:
-
Concentrating solar power
- DFT:
-
Density functional theory
- DLS:
-
Dynamic light scattering
- DSC:
-
Differential scanning calorimetry
- GGA:
-
Generalized gradient approximations
- HMDS:
-
Hexamethyldisiloxane
- HPS:
-
Hot point sensor
- HTF:
-
Heat transfer fluid
- MXene:
-
Ti3C2
- NTU:
-
Number of transfer units
- PAW:
-
Projector augmented wave
- PBE:
-
Perdew–Burke–Ernzerhof
- PDMS:
-
Polydimethylsiloxane
- PTC:
-
Parabolic trough collector
- TMDSC:
-
Temperature-modulated differential scanning calorimetry
- THB:
-
Transient hot bridge
- UV–Vis:
-
Ultraviolet–visible spectroscopy
- VASP:
-
Vienna Ab-initio simulation package
References
Khan, J. & Arsalan, M. H. Solar power technologies for sustainable electricity generation - A review. Renew. Sust. Energ. Rev. 55, 414–425. https://doi.org/10.1016/j.rser.2015.10.135 (2016).
Gómez-Villarejo, R. et al. Ag-based nanofluidic system to enhance heat transfer fluids for concentrating solar power: Nano-level insights. Appl. Energy 194, 19–29. https://doi.org/10.1016/j.apenergy.2017.03.0033 (2017).
Desideri, U., Zepparelli, F., Morettini, V. & Garroni, E. Comparative analysis of concentrating solar power and photovoltaic technologies: Technical and environmental evaluations. Appl. Energy 102, 765–784. https://doi.org/10.1016/j.apenergy.2012.08.033 (2013).
Singh, A. & Baredar, P. Techno-economic assessment of a solar PV, fuel cell, and biomass gasifier hybrid energy system. Energy Rep. 2, 254–260. https://doi.org/10.1016/j.egyr.2016.10.001 (2016).
https://www.wacker.com/h/en-us/medias/HELISOL-5A-en-2024.06.16.pdf.
Bakthavatchalam, B., Habib, K., Saidur, R., Saha, B. B. & Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 305, 112787. https://doi.org/10.1016/j.molliq.2020.112787 (2020).
Rajendran, D. R. et al. Review on influencing parameters in the performance of concentrated solar power collector based on materials, heat transfer fluids and design. J. Therm. Anal. Calorim. 140, 33–51. https://doi.org/10.1007/s10973-019-08759-8 (2020).
Iacobazzi, F., Milanese, M., Colangelo, G., Lomascolo, M. & de Risi, A. An explanation of the Al2O3 nanofluid thermal conductivity based on the phonon theory of liquid. Energy 116, 786–794. https://doi.org/10.1016/j.energy.2016.10.027 (2016).
Jin, C., Wu, Q. B., Yang, G. Q., Zhang, H. Y. & Zhong, Y. F. Investigation on hybrid nanofluids based on carbon nanotubes filled with metal nanoparticles: Stability, thermal conductivity, and viscosity. Powder Technol. 389, 1–10. https://doi.org/10.1016/j.powtec.2021.05.007 (2021).
Jin, X., Guan, H. Q., Wang, R. J., Huang, L. Z. & Shao, C. The most crucial factor on the thermal conductivity of metal-water nanofluids: Match degree of the phonon density of state. Powder Technol. 412, 117969. https://doi.org/10.1016/j.powtec.2022.117969 (2022).
Hasan, H. A., Hatem, A. A., Abd, L. A., Abed, A. M. & Sopian, K. Numerical investigation of nanofluids comprising different metal oxide nanoparticles for cooling concentration photovoltaic thermal CPVT. Clean Eng. Technol. 10, 100543. https://doi.org/10.1016/j.clet.2022.100543 (2022).
Karakas, A., Harikrishnan, S. & Oztop, H. F. Preparation of EG/water mixture-based nanofluids using metal-oxide nanocomposite and measurement of their thermophysical properties. Therm. Sci. Eng. Prog. 36, 101538. https://doi.org/10.1016/j.tsep.2022.101538 (2022).
Banisharif, A., Estellé, P., Rashidi, A., Van Vaerenbergh, S. & Aghajani, M. Heat transfer properties of metal, metal oxides, and carbon water-based nanofluids in the ethanol condensation process. Coll. Surf. A 622, 126720. https://doi.org/10.1016/j.colsurfa.2021.126720 (2021).
Loong, T. T., Salleh, H., Khalid, A. & Koten, H. Thermal performance evaluation for different type of metal oxide water based nanofluids. Case Stud. Therm. Eng. 27, 101288. https://doi.org/10.1016/j.csite.2021.101288 (2021).
Elboughdiri, N. et al. Towards a novel EMHD dissipative stagnation point flow model for radiating copper-based ethylene glycol nanofluids: An unsteady two-dimensional homogeneous second-grade flow case study. Case Stud. Therm. Eng. 45, 102914. https://doi.org/10.1016/j.csite.2023.102914 (2023).
Sundar, L. S. & Shaik, F. Laminar convective heat transfer, entropy generation, and exergy efficiency studies on ethylene glycol based nanofluid containing nanodiamond nanoparticles. Diam. Relat. Mater. 131, 109599. https://doi.org/10.1016/j.diamond.2022.109599 (2023).
Carrillo-Berdugo, I. et al. Optical and transport properties of metal-oil nanofluids for thermal solar industry: Experimental characterization. Perform. Assess., Mol. Dyn. Insights, ACS Sustain. Chem. Eng. 9, 4194–4205. https://doi.org/10.1021/acssuschemeng.1c00053 (2021).
de los-Santos, D. M. et al. NiO nanowire-containing heat transfer nanofluids for CSP plants: Experiments and simulations to promote their application. J. Mol. Liq. 361, 119593. https://doi.org/10.1016/j.molliq.2022.119593 (2022).
Potenza, M., Milanese, M., Colangelo, G. & de Risi, A. Experimental investigation of transparent parabolic trough collector based on gas-phase nanofluid. Appl. EnergY 203, 560–570. https://doi.org/10.1016/j.apenergy.2017.06.075 (2017).
Colangelo, G., Favale, E., Miglietta, P., Milanese, M. & de Risi, A. Thermal conductivity, viscosity and stability of Al2O3-diathermic oil nanofluids for solar energy systems. Energy 95, 124–136. https://doi.org/10.1016/j.energy.2015.11.032 (2016).
Navas, J. et al. MoS2 nanosheets vs nanowires: Preparation and a theoretical study of highly stable and efficient nanofluids for concentrating solar power. J. Mater. Chem. A 6, 14919–14929. https://doi.org/10.1039/c8ta03817a (2018).
Wan, M. H., Xu, B., Shi, L., Zheng, N. B. & Sun, Z. Q. The dynamic stability of silicone oil-based MWCNT nanofluids under high-temperature, high-flux irradiation, and shear-flow conditions. Powder Technol. 424, 118508. https://doi.org/10.1016/j.powtec.2023.118508 (2023).
Aslfattahi, N., Samylingam, L., Abdelrazik, A. S., Arifutzzaman, A. & Saidur, R. MXene based new class of silicone oil nanofluids for the performance improvement of concentrated photovoltaic thermal collector. Sol Energy Mat. Sol. C 211, 110526. https://doi.org/10.1016/j.solmat.2020.110526 (2020).
Gómez-Villarejo, R. et al. Towards the improvement of the global efficiency of concentrating solar power plants by using Pt-based nanofluids: The internal molecular structure effect. Appl. Energy 228, 2262–2274. https://doi.org/10.1016/j.apenergy.2018.07.062 (2018).
Kresse, G. & Hafner, J. Ab-Initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269. https://doi.org/10.1103/PhysRevB.49.14251 (1994).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50. https://doi.org/10.1016/0927-0256(96)00008-0 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186. https://doi.org/10.1103/PhysRevB.54.11169 (1996).
Kresse, G., Vogtenhuber, D., Marsman, M., Kaltak, M., Karsai, F. & Schlipf, M. Vienna Ab-initio Simulation Package (VASP), 5.4.4 https://www.vasp.at (2017).
Tao, A. R., Habas, S. & Yang, P. D. Shape control of colloidal metal nanocrystals. Small 4, 310–325. https://doi.org/10.1002/smll.200701295 (2008).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple (vol 77, pg 3865. Phys. Rev. Lett. 78(1997), 1396–1396. https://doi.org/10.1103/PhysRevLett.77.3865 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104. https://doi.org/10.1063/1.3382344 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465. https://doi.org/10.1002/jcc.21759 (2011).
Kresse, G. & Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition-elements. J. Phys.-Condens. Mat. 6, 8245–8257. https://doi.org/10.1088/0953-8984/6/40/015 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775. https://doi.org/10.1103/PhysRevB.59.1758 (1999).
Monkhorst, H. J. & Pack, J. D. Special points for brillouin-zone integrations. Phys. Rev. B 13, 5188–5192. https://doi.org/10.1103/PhysRevB.13.5188 (1976).
Pack, J. D. & Monkhorst, H. J. Special points for brillouin-zone integrations - Reply. Phys. Rev. B 16, 1748–1749. https://doi.org/10.1103/PhysRevB.16.1748 (1977).
Methfessel, M. & Paxton, A. T. High-precision sampling for brillouin-zone integration in metals. Phys. Rev. B 40, 3616–3621. https://doi.org/10.1103/PhysRevB.40.3616 (1989).
Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the open visualization tool. Model Simul. Mater. Sc. 18, 015012. https://doi.org/10.1088/0965-0393/18/1/015012 (2010).
Wen, D. S., Lin, G. P., Vafaei, S. & Zhang, K. Review of nanofluids for heat transfer applications. Particuology 7, 141–150. https://doi.org/10.1016/j.partic.2009.01.007 (2009).
Su, G. F. et al. Review on factors affecting nanofluids surface tension and mechanism analysis. J. Mol. Liq. 407, 125159. https://doi.org/10.1016/j.molliq.2024.125159 (2024).
Pastoriza-Gallego, M. J. et al. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J. Appl. Phys. 106, 064301. https://doi.org/10.1063/1.3187732 (2009).
Fang, X. D., Xu, Y. & Zhou, Z. R. New correlations of single-phase friction factor for turbulent pipe flow and evaluation of existing single-phase friction factor correlations. Nucl. Eng. Des. 241, 897–902. https://doi.org/10.1016/j.nucengdes.2010.12.019 (2011).
Bellos, E. & Tzivanidis, C. Thermal efficiency enhancement of nanofluid-based parabolic trough collectors. J. Therm. Anal. Calorim. 135, 597–608. https://doi.org/10.1007/s10973-018-7056-7 (2019).
Carrillo-Berdugo, I., Midgley, S. D., Grau-Crespo, R., Zorrilla, D. & Navas, J. Understanding the specific heat enhancement in metal-containing nanofluids for thermal energy storage: Experimental and Ab initio evidence for a strong interfacial layering effect. ACS Appl. Energ. Mater. 3, 9246–9256. https://doi.org/10.1021/acsaem.0c01556 (2020).
Carrillo-Berdugo, I., Grau-Crespo, R., Zorrilla, D. & Navas, J. Interfacial molecular layering enhances specific heat of nanofluids: Evidence from molecular dynamics. J. Mol. Liq. 325, 115217. https://doi.org/10.1016/j.molliq.2020.115217 (2021).
de los Santos, D. et al. Nanofluids based on Pd nanoparticles and a linear silicone-based fluid: Toward highly efficient heat transfer fluids for concentrated solar power. ACS Sustain. Chem. Eng. 12, 2375–2385. https://doi.org/10.1021/acssuschemeng.3c07285 (2024).
Lenert, A. & Wang, E. N. Optimization of nanofluid volumetric receivers for solar thermal energy conversion. Sol. Energy 86, 253–265. https://doi.org/10.1016/j.solener.2011.09.029 (2012).
Iacobazzi, F., Milanese, M., Colangelo, G. & de Risi, A. A critical analysis of clustering phenomenon in Al2O3 nanofluids. J. Therm. Anal. Calorim. 135, 371–377. https://doi.org/10.1007/s10973-018-7099-9 (2019).
Bellos, E. & Tzivanidis, C. Development of analytical expressions for the incident angle modifiers of a linear Fresnel reflector. Sol. Energy 173, 769–779. https://doi.org/10.1016/j.solener.2018.08.019 (2018).
Dudley, V. E., Kolb, G. J., Mahoney, A. R., Mancini, T. R., Matthews, C. W., Sloan, M. & Kearney, D. Test results: SEGS LS-2solar collector. Technical Report https://doi.org/10.2172/70756 (1994).
Bergman, T. L., Lavine, A. S., Incropera, F. P. & Dewitt, D. P. Fundamentals of heat and mass transfer 7th edn. (Wiley, 2011).
Milanese, M., Iacobazzi, F., Colangelo, G. & de Risi, A. An investigation of layering phenomenon at the liquid-solid interface in Cu and CuO based nanofluids. Int. J. Heat Mass Tran 103, 564–571. https://doi.org/10.1016/j.ijheatmasstransfer.2016.07.082 (2016).
Acknowledgements
This work was supported by MCIN/AEI/https://doi.org/10.13039/501100011033 and European Union “NextGenerationEU”/PRTR” [grant number TED2021-132518B-I00]; and by Ministerio de Universidades del Gobierno de España for the allocated budget from the NextGenerationEU programme directed to public universities for the requalification of the Spanish university system, which funds I.C.-B.’s postdoctoral position at the University of Cadiz in the form of a Margarita Salas fellowship [grant number 2021-067/PN/MS-RECUAL/CD]; the University of Cadiz’s high performance computing service for research. Lab diagrams in Fig. 1 were created with the online editor Chemix (https://chemix.org), using I.C.-B.’s ‘Boost’ subscription, which grants permission for publishing and commercial use.
Author information
Authors and Affiliations
Contributions
Conceptualization: Juan Jesús Gallardo; Methodology: Juan Jesús Gallardo, Desireé De los Santos, Iván Carrillo-Berdugo; Investigation: Juan Jesús Gallardo, Desireé De los Santos, Iván Carrillo-Berdugo; Formal analysis: Iván Carrillo-Berdugo; Writing—original draft preparation: Juan Jesús Gallardo, Iván Carrillo-Berdugo; Writing—Review & Editing: Rodrigo Alcántara, Javier Navas; Supervision: Rodrigo Alcántara, Javier Navas; Project administration: Javier Navas; Funding acquisition: Javier Navas.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gallardo, J.J., De los Santos, D., Carrillo-Berdugo, I. et al. On the enhancement of the efficiency of concentrated solar power plants using nanofluids based on a linear silicone fluid and Pt nanoparticles. Sci Rep 15, 3586 (2025). https://doi.org/10.1038/s41598-024-84490-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84490-1