Abstract
Patients with premenopausal breast cancer (preBC) usually have long-term survivorship. However, less is known about the risk pattern of subsequent primary cancer (SPC) and noncancer diseases among them. Here, this study aimed to evaluate the risk of developing SPCs and mortality among preBC survivors. In this population-based, retrospective cohort study, 5-year preBC survivors diagnosed at age 20–59 years during 1992–2014, in the 11 Surveillance, Epidemiology and End Result registries were included. Standardized incidence ratio (SIR) and standardized mortality ratio (SMR) were estimated for SPCs and mortality by cause, respectively. Among 181,947 survivors (mean age at diagnosis, 49.1 years; 65.9% White), there were 12,503 SPC cases, 4,280 SPC-related deaths, and 10,591 noncancer-related deaths. SPC risk was increased compared with the general population (SIR 1.06, [95% CI, 1.04–1.08]). The elevated risk was primarily associated with soft tissue cancer, bones/joints cancer, and acute non-lymphocytic leukemia (SIRs 2.01 [95% CI, 1.73–2.33], 1.78 [95% CI, 1.21–2.53], and 1.68 [95% CI, 1.46–1.93], respectively). Young-onset, Asian survivors, those with hormone receptor-negative BC, and initially treated with chemo-radiotherapy were at high-risk. The risk of dying from SPCs was also increased (SMR 1.07, [95% CI, 1.04–1.10]) and featured with similarly vulnerable subpopulations. Survivors of non-White ethnicity had a higher risk of dying from noncancer diseases. This study highlighted the excess risk of developing and dying from SPCs among preBC survivors, suggesting that targeted healthcare is warranted. Strategies for SPCs and noncancer comorbidity management are in great demand to achieve better long-term survivorship among preBC patients.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most prevalent malignancy among women in the United States (US), with a five-year survival rate of 86–99% for the nonmetastatic stage1. n 2022, an estimated 4.1 million BC survivors were living in the US2. Long-term BC survivorship may be accompanied by physical, psychological, and social effects or impairments induced either by tumor or its treatment3,4, including arm lymphedema5, heart diseases6,7,8,9, osteoporosis10, ovarian suppression11,12, sexual dysfunction and fertility concerns13,14, cognitive impairment and fatigue3,15.
Premenopausal BC (preBC, BC diagnosed before menopause) featured distinct pathogenesis, risk factors, and clinical management compared to postmenopausal BC. BC onset at a younger age tends to be more aggressive. Chemotherapy is more prevalent as part of the treatment regimen, especially when novel treatment regimen is emerging, including antibody drug conjugate16,17, and immune therapy-based regimen18,19,20. The landscape of BC treatment has led to a greater number of chemotherapy-related complications21. Though various tools have been developed to predict the survival of BC patients22,23,24, survivors diagnosed at a younger age are more likely to develop subsequent primary cancers (SPCs)25,26, even decades after their initial diagnosis. This increased risk is likely due to genetic susceptibility and the long-term effects of treatment. A previous study documented that adult-onset BC survivors have a 6% increased risk of SPCs, compared to the general population27. Long-term risk patterns among preBC survivors warrant closer analysis, with greater focus on hormonic, inherited, and treatment-related characteristics. However, few studies have examined the risk of preBC survivors developing SPCs and dying from other causes in depth. The scarcity of data may impede the formulation of management and surveillance strategies for preBC survivors.
Hence, we aimed to determine the risk of developing SPCs and cause of mortality among pre-BC survivors in this study.
Methods
Population and follow-up
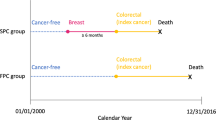
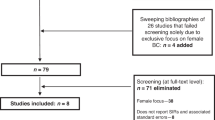
We used data from the Surveillance, Epidemiology, and End Results (SEER) database to estimate the SPC risk and cause of death among preBC survivors. Females aged 20–59 diagnosed with non-metastatic invasive primary BC between 1992 and 2014 were identified from 11 SEER registers. Case with at least five years of survival was classified as preBC survivor (eTable 1). The age limit of 60 aligns with the NCCN guideline definition of menopause28. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines. Ethical review and informed consent were exempted because the data were deidentified and publicly accessible.
Follow-up began five years after the diagnosis of nonmetastatic preBC until death, loss to follow-up, or Dec.31, 2019 (study date-of-end), whichever occurred first. SPCs were identified following SEER multiple primary/histology rules29 (eTable 2), where BC and non-melanoma skin cancer were excluded. Multiple SPCs in a single survivor were allowed. Cause of mortality was classified according to the International Classification of Disease (tenth edition), while death due to non-melanoma skin cancers and miscellaneous cancer (due to they may not die from miscellaneous cancer) were excluded when calculating SPC death (eMethods).
Outcome definition
The primary outcomes were the risks of developing SPCs among preBC survivors. The secondary outcome were the risks of mortality by cause among those survivors, including deaths due to SPCs and deaths due to noncancer diseases. We also analyzed trends in fatalities due to BC, SPCs, and cardiovascular disease (CVD) among preBC survivors, and the incidence trends of primary non-metastatic BC among women aged 20–59 years old.
Statistical analysis
The primary outcomes were assessed using standardized incidence ratio (SIR) and absolute excess incidence (AEI) in comparison to the general population (Supplementary Methods). For the secondary outcomes, standardized mortality ratio (SMR) and absolute excess mortality (AEM) were calculated. Additionally, the percentages of mortality attributable to BC, SPCs, and CVD (top-three leading causes) were calculated annually to determine their trends over time (eMethods).
The analyses for primary and secondary outcomes were p conducted for the overall population and stratified by age at diagnosis, race/ethnicity, latency, molecular characteristics (hormone receptor [HR] and human epidermal growth factor receptor-2 [HER2]), and treatment (chemotherapy and radiotherapy). Chemotherapy and radiotherapy were classified as “yes” or “no/unknown”, with the latter indicating no evidence of treatment in the medical records examined. Notably, when stratified by treatment, the relative risk (RR) was estimated instead of SIR, and adjusted for age and latency using multivariable Poisson regression or negative binary regression (when dispersion was detected) (eTable 3). The heterogeneities of SIRs and RRs were evaluated using multivariable Poisson regression and likelihood ratio tests (eMethods)30,31. The trends in non-metastatic BC incidence were quantified by the average annual percentage change (AAPC) during 2010–2019, which was generated by applying delay-adjusted annual incidence (age-standardized to 2000 US population) into a Joinpoint regression model. (eMethods).
All analyses were performed using SEER*Stat Version 8.4.0 (National Cancer Institute), Joinpoint Regression Program (National Cancer Institute) version 4.9.1.0, and R statistics software. All P-values were 2-sided, and statistical significance was claimed when less than 0.05.
Results
Trends in incidence
BC incidence trends are shown in Fig. 1 and eTable 4–5. Overall, the incidence significantly increased in 2010–2019 (AAPC, 1.0%), with the most rapid increase among Asians (2.1%). A rising trend was observed for the majority of registries.
Incidence trend in premenopausal breast cancer during 2004–2019. (a) Trends stratified by age at diagnosis, race/ethnicity, summary stage at diagnosis; subtype. (b) Stage and subtype-specific incidence trends in premenopausal breast cancer, stratified by age at diagnosis and race/ethnicity. Abbreviations: NHW, non-Hispanic White; NHB, non-Hispanic Black; NHAPI, non-Hispanic Asian and Pacific Islander; HR, hormone receptor; HER2, Human epidermal growth factor receptor 2; AAPC, average annual percentage change. Notes: AAPC during 2010-2019 were labeled for each subgroup in panel (a); Asterisk represents that the AAPC during 2010-2019 was significantly different from zero.
BC mainly featured primarily by its localized stage and HR+/HER2- subtype (incidence, 59.4 and 60.5, respectively), and the trend of stage-specific and subtype-specific incidence varied by age and race/ethnicity during 2010–2019 (Fig. 1 and eTable 4). The elevating trend was most pronounced for localized BC (AAPC, 1.6%), while the increase in regional BC was limited in Asians (1.4%). The incidence in HR + BC remarkably inflated (1.6% and 2.1% for HR+/HER2 + and HR+/HER2- BC, respectively), while Asians and Hispanics occupied the majority of the increases for the HR+/HER2- BC (3.3% and 3.5%, respectively). A significant increase in triple-negative BC (TNBC) incidence was only identified among women aged 20–39 years and Hispanics (1.5% and 1.2%, respectively) (all P < 0.05). Sensitivity analyses confirmed the findings (eTable 6).
Risk of subsequent primary cancer
After a mean follow-up of 9.4 years, a total of 181,947 preBC survivors (mean age at diagnosis, 49.1 years old; 65.9% White) experienced 12,503 SPCs, which corresponded to 6% higher risk (SIR, 1.06 [95% CI, 1.04–1.08], Table 1) compared with the general population. 14 out of 29 types of cancers exhibited statistically increased risks, with the highest SIR for soft tissue cancer, followed by bones/joints cancer, and acute non-lymphocytic leukemia (ANLL) (2.01, 1.78, and 1.68, respectively, Table 1).
The risk varied by age and race/ethnicity (Fig. 2 and eTable 7–8). Survivors diagnosed at age 20–39 years old have a higher risk compared with their counterparts (SIRs, 1.32 vs. 1.05). The highest SIRs were seen for soft tissue cancer for both subpopulations (3.67 and 1.89 for those diagnosed at age 20–39 and 40–59 years, respectively). Asian survivors were at the highest risk, followed by Black women (1.36 and 1.16, respectively). Asian and Black survivors had the highest SIRs for ANLL (2.56 and 2.38, respectively), while White and Hispanic survivors had a higher risk of developing soft tissue cancer (2.0) and gastric cancer (2.12), respectively. Notably, an excess risk of subsequent ovarian cancer was prevalent across all racial/ethnic groups, with the highest SIR (1.86) for Hispanic survivors.
Standardized incidence ratio and relative ratio of developing subsequent primary cancer by age, race/ethnicity, hormone receptor status and initial treatment among premenopausal breast cancer survivors. Abbreviations: HR, hormone receptor. Notes: Relative risk was demonstrated when stratified by initial treatment, comparing with survivors without initial treatments; Estimates are not shown for subpopulations of observed events less than 5; Asterisk represents that the SIR or RR was significantly different among subgroups, where “*” indicates a P < 0.05, “**” indicates a P < 0.01, and “***” indicates a P < 0.001. “#” indicates a different scale from the other panels is used.
The risk of developing SPCs also associated with molecular characteristics and initial therapy (Fig. 2 and eTable 9–12). HR-negative BC survivors were more likely to develop SPCs (SIR, 1.18), with the highest SIR for ovarian cancer (2.54). Additionally, the excess risk among TNBC survivors was primarily related to ANLL (SIR, 7.11). Among these survivors initially treated with chemotherapy alone, an elevated RR was observed (RR, 1.17 [95% CI, 1.11–1.24]), with the highest RR for ANLL (1.90 [95% CI, 1.23–2.93]). Greater excess RR was also found when combining radiotherapy with chemotherapy (1.22 [95% CI, 1.16–1.29]), such as ANLL (2.21) and soft tissue cancer (1.73). Survivors who received radiotherapy alone also showed increased RR (1.06, [95% CI, 1.01–1.12]), with remarkable RRs for soft tissue cancer (1.8) and lung cancer (1.23).
The risk of developing the majority SPCs modified over time, with the exception of head/neck, lung, soft tissue cancer, and ANLL after 15 years postdiagnosis (eTable 13). Additionally, the risk of developing SPCs was not affected by the eras of chemotherapy and radiotherapy (Fig. 2 and eTable 14–15).
The AEI is shown in Table 1, eTables 7, 8, 9, 10, 11, 12, 13, 14 and 15 and eFigure 2. Lung cancer had the highest AEI among the majority of the subpopulations (ranging from 1.5 to 3.1). However, the highest AEI was observed for ovarian cancer among those diagnosed at age 20–39 years (2.6), Hispanic survivors (2.4), and survivors with HR-negative BC (4.1), whereas the highest AEI for corpus/uterus cancer was observed among Black (2.9) and Asian (3.9) survivors. Sensitivity analyses showed the consistency of the results (eTables 16, 17, 18, 19 and 20).
Risk of death by cause of death
Among 28,501 (2.7% with unknown cause) death, morality due to BC decreased from 88.7 to 42.3% during 1997–2019, while deaths due to SPCs and CVD nearly tripled (Fig. 3). The risk of mortality from SPCs was higher than expected for 8 of 29 cancers (SMR, 1.07, [95% CI, 1.04 to 1.10], Table 2), with the highest SMRs for acute lymphocytic leukemia (ALL), followed by malignancies of bones/joints and soft tissue (2.55, 1.97 and 1.96, respectively).
Cause of mortality by age, race/ethnicity, hormone receptor status and initial treatment among premenopausal breast cancer survivors. (a) Death due to subsequent primary cancers; (b) Death due to noncancer diseases; (c) Trends in percentage of death due to breast cancer, subsequent primary cancer and cardiovascular diseases. Abbreviations: NH-White, non-Hispanic White; NH-Black, non-Hispanic Black; NH-API, non-Hispanic Asian and Pacific Islander; HR, hormone receptor; LIHC, liver and intrahepatic bile duct cancer; ONS, other nervous system; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; ALL, acute lymphocytic leukemia; ANLL, acute non-lymphocytic leukemia; CML, chronic myeloid leukemia; DM, diabetes mellitus; AD, Alzheimer’s disease; CVD, cardiovascular disease; CLD, chronic liver disease; COD, cause of death. Notes: Death due to miscellaneous cancer was not included in death cause of other cancers in panel (a). Standardized mortality ratio or relative risk in death due to breast cancer, miscellaneous cancer and non-melanoma skin cancer were not shown; Relative risk was demonstrated when stratified by initial treatment, comparing with survivors without initial treatments; Asterisk represents that the SIR was significantly different from zero; The gray cells indicate that the observed sample size is less than 5 and estimates are not shown due to confidentiality consideration.
The risk of dying from SPCs varied by age and race/ethnicity (Fig. 3 and eTable 21–22). Survivors diagnosed at age 20–39 years were at greater risk compared with their counterpart (SMR, 1.65 vs. 1.05), with the highest SMR for ovarian cancer among the former (4.76) and ALL for the latter (2.51). Black (1.19) and Asian (1.54) survivors posed a significant excess death risk due to SPC. The highest SMRs were observed for ALL among White (1.97), Black (7.77), and Hispanic (4.13) survivors, while esophageal cancer has the largest SMR among Asian survivors (3.05). In addition, Hispanic survivors exhibited substantial excess mortality risk associated with gastric cancer and liver cancer (SMRs, 3.63 and 2.09, respectively).
The mortality risk from SPCs is also associated with molecular characteristics and initial therapy (Fig. 3 and eTable 23–26). Survivors with HR-negative BC had an increased risk (SMR, 1.27), which was primarily related to ovarian cancer (2.98). Survivors initially treated with chemotherapy alone or combined with radiotherapy tended to have an elevated RR for mortality from other cancers (1.12 [95% CI, 1.02 to 1.23] and 1.21 [95% CI, 1.12 to 1.31], respectively). Figure 3 and eTable 27–29 depict the risk stratified by latency and treatment era.
Death risks associated with other noncancer causes are displayed in Fig. 3; Table 2 and eTables 21, 22, 23, 24, 25, 26, 27, 28 and 29. Significantly elevated SMRs were observed for Alzheimer’s disease among Black survivors (SMR, 1.67), diabetes (1.33) and chronic liver diseases (1.78) among Hispanic survivors, and pneumonia and influenza for Asian survivors (1.92). The increased risk was not observed for CVD in either the overall population or any subpopulations; however, Asian survivors had a higher risk for the disease of heart (1.20).
The AEM is demonstrated in eFigure 3 and eTable 21–29. The highest AEM was observed for ovarian cancer among the overall population (0.8), survivors diagnosed at age 20–39 years (2.1), White (0.6), Hispanic survivors (1.0), and those HR-negative BC (3.2). Also, pancreatic cancer contributed to a substantial mortality burden, with an AEM of 0.7 in the overall population. Specifically, a substantial AEM was observed for chronic liver disease (1.3) and disease of heart (1.5) in Hispanic and Asian survivors, respectively. Sensitivity analyses illustrated the robustness of our findings (eTable 30–34).
Discussion
Utilizing the population-based data in the US, we found that preBC survivors had a higher risk of developing or dying from SPCs, compared to the general population. The risk was more pronounced among survivors diagnosed at a younger age, Asian survivors, those with HR-negative BC, and those treated with chemotherapy. Excessive death risks associated with certain noncancer diseases were observed among non-White survivors. In addition, the percentage of mortality attributable to SPC and CVD has rapidly increased in the past few decades.
The increased risk associated with SPCs among BC survivors has been documented in prior studies27,32,33. Also, survivors of BC have been shown to have an elevated risk of co-morbidity, particularly CVD6,7,8,9,34,35. However, prior to our investigation, no research that systematically described the risk pattern of SPC and other non-cancer diseases in relation to preBC.
Chemotherapy was associated with an increased risk of developing SPCs, with radiotherapy further compounding this risk, primarily driven by subsequent ANLL. Convincing evidence has demonstrated a dose-dependent risk of subsequent leukemia associated with alkylating agents36, cyclophosphamide and/or anthracycline-based regimen37,38,39, and topoisomerase-II inhibitor-based chemotherapy39 among BC patients. The extraordinary excess risk of ANLL observed in our study, particularly among TNBC patients, may be attributable to dose-intensified chemotherapy regimens. Similar to prior observations of adjuvant therapy-associated marrow neoplasms40, an even higher relative risk was observed for ANLL when radiotherapy and chemotherapy were combined. The poor prognosis for treatment-related leukemia underscores the urgent need for optimized chemotherapy combinations or dose reductions in preBC survivors. Consistent with other studies, excessive SPC risks were observed for melanoma32 and lung cancer41,42 among preBC survivors treated with radiotherapy alone. Elevated risks of developing subsequent esophageal and soft tissue cancers were observed when radiotherapy and chemotherapy combined. These SPCs often occurred near or within the radiotherapy field, emphasizing the need for heightened protection of adjacent organs during treatment.
Modified health factors could alter the treatment-associated excess SPC risk. A Connecticut study demonstrated that smoking increased the risk of subsequent lung cancer to radiotherapy among BC survivors43. Also, research BC survivors with a history of hypertension or diabetes or both face a fivefold to eightfold higher risk of developing angiosarcoma33. Due to the extensive use of chemotherapy and radiotherapy in all stages of BC, these modifiable health factors are of utmost importance for the SPC outcomes and long-term prognosis of preBC survivors.
The tumor spectrum with markedly elevated SPC risk varied across latency periods, with most risks diminishing over time. However, even 15 years postdiagnosis, the SPC risks of head/neck, lung, soft tissue, and ANLL remained substantially elevated. PreBC survivors should prioritize long-term follow-up and screening for these tumors. Specifically, preBC survivors should screen for these SPCs with high risk during follow-up, including performing low-dose computer tomograph for lung cancer and blood test for ANLL more frequently, especially for those with germline mutation.
Moreover, the pathogenetic germline mutation among BC survivors was identified to be associated with an increased SPC risk44. Generally, BC-associated pathogenetic mutations were more prevalent among young-onset survivors, which is consistent with the greater excess risk of developing SPCs among them. In addition, a higher prevalence of BRCA mutations was observed among Hispanic ethnicity45,46 and HR-negative BC47, which contributed in part to the higher risk of subsequent ovarian cancer in our study, compared with other counterparts. Meanwhile, the health resource access may differ between ethnicity and racial groups, these structural causes might result in the racial disparities in cancer screening and diagnosis48.
Infection may be associated with certain types of SPC risk, which may owe to compromised immunity. For example, the higher prevalence in Helicobacter pylori infection among African Americans and Hispanic ethnicity49 might contribute to the increased risk of subsequent gastric cancer. Lower rates of hepatitis C virus treatment among Hispanic carriers could elevate the risk of subsequent liver cancer50. Besides, other lifestyle factors also contributed to the increased risks, such as alcohol consumption, smoking, physical activity, and mental health.
Our findings suggested an excess mortality risk due to noncancer disease among Black, Hispanic and Asian survivors, including heart disease, Alzheimer’s disease, diabetes, chronic liver diseases, and pneumonia and influenza. Although the cause for the disproportionate distribution of comorbidity death among different races/ethnicities is unclear, it may be related to structural barriers in health equity51. Also, our result demonstrated a rapidly rising trend in the proportion of CVD death among preBC survivors, who were a vulnerable population for treatment-related CVD6,7,8,35. Additionally, comorbidity with diabetes indicated a worse survival34, while pneumonia and influenza may associate with a compromised immunity in survivors. To improve the prognosis for survivors of noncancerous diseases, additional efforts, including advanced therapies, heightened protection during treatment, and expanded surveillance, are required.
Divergent incident trends were found, with Asian and Hispanic women experiencing a more rapid increment. Besides, the incidence of TNBC increased dramatically among younger female and Hispanic women. These escalating tendencies warrant alarm. Asian survivors, survivors diagnosed at a younger age, and those with HR-negative preBC were subjected to an excess risk of developing and dying from SPC, while Asian and Hispanic survivors also encounter an additional excess risk of dying from other non-cancer diseases. Increasing preBC incidence in these subpopulations may result in disease burden, necessitating close surveillance of SPCs and expanded medical resources. These disparities might be associated with the difference in cancer screening rate, healthcare access, and socioeconomic status48,52. For example, the neighborhood socioeconomics could influence the cancer survival among Black and Hispanic individuals52. These systemic barrier factors above, take together, could result in a relative low quality of healthcare among the minorities, beyond genetics.
Integration of risk estimate by age, race/ethnicity, HR status, and initial treatment, and the demonstration of risk profile over time will be crucial for developing of individualized policies for preBC survivors. Particular SPC prevention strategies that target preBC survivors include relevant cancer screenings, genetic testing and counseling on hereditary genetic predisposition, mitigating behavioral and lifestyle risk factors, and immunization. Not only oncologists but also primary care physicians should educate preBC survivors to avoid unhealthy lifestyle behaviors and cancer-related infection. Substantial SPC risk and cardiotoxicity challenges posed by treatment should be addressed.
Here, our study held significant potential to reshape post-cancer care strategies for preBC survivors. By identifying the elevated risks of SPCs and non-cancer mortality, our findings highlight the urgent need for targeted surveillance and public health policies. These strategies will guide healthcare resource allocation and improve long-term outcomes for preBC survivors. Personalized approaches, informed by genetic profiling, will enable the identification of high-risk individuals. Further research is warranted to analyze genetic, lifestyle, and environmental factors contributing to vulnerability in preBC survivors, ultimately aiding in the development of preventive strategies and improving survival.
Several limitations should be noted. First, the SEER database did not record the information on menopause status, so the precise definition of premenopausal onset BC cannot be determined for these survivors. We adopted the age of 60 years was chosen as the cutoff to assure conformity based on the NCCN guidelines28. Also, sensitivity analyses were performed for age cutoffs of 55 years according to the epidemiological evidence53. Second, we excluded survivors with distant stages due to their poor survival, although SPCs could still occur in this population. Sensitivity analyses were performed to address this. Third, we did not stratify the risk by chemotherapy and radiotherapy regimen. Forth, the underlying the causes of mortality were not analyzed because these data were not available in SEER database. This may underestimate the risk. Fifth, the unmeasured confounders, such as lifestyle variables including physical activity, diet quality, and sleep pattern, were not included in this study and might influence the long-term outcomes.
The preBC survivors in the US displayed an elevated risk of developing and dying from SPCs. The risk of mortality from noncancer disease was elevated among non-White survivors of multiple diseases. The portion of death attributable to SPC and CVD increased, while that attributable to BC decreased. The incidence of preBC is rising among those at risk for developing and dying from SPCs and other noncancer diseases identified in this study. Findings highlighted the imperative and long-term necessity of placing a high priority on the surveillance of SPCs and the allocation of medical resources for preBC survivors.
Data availability
The datasets generated and analyzed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) database, shown at: https://seer.cancer.gov/. The availability of data, code, and other materials underlying research publications offers greater transparency, better trust in the literature and better reproducibility. Reuse of data offers enormous potential for corroboration of scientific findings and further discoveries. Making available the data underlying research publications supports the Journal’s educational and scholarly mission.
Abbreviations
- BC:
-
Breast cancer
- PreBC:
-
Premenopausal breast cancer
- US:
-
United States
- SPC:
-
Subsequent primary cancer
- SEER:
-
Surveillance, Epidemiology and End Results
- CVD:
-
Cardiovascular disease
- SIR:
-
Standardized incidence ratio
- AEI:
-
Absolute excess incidence
- SMR:
-
Standardized mortality ratio
- AEM:
-
Absolute excess mortality
- HR:
-
Hormone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- RR:
-
Relative risk
- AAPC:
-
Average annual percentage change
- TNBC:
-
Triple-negative breast cancer
- ANLL:
-
Acute non-lymphocytic leukemia
- ALL:
-
Acute lymphocytic leukemia
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48 (2023).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72 (5), 409–436 (2022).
Runowicz, C. D. et al. American Cancer Society/American Society of clinical oncology breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 66 (1), 43–73 (2016).
Loprinzi, C. L., Wolf, S. L., Barton, D. L. & Laack, N. N. Symptom management in premenopausal patients with breast cancer. Lancet Oncol. 9 (10), 993–1001 (2008).
DiSipio, T., Rye, S., Newman, B. & Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 14 (6), 500–515 (2013).
Guha, A. et al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur. Heart J. 43 (4), 300–312 (2022).
Greenlee, H. et al. Risk of Cardiovascular Disease in Women with and without breast Cancer: the pathways Heart Study. J. Clin. Oncol. 40 (15), 1647–1658 (2022).
Darby, S. C. et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl. J. Med. 368 (11), 987–998 (2013).
Abdel-Qadir, H. et al. Association of early-stage breast Cancer and subsequent chemotherapy with risk of Atrial Fibrillation. JAMA Netw. Open. 2 (9), e1911838 (2019).
VanderWalde, A. & Hurria, A. Aging and osteoporosis in breast and prostate cancer. CA Cancer J. Clin. 61 (3), 139–156 (2011).
Leon-Ferre, R. A., Majithia, N. & Loprinzi, C. L. Management of hot flashes in women with breast cancer receiving ovarian function suppression. Cancer Treat. Rev. 52, 82–90 (2017).
Kim, H. A. et al. Adding ovarian suppression to tamoxifen for premenopausal breast Cancer: a Randomized Phase III Trial. J. Clin. Oncol. 38 (5), 434–443 (2020).
Oktay, K. et al. Fertility preservation in patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 36 (19), 1994–2001 (2018).
Carter, J. et al. Interventions to address sexual problems in people with Cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J. Clin. Oncol. 36 (5), 492–511 (2018).
Williams, A. M. et al. Fatigue, anxiety, and quality of life in breast cancer patients compared to non-cancer controls: a nationwide longitudinal analysis. Breast Cancer Res. Treat. 187 (1), 275–285 (2021).
Schipilliti, F. M. et al. Datopotamab deruxtecan: a novel antibody drug conjugate for triple-negative breast cancer. Heliyon 10 (7), e28385 (2024).
Caputo, R. et al. Sacituzumab Govitecan for the treatment of advanced triple negative breast cancer patients: a multi-center real-world analysis. Front. Oncol. 14, 1362641 (2024).
Guven, D. C. et al. Immune checkpoint inhibitor-related hearing loss: a systematic review and analysis of individual patient data. Support Care Cancer. 31 (12), 624 (2023).
Rizzo, A. et al. KEYNOTE-522, IMpassion031 and GeparNUEVO: changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol. 18 (18), 2301–2309 (2022).
Rizzo, A. & Palmiotti, G. Neoadjuvant chemoimmunotherapy in early triple-negative breast cancer: a new kid on the block? Immunotherapy 14 (10), 755–758 (2022).
Rizzo, A. et al. Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: the MOUSEION-02 study. Expert Opin. Drug Metab. Toxicol. 17 (12), 1455–1466 (2021).
Sahin, T. K., Rizzo, A., Aksoy, S. & Guven, D. C. Prognostic significance of the Royal Marsden Hospital (RMH) score in patients with Cancer: a systematic review and Meta-analysis. Cancers (Basel) 16(10), 1835 (2024).
Vitale, E., Rizzo, A., Santa, K. & Jirillo, E. Associations between Cancer Risk, inflammation and metabolic syndrome: a scoping review. Biology (Basel) 13(5), 352 (2024).
Viscardi, G. et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur. J. Cancer. 177, 175–185 (2022).
Bright, C. J. et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (teenage and young adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 20 (4), 531–545 (2019).
Sung, H. et al. Subsequent primary Cancer risk among 5-Year survivors of adolescent and young adult cancers. J. Natl. Cancer Inst. 114 (8), 1095–1108 (2022).
Sung, H., Hyun, N., Leach, C. R., Yabroff, K. R. & Jemal, A. Association of First Primary Cancer with risk of subsequent primary Cancer among survivors of adult-onset cancers in the United States. Jama 324 (24), 2521–2535 (2020).
NCCN Clinical Practice Guidelines in Oncology, Breast Cancer, Version 4. [ (2023). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf]
Multiple Primary and Histology Coding Rules [ (2007). https://seer.cancer.gov/tools/mphrules/]
Rostgaard, K. Methods for stratification of person-time and events - a prerequisite for Poisson regression and SIR estimation. Epidemiol. Perspect. Innov. 5, 7 (2008).
Yasui, Y. et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am. J. Epidemiol. 158 (11), 1108–1113 (2003).
New Malignancies Among Cancer Survivors. SEER Cancer Registries, 1973–2000 [https://seer.cancer.gov/archive/publications/mpmono/MPMonograph_complete.pdf]
Veiga, L. H. S. et al. Treatment-related thoracic soft tissue sarcomas in US breast cancer survivors: a retrospective cohort study. Lancet Oncol. 23 (11), 1451–1464 (2022).
Rao Kondapally Seshasai, S. et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl. J. Med. 364 (9), 829–841 (2011).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-Positive breast Cancer. N Engl. J. Med. 377 (2), 122–131 (2017).
Curtis, R. E. et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N. Engl. J. Med. 326 (26), 1745–1751 (1992).
Smith, R. E., Bryant, J., DeCillis, A. & Anderson, S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical adjuvant breast and Bowel Project experience. J. Clin. Oncology: Official J. Am. Soc. Clin. Oncol. 21 (7), 1195–1204 (2003).
Praga, C. et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J. Clin. Oncology: Official J. Am. Soc. Clin. Oncol. 23 (18), 4179–4191 (2005).
Le Deley, M. C. et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J. Clin. Oncol. 25 (3), 292–300 (2007).
Wolff, A. C. et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J. Clin. Oncol. 33 (4), 340–348 (2015).
Wang, Y., Li, J., Chang, S., Dong, Y. & Che, G. Risk and influencing factors for subsequent primary Lung Cancer after treatment of breast Cancer: a systematic review and two Meta-analyses based on four million cases. J. Thorac. Oncol. 16 (11), 1893–1908 (2021).
Huang, Y. J. et al. Radiation Therapy for invasive breast Cancer increases the risk of second primary lung Cancer: a Nationwide Population-based cohort analysis. J. Thorac. Oncol. 12 (5), 782–790 (2017).
Kaufman, E. L., Jacobson, J. S., Hershman, D. L., Desai, M. & Neugut, A. I. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J. Clin. Oncol. 26 (3), 392–398 (2008).
Chen, F. et al. Genetic risk of second primary Cancer in breast Cancer survivors: the multiethnic cohort study. Cancer Res. 82 (18), 3201–3208 (2022).
Kurian, A. W. et al. Genetic testing and results in a Population-based cohort of breast Cancer patients and ovarian Cancer patients. J. Clin. Oncology: Official J. Am. Soc. Clin. Oncol. 37 (15), 1305–1315 (2019).
Hirko, K. A. et al. The impact of race and ethnicity in breast cancer-disparities and implications for precision oncology. BMC Med. 20 (1), 72 (2022).
Copson, E. R. et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 19 (2), 169–180 (2018).
Mishra, S. I., DeForge, B., Barnet, B., Ntiri, S. & Grant, L. Social determinants of breast cancer screening in urban primary care practices: a community-engaged formative study. Womens Health Issues. 22 (5), e429–438 (2012).
Nguyen, T. et al. The prevalence of Helicobacter pylori remains high in African American and hispanic veterans. Helicobacter 20 (4), 305–315 (2015).
Wong, R. J. et al. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am. J. Gastroenterol. 113 (9), 1329–1338 (2018).
Binkley, J. M. et al. Racial disparity in breast cancer survivorship: themes from a series of four national healthcare provider live virtual forums. J. Cancer Surviv. 17(4), 1008–1016 (2023).
Ellis, L. et al. Racial and ethnic disparities in Cancer Survival: the contribution of Tumor, Sociodemographic, Institutional, and Neighborhood characteristics. J. Clin. Oncol. 36 (1), 25–33 (2018).
Collaborative Group on Hormonal Factors in Breast C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 13 (11), 1141–1151 (2012).
Funding
This study was funded by the Changzhou medical innovation team Project (CCX201807).
Author information
Authors and Affiliations
Contributions
MY, ZW and JX wrote the main manuscript text. JD, CZ and XQ prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, M., Wang, Z., Xue, J. et al. Subsequent primary cancer risk and mortality among premenopausal breast cancer survivors. Sci Rep 15, 10829 (2025). https://doi.org/10.1038/s41598-024-84606-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84606-7
Keywords
This article is cited by
-
Mandibular metastasis of invasive ductal carcinoma of the breast: a case report
Journal of Medical Case Reports (2025)