Abstract
In modern knee arthroplasty, surgeons increasingly aim for individualised implant selection based on data-driven decisions to improve patient satisfaction rates. The identification of an implant design that optimally fits to a patient’s native kinematic patterns and functional requirements could provide a basis towards subject-specific phenotyping. The goal of this study was to achieve a first step towards identifying easily accessible and intuitive features that allow for discrimination between implant designs based on kinematic data. A squat-cycle was simulated on eight fresh frozen specimens mounted in a weight-bearing knee rig, each initially tested under native conditions, and then after implantation with four different implant types (CR/CS, MS, LS, and PS). The kinematic signals of these five configurations were compared to determine whether key differences between implants could be detected leveraging two methodological approaches: (1) statistical parametric mapping to directly compare waveforms and (2) simple paired t-tests to compare the three-dimensional coordinates of the functional centres of rotation determined using a previously published REference FRame Alignment Method (REFRAME). While statistical parametric mapping of the kinematic data revealed only small differences in certain comparisons (e.g. LS vs. PS, and MS vs. LS) under lenient statistical testing conditions, the application of REFRAME showed clear differences between implants (for all implant combinations except for CR/CS vs. LS), even under conservative statistical testing. Since for most implant combinations, significant differences in the centres of rotation were found using REFRAME, this approach could present a suitable tool for discriminating between the kinematics of different implant types. Preoperative assessment of joint kinematics, combined with this REFRAME application, could therefore provide a key approach for improved clinical selection of implant type.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) has evolved considerably in recent decades1,2. No longer restricted to the use of Walker, Ranawat and Insall’s total condylar prosthesis design3 in a neutral mechanical alignment, orthopaedic surgeons today are free to choose from a variety of options. Implant designs with different types of bearing mobility are available (i.e. fixed vs. mobile bearing), in addition to different extend of constraint (e.g. cruciate retaining, posterior stabilised, etc.)4. Moreover, surgical protocols can be adapted based on the decision to opt for or against cemented fixation and patella resurfacing4, as well as based on the selected limb alignment technique (mechanical vs. kinematic alignment)5. Unfortunately, despite these notable improvements, up to 20% of patients continue to express dissatisfaction with the clinical outcome6,7,8 of TKA procedures.
Having a wide array of implant designs and surgical techniques at our disposal certainly has the potential to be advantageous, as they offer clinicians the flexibility to adapt treatment protocols to better address patient-specific needs. However, successfully tailoring surgical intervention to a particular patient requires an understanding of the effects that the different choices have on post-operative patient function. Previous studies have attempted to tackle this challenge by focusing on the identification of patient “phenotypes” (i.e. patient subgroups or clusters) in healthy populations9,10,11, as well as among patients affected by osteoarthritis12,13, and even among TKA patients specifically14. Surgeons could then adapt treatment protocols by selecting the optimal components for implantation with the underlying goal of recreating the healthy native kinematic patterns associated with a patient’s phenotype. Such an approach could provide the fundamental benefit of recreating a loading environment where the surrounding soft tissues are not subject to overloading15,16,17, hence avoiding unnecessary pain.
A TKA approach that is based on patient phenotyping could prove benefits over the more standard use of a one-implant-design-fits-all approach, by recognising patient-specific characteristics, while managing to avoid the considerable time and expense—as well as biomechanical and biotribological limitations—associated with custom-made implants. Most approaches seeking to identify kinematics-driven phenotypes tend to cluster subjects directly based on the kinematic curves of their tibio-femoral joint e.g. over a level walking cycle9,10,11,12. A key limitation of this phenotyping approach is that kinematic signals are inherently highly dimensional, complex, and subject to considerable intra- and inter-subject (as well as -session and -observer) variability18,19,20. Recent studies have highlighted the extent to which the shape and magnitude of kinematic signals are easily influenced by even minor variations in the position and/or orientation of local reference frames21,22,23,24. This is especially critical since different sources of variability are introduced into any motion capture process (e.g. gait lab or intraoperatively), and these will therefore critically limit our ability to robustly phenotype an individual. For example, the intra- and inter-observer variability for identifying a trans-epicondylar axis has been reported to be as high as ~ 5° and 9°, respectively25. Similarly, others found that the angular deviation between knee flexion/extension axes, which had been identified radiographically using different approaches, was approximately 3° on average26. Moreover, the levels of uncertainty affecting joint axes determined from e.g. optical markers placed on the skin, without access to direct physical visualisation (intra-operatively) or radiographic images of the underlying bones, can feasibly be even higher, especially considering the effect of soft-tissue artefact27. Importantly, however, even small errors in the resulting reference frames are known to fundamentally alter the interpretation of the underlying movement patterns21. As a result, our current ability to phenotype an individual for suitable implant selection critically depends on having a reliable representation of the joint movement patterns.
In this study, we explore the foundations for a novel phenotyping approach by moving the focus away from the kinematic signals to instead concentrate on the extraction of a key feature from these signals. The proposed conceptual foundation for a future phenotyping framework manages to reduce dimensionality and simplify interpretation, while relying on a feature that, rather than being limited to a discrete timepoint, successfully encompasses joint behaviour throughout the entire gait cycle: the joint’s functional centre of rotation (COR). Previously analysed for natural knees in cadaveric specimens over passive flexion28, in vivo during level walking with optical motion capture29, as well as during several activities of daily living using moving video-fluoroscopy30, the position of the COR of the knee in the transverse plane can act as a valuable characterisation of the relative motion of the femoral condyles relative to the tibial plateau. While studies investigating the position of these CORs in knee arthroplasty implants have been performed previously31,32,33, their in vivo nature restricted their ability to control for pre-existing differences between subjects’ native knee CORs.
By leveraging the recently published REference FRame Alignment MEthod (REFRAME)24, our approach focuses on utilising the position of the femoral centre of rotation as a relevant feature for phenotyping an individual. In this context, the REFRAME approach is able to identify a cross-talk-free representation of tibio-femoral translations, thereby identifying the joint’s functional COR. Here, in this proof-of-concept study, we investigate the extent to which REFRAME can be applied to tibio-femoral kinematic signals in order to discriminate between the kinematics of different implant designs. We hypothesised that the position of the functional femoral COR would differ between implant designs. For example, we predicted that a medial-stabilised (MS) design would lead to a medially placed rotation centre. Analogously, a lateral-stabilised (LS) implant would result in a rotation centre that is located laterally within the joint. On the other hand, the functional rotation centre for a posterior-stabilised (PS) implant was predicted to be positioned more posteriorly and distally, likely due to the femoral rollback during flexion enforced by its geometry. Finally, we expected a cruciate retaining/cruciate sacrificing (CR/CS) implant to produce a greater distribution of COR positions, given the comparably less constrained geometry associated with this design. Assessing these effects ex vivo (i.e. using cadaveric specimens) allowed us to evaluate how each subject’s native kinematics were affected by the use of four different implant designs.
Methods
Experimental setup
Eight fresh frozen cadaver knee specimens (4 female; 3 right; aged 88.4 ± 4.6 years; Table 1) were tested on an established force-controlled knee rig34,35,36 in a study approved by the ethics committee of the LMU Munich (ID 58-16). Informed consent for donation to scientific research had been signed before death by the donors or after death by their relatives and all methods were performed in accordance with the relevant guidelines and regulations. Two additional specimens that were initially also tested as part of this cohort were excluded from analyses as specimen characteristics hindered the implantation of certain prosthesis designs and/or the execution of parts of the full experimental protocol.
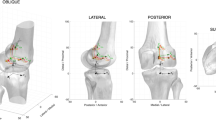
The fibula head of every specimen was attached to the proximal tibia using a cortical screw. The femur and the tibia were embedded into metal pots with epoxy resin (RenCast FC 52/53 Isocyanate & FC 53 Polyol, Huntsman Advanced Materials GmbH, Texas, USA) after cutting them 20 cm proximally and 22 cm distally from the epicondylar line. The vastus medialis, vastus lateralis, M. semitendinosus, and biceps femoris were attached to metallic finger traps (Bühler-Instrumente Medizintechnik GmbH, Tuttlingen, Germany) to apply constant muscle forces of 20 N during the entire load cycle (Fig. 1). The tibial fixation component consisted of a mobile bearing that allowed for tibial rotations and was free to translate mediolaterally. This general setup has been described in detail in prior studies36,37,38.
A squat with the minimum and maximum angles of 30° and 130° of knee flexion was executed with a constant flexion angular velocity of 3°/s and a ground reaction force of 50 N, induced by the controlled muscle force applied to the rectus femoris. Two angular sensors (8820, Burster, Gernsbach, Germany) were used to measure flexion angle; one was placed at the hip and the other at the ankle joint. A deep squat movement was simulated in the native knee (i.e. prior to implantation), as well as post-implantation of the new oneKNEE® implant platform system including the design variants CR/CS, MS, LS and PS (Aesculap AG, Tuttlingen, Germany), which were sequentially implanted into every specimen. A single valid trial was performed per specimen per implant design (the repeatability of the knee simulator used in this study has been previously shown to be < 0.8 mm for AP-translation and < 0.4° for internal external rotation39). The CR/CS, MS and LS implants of this new implant platform system use the same metallic tibial and femoral components. Therefore, switching between these configurations only required switching the polyethylene insert on the tibial component. Implantations of knee prostheses were performed by two senior orthopaedic knee surgeons (PEM, HW), following a protocol that has been described previously36,37,38. The implantation relied on a tibia-first technique using intramedullary alignment. The implant components were medio-laterally centred on the bone cuts of the tibial and femoral bones. All configurations analysed in this study had an intact posterior cruciate ligament (PCL) and native patella (i.e. no resurfacing) except for the PS-implant, which required PCL resection. Given the order of the experimental protocol, PS-implanted specimens had also been subject to patellar resurfacing.
Kinematic measurements
Optical marker data (Aramis, GOM, Braunschweig, Germany) was collected to measure the three-dimensional (3D) motion of the tibia and femur throughout each simulated squat. To calculate joint rotations and translations during the activity cycle, a local segment frame was defined for the femur, and another for the tibia. Four anatomical bony landmarks were identified on the femoral and tibial segment to define the segments’ coordinate frames. The position of the femoral origin was given as the midpoint between the medial and lateral epicondyles. The medio-lateral (ML) flexion/extension axis of the femur was defined by the vector between these two points, directed laterally. The antero-posterior (AP) femoral axis was directed anteriorly, in a direction perpendicular to both the flexion/extension axis and a vector from the fossa intercondylaris pointing in the direction of the femoral bone axis. Finally, the proximo-distal (PD) axis pointed proximally and was oriented orthogonally to both the flexion/extension axis and the AP femoral axis, with proximal as positive. For the local reference frame of the tibia, the origin was given as the centre of the tibial intercondylar eminence, while the directions of the axes were analogously defined, using the medial and lateral tibial condyles, the eminentia intercondylaris and a point on the long axis of the tibia at the bone cut. The orientation and position of these local tibial and femoral reference frames relative to the respective bones was maintained for each specimen over all trials (i.e. even after each prosthesis implantation).
The tibio-femoral rotations were then calculated as an intrinsic extension-adduction-internal rotation Cardan sequence of the tibia relative to the femur (a definition comparable to the Grood and Suntay approach)40,41,42. On the other hand, tibio-femoral translations were given by the vector describing the position of the femoral origin relative to the tibial origin, along the tibial reference frame axes. Left knees were mirrored into right knees, in order to ensure consistent clinical interpretation of the positive rotations and translations (e.g. a positive rotation around the antero-posterior axis always represents knee adduction).
Femoral rotation centre calculation using REFRAME
REference FRame Alignment MEthod (REFRAME) is an approach intended to optimise the orientations22 and/or positions24 of local segment reference frames in order to enable a repeatable comparison of joint kinematic signals. Within REFRAME, objective criteria for joint rotations and translations are defined to target specific characteristics of the joint motion, and then minimised by rotating and/or translating the underlying local reference frames. Since segment rotations were not a critical aspect of the study results, orientations of the reference frames were not altered in the implementation of REFRAME, hence allowing a relationship to the anatomical landmarks to be maintained. After the relative positions of each femoral frame origin were calculated, REFRAME was applied to minimise the variance of joint translations in all three dimensions. In this study, the new “REFRAMEd” position of the femoral frame origin after minimisation of the variance of all three components of the translation vector corresponded with the position of the femoral centre of rotation relative to the tibia. To account for the effects of differences in knee dimensions across subjects, translation values were scaled according to each subject’s femoral width, calculated as the distance between the femoral epicondyles.
Statistical analyses
Analysis of statistically significant differences between kinematic time-series signals in all six degrees of freedom (DOFs) relied on statistical parametric mapping (SPM)43, a method used for the statistical analysis of vector fields where the threshold for rejecting the null hypothesis is corrected by field size and field smoothness44,45. This approach was used to determine whether statistically significant differences exist between the raw kinematic signals (and for which time periods). Here, SPM analyses using two-tailed paired t-test comparisons were executed for all possible pairwise comparisons of the oneKNEE® implant designs (e.g. MS vs. LS, MS vs. PS, LS vs. PS, etc.), as well as all individual implant designs vs. the native condition. Significance levels were initially not corrected to account for multiple comparisons, hence allowing a lenient analysis, but then examined more conservatively using Bonferroni correction46.
In order to establish differences in centre of rotation positions between implants and the native condition, paired t-tests were performed to evaluate the differences between all possible pairwise comparisons of the REFRAMEd femoral origin positions of each implant design, along each of the three dimensions, respectively. Since this study sought to assess the global differences in the position of the rotation centres between implant designs, the increased likelihood of type I errors (i.e. the identification of false positives where an observed significant difference does not actually exist) resulting from multiple comparisons needed to be addressed. Here, we used a conservative Bonferroni correction approach, correcting the threshold for significance of p-values by a factor of three (for every pairwise comparison of two different implants, or any implant vs. native condition, differences are assessed in each of three x, y, and z directions)46. This resulted in using local significance levels of 0.017 and 0.003 for the single tests aiming for global significance levels of 0.05 and 0.01, respectively. REFRAME implementation, as well as all statistical analyses, were executed in MATLAB (R2023a, MathWorks Inc., Natick, MA, USA).
Results
Time-series signals
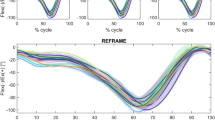
Plots of the raw time-series kinematic signals revealed no obvious differences between the native condition and the different implant types in flexion/extension and ab/adduction (Fig. 2). However, the native int/external rotation movement pattern did appear to deviate from that obtained after implantation with each implant type, especially over the first half of the activity cycle. These observed differences were found to be statistically significant by SPM analysis for all implants except for the PS design (supplementary material, Figs. S1–S4). Regarding joint translations, results showed the relative medio-lateral position between the femoral and tibial origins to be fairly constant throughout the entire cycle. However, a steady offset was noticeable between values for the native condition versus all implant types, where the former displayed more medially placed femoral origins in comparison to the latter. SPM analysis found this offset to be statistically significant between the native condition and all implant types, throughout the entire load cycle.
Mean kinematic signals ± standard deviation across all eight specimens for the analysed implant configurations in anatomical frames. All rotations are shown in the top row as extension, adduction, and internal rotation of the tibia as positive values. Translations of the femur relative to the tibia are shown in the bottom row with positive directions as lateral, anterior, and proximal.
Comparisons between implant designs found statistically significant differences in int/external rotation in less than half of the load cycle for CR/CS vs. LS, LS vs. PS, and MS vs. LS (supplementary material, Figs. S6–S8). Other than that, a small portion of the load cycle was also shown to be significantly different in AP-translation for the comparison of CR/CS and LS (supplementary material, Fig. S6). For the remaining comparisons between implants (CR/CS vs. MS, CR/CS vs. PS, and MS vs. PS) and for all other DOFs, SPM analysis detected no statistically significant differences (supplementary material, Figs. S5, S7 and S9). In a more conservative analysis, a Bonferroni correction to the significance levels was added to account for multiple comparisons (six comparisons; one for each DOF), reducing α from 0.05 to 0.0083. This resulted in the detection of no significant differences, except for a difference in int/external rotation for MS versus LS (supplementary material, Figure S13) and ML-translation of native knees versus CR/CS and PS implants (supplementary material, Figs. S11, S12).
Centres of rotation
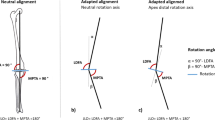
The implementation of REFRAME to minimise translation variances led to a change in the position of the local femoral frame origin for all subjects and trials (Fig. 3). On average, native and MS-implanted knee joints exhibited medially located femoral origins, while knees with a CR/CS, LS or PS implant had more laterally located origins. Moreover, the femoral origin for PS implants was on average located more distally and posteriorly compared to other implants. The results of the paired t-tests revealed the position of the femoral origins to be statistically significantly different in at least one of three dimensions for all comparison pairs, with the exception of CR/CS vs. LS (Table 2).
Schematic of the proximal femur showing REFRAMEd functional femoral centres of rotation for all specimens and implant designs in proximal view (top) and lateral view (bottom). Individual specimen centres are represented by hollow circles, while the mean origin across specimens for each implant design is shown as a solid circle (corresponding standard deviations are shown as shaded ellipses, aligned with the anatomical femur axes to allow directional analysis). The anatomical femoral origin (i.e. the midpoint between lateral and medial epicondyles is shown as \(\times\).
Discussion
In this study, we presented and evaluated a method for feature extraction from tibio-femoral kinematic signals that leverages an implementation of the REFRAME approach. The overarching aim was to determine whether tibio-femoral kinematics are able to indicate clear associations with implant design, towards a solution for individualised implant selection. These associations were explored at both the kinematic signal level, using SPM analysis, as well as at the COR level, using REFRAME optimisation and simple paired t-tests. We hypothesised that implanting the same subject with different implants would lead to different positions of the COR, and that these differences could be clearly distinguished using REFRAME and statistical analyses. To achieve this goal, eight cadaveric knee specimens from human donors were tested, and an optical marker-based system was used to measure tibio-femoral kinematics over the progression of a simulated deep squat cycle. Specimens were tested first in a native anatomy condition, and then again after being sequentially implanted with a series of different implant designs (CR/CS, MS, LS and finally PS) from the oneKNEE® implant platform system. Although a preliminary analysis of the raw kinematic signals using SPM detected limited significant differences between implant designs, it was only with the additional understanding provided by REFRAME optimisation of femoral CORs that we were able to find potential kinematic phenotypes and differentiate between implant designs.
Different total knee prosthesis designs (e.g. MS, LS, PS, etc.) have been developed to better accommodate individual needs, towards recreating the native tibio-femoral functionality of different patients31. In line with several studies investigating whether significant differences in joint kinematics are present in different populations12,47,48,49, the first stage of our analysis used SPM to directly compare the raw kinematic signals (i.e. joint angle/displacement curves plotted over each activity cycle) corresponding to the different implant designs. Geometrical differences between various implant designs are intended to promote specific kinematic patterns, and we therefore expected to observe certain significant differences between them. For example, in the transverse plane, an MS design is intended to pivot around the medial condyle, while an LS design would rather pivot around the lateral condyle. Consequently, we anticipated differences in AP translations of the anatomical frame origin between these designs (supplementary material, Fig. S14). An SPM analysis with Bonferroni correction to account for multiple comparisons, however, only identified significant differences between the int/external rotation of MS versus LS, and the ML translation of native knees vs. CR/CS and PS implants. Given that some researchers have previously argued the Bonferroni correction to be overly conservative in nature50, we also considered the differences that would have been considered statistically significant without adapting the significance level to account for multiple comparisons. In that case, although more of the measured differences would have been considered statistically significant, these differences were still mostly associated with int/external rotation and ML translation. The only significant difference in AP translation was detected between CR/CS and LS, occurring only for a fraction of the load cycle. These results suggest that no straightforward or intuitive method exists to relate the “significant” differences between implant geometries that were observed based on SPM analysis to the intended motion patterns their designs are meant to promote. On the other hand, our use of REFRAME to determine the location of the femoral COR relative to the original anatomically defined origin (i.e. the midpoint of the condyles) clearly detected differences that directly reflected the design and intended function of the implants considered. Not only did the REFRAME approach considerably simplify the analysis (by considering a single 3D vector vs. 100 individual timepoints of a load cycle for each degree of freedom), but it also achieved this while still encompassing movement information contained throughout the entire activity cycle, rather than only one (or a handful of) discrete timepoint(s).
The REFRAME methodology was able to detect numerous differences between implant kinematics (Fig. 3), several of which empirically corroborated our initial hypotheses. Here, we originally predicted that significant differences would be distinguishable in the position of the CORs between MS and LS implants (MS would have a medial COR, while LS would have a lateral one; see supplementary material, Figure S14). This difference was corroborated by our results and determined to be statistically significant at a 0.05 significance level, even after Bonferroni correction to 0.017 (Table 2). Analogously, we anticipated that the COR of PS implants would be located more posteriorly and distally within the femur than for the other designs (supplementary material, Fig. S15). Again, not only did a preliminary analysis of our results support this hypothesis (Fig. 3), but the observed differences were also determined to be statistically significant in the AP direction for PS vs. all implant designs (including native configuration), and in the PD direction against CR/CS (at a Bonferroni-corrected significance level of 0.05).
It is well known that kinematic differences between implants exist49,51,52, but interpreting the differences for easy clinical access is known to be challenging21. Our findings demonstrate that the proposed REFRAME implementation enables a more straightforward (one 3D vector vs. 100 timepoints for each degree of freedom) and robust (present even after conservative statistical analysis) identification of kinematic differences than a direct assessment of raw kinematic signals using SPM analysis. This fundamental understanding of implant-specific features now becomes relevant when we specifically use this information towards subject-specific implant selection. Previous work has already established how sensitive the shape and magnitude of kinematic signals actually are to coordinate frame definitions; a challenge exacerbated by both the lack of consensus in the field on how to interpret kinematic signals, as well as the considerable variability associated with reproducing even a single joint axis method. We therefore propose that a phenotyping approach that shifts the focus from the direct analysis of kinematic signals (Fig. 2) towards the extraction of relevant kinematic features (Fig. 3) has the potential to improve analysis robustness and thereby provide a clinically suitable phenotyping approach to total joint arthroplasty.
It is important to note the possible limitations of this study. First, it is important to acknowledge that studies working towards the application of functional phenotypes to inform implant selection and treatment planning hinge on multiple key fundamental assumptions, including (1) that functional phenotypes discriminating between individuals with different knee motion patterns exist, (2) that pathological knees belonging to different functional phenotypes would be optimally treated with different intervention strategies, (3) that different implant designs can target the needs of different functional phenotypes, and (4) that optimising implant selection would improve TKA satisfaction rates. Although recent studies (e.g.12,13,14) have been able to provide early evidence supporting these hypotheses, additional work is still necessary to conclusively substantiate these conjectures.
Second, it is unclear to what extent the ex vivo kinematics of eight cadaveric specimens are able to represent the in vivo movement patterns of the general population. Here, it is precisely the ex vivo nature of our study that has enabled us to measure the kinematics of each specimen after sequential implantation of different prosthesis designs. This experimental set up therefore allowed us to investigate differences in the kinematics that are induced by the different implant designs on different knees, and evaluate whether these differences are influenced by each specimen’s native e.g. COR position. While reproduction of in vivo loading and boundary conditions was clearly a limiting factor in this study, the specific setup has allowed us to assess implant-imposed kinematic differences between subjects. Moreover, previous research has demonstrated that an increase of the applied loads does not imply superior (or even different) qualitative outcomes; it could, however, represent an unnecessarily high stress to both the specimens (which needed to endure multiple implantations and tests) and the equipment53,54. Additional investigation is clearly required to understand whether these implant kinematics are also replicated in patients in vivo, as well as to explore how the foundational principles presented here can be best implemented to develop a practical framework that can realistically be used in clinics. We expect the development of a robust model to predict the optimal implant geometry for a specific patient will represent one of the major challenges in such future work.
A third potential limitation is the sequential nature of the implantation process. While the switch between CR/CS, MS and LS only required exchanging the polyethylene inlay (thereby not affecting the bony and ligamentous structures), the switch to the PS implant involved additional bone cuts and changing the metallic components; therefore, PS had to be the last design to be implanted and the additional cuts could have led to systematic differences. The sequence of implantation was thus not randomised in this study, but future work should explore whether our results hold when using a random sequence for at least CR/CS, MS and LS. Finally, only one activity was examined in this investigation.
While the aforementioned limitations should be directly addressed in further studies, the simulation of squat kinematics alone has already nicely provided a proof-of-concept demonstration that the REFRAME approach can deliver easy access to relevant and reliable kinematic characteristics. Our study provides further evidence that the position of the knee centre of rotation can play a crucial role in discriminating between different kinematic patterns, and is a valuable marker to compare not only implant geometries but prospective patient functional phenotypes. As such, REFRAME optimisation demonstrates clear early potential to support a future pre-operative phenotyping framework towards improved implant selection and, in the long-term, hopefully better TKA satisfaction rates.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Ranawat, A. S. & Ranawat, C. S. The history of total knee arthroplasty. In The knee joint: Surgical techniques and strategies (eds Bonnin, M. et al.) 699–707 (Springer Paris, 2012).
Gkiatas, I. & Sculco, P., The history of total knee arthroplasty. 3–14 (2022)
Insall, J., Ranawat, C. S., Scott, W. N. & Walker, P. Total condylar knee replacement: Preliminary report. Clin. Orthopaed. Related Res. 120, 149–154 (1976).
Marques, E. M. R. et al. Choice between implants in knee replacement: Protocol for a bayesian network meta-analysis, analysis of joint registries and economic decision model to determine the effectiveness and cost-effectiveness of knee implants for nhs patients—the knee implant prostheses study (knips). BMJ Open 11(1), e040205 (2021).
Rivière, C. et al. Alignment options for total knee arthroplasty: A systematic review. Orthop. Traumatol. Surg. Res. 103(7), 1047–1056 (2017).
Overgaard, A., Lidgren, L., Sundberg, M., Robertsson, O. & W-Dahl, A.,. Patient-reported 1-year outcome not affected by body mass index in 3,327 total knee arthroplasty patients. Acta Orthop. 90(4), 360–365 (2019).
Bryan, S. et al. Revisiting patient satisfaction following total knee arthroplasty: A longitudinal observational study. BMC Musculoskelet. Disord. 19(1), 423 (2018).
Choi, Y. J. & Ra, H. J. Patient satisfaction after total knee arthroplasty. Knee Surg. Relat. Res. 28(1), 1–15 (2016).
Mezghani, N. et al. Healthy knee kinematic phenotypes identification based on a clustering data analysis. Appl. Sci. 11(24), 12054 (2021).
Mezghani, N. et al. Phenotypes in 3d knee kinematics of healthy individuals: Identification and characterization: 1160. Med. Sci. Sports Exerc. 54, 284–284 (2022).
Zgolli, F., et al., Kinematic data clustering for healthy knee gait characterization.. 239–242.
Petersen, E. T. et al. Patients with knee osteoarthritis can be divided into subgroups based on tibiofemoral joint kinematics of gait—An exploratory and dynamic radiostereometric study. Osteoarthr. Cartil.ge 30(2), 249–259 (2022).
van Spil, W. E. et al. A consensus-based framework for conducting and reporting osteoarthritis phenotype research. Arthr. Res. Therapy 22(1), 54 (2020).
Young-Shand, K. L., Roy, P. C., Dunbar, M. J., Abidi, S. S. R. & Astephen Wilson, J. L. Gait biomechanics phenotypes among total knee arthroplasty candidates by machine learning cluster analysis. J. Orthop. Res. 41(2), 335–344 (2023).
Hosseini Nasab, S. H. et al. Loading patterns of the posterior cruciate ligament in the healthy knee: A systematic review. PLOS One 11(11), e0167106 (2016).
Asano, H., Muneta, T. & Sekiya, I. Soft tissue tension in extension in total knee arthroplasty affects postoperative knee extension and stability. Knee Surg. Sports Traumatol. Arthrosc. 16(11), 999–1003 (2008).
Becker, R., Hirschmann, M. T. & Karlsson, J. The role of ligament tension and sensomotoric system in total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 25(6), 1663–1665 (2017).
Kadaba, M. P. et al. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J. Orthopaed. Res. 7(6), 849–860 (1989).
McGinley, J. L., Baker, R., Wolfe, R. & Morris, M. E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 29(3), 360–369 (2009).
Chau, T., Young, S. & Redekop, S. Managing variability in the summary and comparison of gait data. J. NeuroEng. Rehabilit. 2(1), 22 (2005).
Postolka, B. et al. Interpretation of natural tibio-femoral kinematics critically depends upon the kinematic analysis approach: A survey and comparison of methodologies. J. Biomech. 144, 111306 (2022).
Ortigas-Vásquez, A. et al. A frame orientation optimisation method for consistent interpretation of kinematic signals. Sci. Rep. 13(1), 9632 (2023).
Ortigas-Vásquez, A. et al. A framework for analytical validation of inertial-sensor-based knee kinematics using a six-degrees-of-freedom joint simulator. Sensors 23(1), 348 (2022).
Ortigas-Vásquez, A., et al. A reproducible and robust representation of tibiofemoral kinematics of the healthy knee joint during stair descent using reframe—part i: Reframe foundations and validation. (2024). Preprint on Research Square.
Jenny, J. Y. & Boeri, C. Low reproducibility of the intra–operative measurement of the transepicondylar axis during total knee replacement. Acta Orthopaed. Scand. 75(1), 74–77 (2004).
Yin, L. et al. Identifying the functional flexion-extension axis of the knee: An in-vivo kinematics study. PLOS One 10(6), e0128877 (2015).
Taylor, W. R. et al. On the influence of soft tissue coverage in the determination of bone kinematics using skin markers. J. Orthopaed. Res. 23(4), 726–734 (2005).
Freeman, M. A. R. & Pinskerova, V. The movement of the normal tibio-femoral joint. J. Biomechan. 38(2), 197–208 (2005).
Koo, S. & Andriacchi, T. P. The knee joint center of rotation is predominantly on the lateral side during normal walking. J. Biomech. 41(6), 1269–1273 (2008).
Thomeer, L. et al. Six-degree-of-freedom tibiofemoral and patellofemoral joint motion during activities of daily living. Ann. Biomed. Eng. 49(4), 1183–1198 (2021).
Kour, R. Y. N., Guan, S., Dowsey, M. M., Choong, P. F. & Pandy, M. G. Kinematic function of knee implant designs across a range of daily activities. J. Orthopaed. Res. 41(6), 1217–1227 (2023).
Gray, H. A. et al. Comparison of posterior-stabilized, cruciate-retaining, and medial-stabilized knee implant motion during gait. J. Orthopaed. Res. 38(8), 1753–1768 (2020).
Banks, S. A. & Hodge, W. A. 2003 hap paul award paper of the international society for technology in arthroplasty. Design and activity dependence of kinematics in fixed and mobile-bearing knee arthroplasties. J. Arthroplast. 19(7), 809–16 (2004).
Steinbrück, A. et al. The effect of trochlea tilting on patellofemoral contact patterns after total knee arthroplasty: An in vitro study. Arch. Orthopaed. Trauma Surg. 134(6), 867–872 (2014).
Steinbrück, A. et al. Patellofemoral contact patterns before and after total knee arthroplasty: An in vitro measurement. BioMed. Eng. OnLine 12, 58 (2013).
Steinbrück, A. et al. Femorotibial kinematics and load patterns after total knee arthroplasty: An in vitro comparison of posterior-stabilized versus medial-stabilized design. Clin. Biomech. 33, 42–48 (2016).
Steinbrück, A. et al. A lateral retinacular release during total knee arthroplasty changes femorotibial kinematics: An in vitro study. Arch. Orthopaed. Trauma Surg. 138(3), 401–407 (2018).
Bauer, L. et al. Secondary patellar resurfacing in tka: A combined analysis of registry data and biomechanical testing. J. Clin. Med. 10(6), 1227 (2021).
Schroeder, C. Die biomechanik des natürlichen und protesenversorgten kniegelenks unter einbezug der prothesenausrichtung, in Medizinischen Fakultät (Ludwig-Maximilians-Universität München, 2014).
Grood, E. S. & Suntay, W. J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 105(2), 136–144 (1983).
MacWilliams, B. A. & Davis, R. B. Addressing some misperceptions of the joint coordinate system. J. Biomech. Eng. 135(5), 54506 (2013).
Sheehan, F. T. & Mitiguy, P. In regards to the “isb recommendations for standardization in the reporting of kinematic data”. J. Biomech. 32(10), 1135–1136 (1999).
Pataky, T. C. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J. Biomech. 43(10), 1976–1982 (2010).
Penny, W., Friston, K., Ashburner, J., Kiebel, S., & Nichols, T., Statistical parametric mapping: The analysis of functional brain images. (2007).
Pataky, T. C., Robinson, M. A. & Vanrenterghem, J. Vector field statistical analysis of kinematic and force trajectories. J. Biomech. 46(14), 2394–2401 (2013).
Armstrong, R. A. When to use the bonferroni correction. Ophthalmic Physiol. Opt. 34(5), 502–508 (2014).
Sole, G., Pataky, T., Tengman, E. & Hager, C. Analysis of three-dimensional knee kinematics during stair descent two decades post-acl rupture—data revisited using statistical parametric mapping. J. Electromyogr. Kinesiol. 32, 44–50 (2017).
Yona, T., Kamel, N., Cohen-Eick, G., Ovadia, I. & Fischer, A. Scoping review of one-dimension statistical parametric mapping in lower limb biomechanical analysis. medRxiv 49, 1345 (2023).
Postolka, B. et al. Isb clinical biomechanics award winner 2021: Tibio-femoral kinematics of natural versus replaced knees—A comparison using dynamic videofluoroscopy. Clin. Biomech. 96, 105667 (2022).
Perneger, T. V. What’s wrong with bonferroni adjustments. BMJ 316(7139), 1236–1238 (1998).
Schütz, P. et al. Kinematic evaluation of the gmk sphere implant during gait activities: A dynamic videofluoroscopy study. J. Orthopaedic Res. 37(11), 2337–2347 (2019).
Angerame, M. R., Holst, D. C., Jennings, J. M., Komistek, R. D. & Dennis, D. A. Total knee arthroplasty kinematics. J. Arthroplasty 34(10), 2502–2510 (2019).
Müller, O., Lo, J., Wünschel, M., Obloh, C. & Wülker, N. Simulation of force loaded knee movement in a newly developed in vitro knee simulator. Biomed. Tech. (Berl) 54(3), 142–149 (2009).
Victor, J. et al. An experimental model for kinematic analysis of the knee. J. Bone Joint. Surg. Am. 91(Suppl 6), 150–163 (2009).
Author information
Authors and Affiliations
Contributions
Conceptualization: AS, AOV, WRT and MW; methodology: AS, AOV, CT and MW; execution of the tests: AOV, CT, PEM, HW and MW; resources: AM, TMG and MW; data curation: AS, AOV, CT and MW; writing—original draft preparation: AS, AOV and WRT; writing—review and editing: AS, AOV, CT, PEM, HW, AM, TMG, WRT and MW; visualization: AS and AOV; supervision: AM, TMG, WRT and MW; project administration: AM, TMG and MW; funding acquisition: AM, TMG and MW. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.Braun Aesculap AG (Tuttlingen, Germany) provided research support for this study. Some of the authors (AS, AOV, AM and TMG) are employees of B.Braun Aesculap AG (Tuttlingen, Germany). AOV, AS, and AM are all listed as co-inventors on a pending patent application submitted by B.Braun Aesculap AG under number DE102022125697A1, which claims a system for standardising axis orientation and position in kinematic data, used in this study. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sauer, A., Ortigas-Vásquez, A., Thorwaechter, C. et al. Conceptual foundations of a REFRAME-based approach to discriminate across total knee implant designs based on the positions of functional centres of rotation. Sci Rep 15, 834 (2025). https://doi.org/10.1038/s41598-024-84522-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84522-w
Keywords
This article is cited by
-
Update 2025: Biomechanik und Kinematik nach Knie-TEP
Die Orthopädie (2025)