Abstract
The objective of the study was to determine the efficacy of white wormwood on helminthes in beef cattle production. Water extracts of white wormwood of different levels of phytotoxicity were used to treat female adult H. contortus over 8 h under controlled laboratory conditions. The experiment was designed in a completely randomized design with six treatments replicated three times. Treatments 3–6 showed a reduction in worm motility over time (P < 0.05) while it did not change much for T1 and T2 (P < 0.05). Even after 8 h of incubation, more than 50% of the worms were still active in T1, T2 and T3. Meaningful reductions in activity were observed in T4 from 6 to 8 h, T5 form 4–8 h and T6 form 2–6 h. The highest mortality was observed in T4, T5 and T6, 8 h after incubation, however only T6 totally killed all worms 6 h after incubation. Treatments 4 and 5 only achieved 80 and 82% mortality respectively, 8 h post incubation. It is therefore concluded that wormwood aqueous extracts at 5 mg/mL and 10 mg/mL have the capability to immobilize, inactivate and kill mature H. contortus worms from beef cattle.
Similar content being viewed by others
Introduction
Internal parasites in ruminants are a constant periodic problem impacting animal productivity in all livestock herds and species. They have become more difficult to manage in small ruminants and beef cattle because of the parasites’ increasing resistance to all available chemical dewormers1. The need for alternative dewormers is paramount, and research on medicinal plants is gaining momentum2. While some internal parasites cause noticeable disease, their most debilitating effects are economic in nature, coupled with social deprivation in animals. Helminths affecting cattle are a major problem to livestock production3. They can cause extensive internal damage without the farmer even realizing that the animals are heavily infested. Nematodiasis causes weakness, loss of appetite, decreased feed efficiency, and reduced weight gain4,5,6. Once affected, the animal’s immune system is weakened, thereby increasing the host susceptibility to other pathogens and secondary infection. It is therefore important to find effective, environmentally friendly and affordable remedies to intestinal parasites. Commercial anthelmintic drugs have been used and abused for a long time, Jain & Basniwal7 and Vargason et al.9 have reported side effects in both humans and animals. Furthermore, synthetic dewormers are only a short-term solution, thus, grazing livestock are always exposed to parasites, constantly being re-infected, thereby increasing the cost of parasitic management.
In addition, smallholder livestock producers that dominate the livestock industry in developing countries cannot afford the costs associated with anthelmintic drugs, therefore alternative methods are germane for this sector. Conventional dewormers are also blamed for environment pollution10, while natural products are safe to use and harmonious with other biological systems. To this end we propose the use of wormwood in the control of H. contortusin beef cattle. Wormwood is a locally growing plant in Zimbabwe, that is traditionally used to treat human ailments10,11,12,13. Its use in livestock production is growing but still limited, a number of results have been reported mostly for sheep and goats, with very few studies focusing on larger ruminants like cattle.
Wormwood belongs to the Asteraceae family, and has a prominent position in the herbal de-worming literature (Tariq et al., 2009). According to Nyagumbo et al.11; Prichard & Geary15, and Sotenjwa et al.14wormwood has a high value in several fields, as food plants, and has anti-coccidian properties16. A report by Beshay17 showed that, during the past century, the use of artemisia had declined, in addition, the medicinal properties of wormwood has not been well studied in Zimbabwe or the SADC region, yet the challenge of internal parasites is enormous. Furthermore, studies involving larger ruminants like beef or dairy are few. Therefore, the objective of this study was to evaluate the effects of wormwood on H. contortus in beef cattle.
Materials and methods
All methods were performed in accordance with the relevant guidelines and regulations of the Department of Veterinary Services, Zimbabwe.
Study site
An in vitro experiment was conducted at the Central Veterinary Services laboratories, 6 km to the NE of Harare, the capital city of Zimbabwe. The experiment was conducted in the parasitology laboratory.
Preparation of extracts
The leaves of wormwood were collected from the National Botanical Gardens in Harare, Zimbabwe in May 2019. The wormwood leaves were initially air dried in a well aerated room protected from sun and dust. They were then further oven dried for 72 h at 40 °C to achieve a moisture content of 13%. The leaves were grounded using pestle and motor and then passed through a 3 mm screen to form a powder. The powder (100 g) was soaked in distilled water (500 ml) in a glass flask and allowed to macerate for 48 h at room temperature with intermittent shaking. After 48 h, the contents were sieved through Whatman number one filter paper. The filtrate was concentrated at 60 °C into semisolid viscous mass using a vacuum rotary evaporator (Bioevopeak Co Ltd, USA). The semisolid extract was stored in airtight container at refrigerated condition − 20 °C till further use.
Preparation of helminths
A simple random sample of animals was conducted at four randomly selected abattoirs; Surrey, Tillisa, CSC and Winsor. The abattoirs mainly rely on cattle deliveries from small holder farmers’ and have a capacity to slaughter a minimum100 cattle per day. We purposively selected animals from small holder or communal farmers because their parasite and or animal health management practices are below average or non-existent at all. Thus chances of finding internal parasites was guaranteed. Faeces were collected from 15 randomly selected animals at each abattoir according to Coles et al.18. The faeces were screened for H. contortus eggs through the sedimentation and floatation method (Hansen and Perry, 1994). Supernatants were collected in 12 test tubes and sent to the Central Veterinary Lab (CVL) in a secure container at room temperature. Analysis of the supernatants was done with the assistance of senior scientists at the CVL and the presence of H. contortuswas established. The eggs were incubated under optimum favourable laboratory conditions to facilitate their hatching2. Mature, live H. contortus were then taken for in-vitro anthelminthic analysis in the parasitology laboratory at CVL.
In-vitroanthelmintic test.
The protocols developed by Tariq et al.19 were used to determine the effect of different aqueous concentrations of wormwood extracts on H. contortus. Mature worms from the helminthes test were suspended in phosphate buffered saline before exposure to each of the treatments in an incubator at controlled temperature (37oC). The worms were observed for motility, activeness and mortality at 30 min intervals and finally time taken for complete immobilisation of helminths was recorded.
Data collection
Time to complete immobilisation of intestinal parasites was determined after incubation. Motility of worms was determined according to Tariq et al.19. The mortality index was calculated as (total number of immobile worms/total number of worms per ml). The anthelminthic activity of plant extracts was performed using adult immersion test according to Chinemana et al., (1985). Antiparasitic effects of each dilution were tested by immersing a sample of 30 parasites in a test tube containing 3–5mls of the extracts for 8 h. The inhibition of motility, activeness and mortality of the worms was observed at an interval of 30 min, 1, 2, 4, 6, 8 h. Enumeration of helminths was done using stereomicroscope and a McMaster slide. The number of motile (alive) and non-motile (dead) worms were counted and recorded for each concentration and replicate. Death of worms was ascertained by absence of motility. The total time to complete immobilisation and death of intestinal parasites was determined after incubation. Death of worms was confirmed according to a method by (Goel et al.2 that is response to heat shock at 50 °C in 0.8% saline for 10s.
Treatments
Distilled water (T1) was considered the negative control, Albendazole (T6) (Albendazole-2.5, Ethicare Animal Health New Delhi, India) was used as the positive control, while four concentrations of the wormwood extract T2;1 mg/mL, T3; 2.5 mg/mL, T4; 5 mg/mL and T5;10 mg/mL were used. Each treatment was replicated three times giving a total of 18 experimental units.
Experimental design
A total of 30 active mature worms were inserted in each of the eighteen test tubes containing either graded levels of aqueous concentrations of wormwood, distilled water or albendazole in a a completely randomised design (CRD) with six treatments (T1 – T6). Motility, activeness and mortality of H. contortus worms was observed and the results were recorded over time.
Statistical analysis
Data was analysed using JMP statistic software (IBM SAS version 14). Time series analysis was carried out to interrogate the effects of the interaction between treatment and time on motility, activeness and mortality of helminths. The effects were further analysed using mixed Restricted Estimate of maximum likelihood (REML) model where time, treatment and their interactions where treated as fixed effects. Replicate effects where nested within the treatment and were treated as random factors. Post hoc was done to separate the means using the Tukey’s HSD test at P < 0.05.
Results
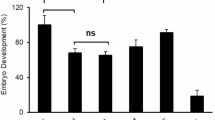
Prior to the study, a short desktop survey was done to rank the most prevalent internal parasites for ruminant animals with devastating effects according to records at the Department of Veterinary Services Zimbabwe, and the results are presented in Table 1. The results showed that H. contortus was the main problematic parasite, giving us the justification for the current study. The most affected animals were; sheep, goats and cattle in that order. Only cattle were slaughtered in large enough numbers (at all the four abattoirs) to warrant an effective study, hence were used in the current study. The general effects of treatments over time were evaluated and results are presented in Fig. 1. Motility and activeness decreased over time, while overall mortality increased (P < 0.05). Motility decreased (P < 0.05) with time for T3 –T6 (Table 2) while it did not change for T1 and T2 (P < 0.05). Within 30 min of incubation, T5 and T6 showed a reduction in motile worms which continued to zero after 8 h (P < 0.05). No worms were motile after 6 h for T6 while T5 had zero motility at 8 h, and it took 6 h for T4 to reduce motility to 50%. Although motility was reduced in T2 and T3, it needed more than 8 h to immobilise at least 50% of the worms. Treatments and time were significant (P˂0.05) in controlling the activeness of H. contortus (Table 3). Activeness did not change (P > 0.05) over time for T1 and T2, while significant reductions were observed for T3 – T6 (P < 0.05). Even after 8 h of incubation, more than 50% of the worms were still active in T1, T2 and T3. Meaningful reductions in activity were observed in T4 from 6 to 8 h, T5 form 4–8 h and T6 form 2–6 h. For T6 after 6 h, no worms were observed active. Mortality increased (P < 0.05) over time for T3, T4, T5 and T6 (Table 4), approximately one worm died in T1 over 8 h of incubation. The highest mortality was observed in T4, T5 and T6, 8 h after incubation, however only T6 totally killed all worms 6 h after incubation. Treatments 4 and 5 only achieved 80 and 82% mortality respectively, 8 h post incubation.
M = Males; F = Females; IS = Infectious stage: minimum number of days for parasite to reach infectious larvae stage (L3) after hatching of eggs; PP = Prevalent stage: period up to appearance of first eggs in dung after host is infected. a infectious stage of Bunostomum is not yet known.
Discussion
The prevalence of H. contortusamong ruminant animals in the current study was not obvious, however, several studies1,2,20have shown similar results especially among small ruminant animals. Our results are in agreement to Pfukenyi & Mukaratirwa21, and Zvinorova et al.22 who promoted that H. contortus is the most prevalent GIN in Zimbabwe. As reported by Hansen and Perry (1994), Tariq et al.19 and Goel et al.2, several plants have anthelminthic properties, and were in fact a part of the traditional husbandry before synthetic dewormers were commonly adopted. Furthermore, veterinary research zeroed in on deworming plants based on this conventional wisdom. Undoubtedly, there is renewed interest in pesticidal plants, due to notable environmental impacts of synthetic chemicals and anthelmintic resistance15,24,25,26. In the current study, higher concentrations of wormwood inactivated, immobilised and killed H. contortusworms, we couldn’t find similar studies in beef production, however the genus was used in sheep27, pigs28and humans12 for promoting growth, immunity and reproductive remedies respectively.
Artemisia are rich sources of sesquiterpene lactones and flavonoids that have reported anthelmintic activity29. For instance, santonin was used to treat intestinal worms before the advent of synthetic anthelmintics, and has recently been used as a reference anthelmintic for in-vitro studies (Barrón-Bravo et al., 2020). In other studies, the aqueous extract and essential oil of A. herba-alba expressed antihemanial activity against Leishmania species30. It is known that phenolic, flavonoid, thiophene and terpenoids are the main secondary metabolites of Artemisiaspecies29. However, the mechanism by which these phytochemicals work is partially understood. Bisht et al.32, postulates that they target important physiological processes such as respiration, enzyme activities and alter the micro and ultra-structure of the cell, thereby causing an alteration of protein and nucleic acid biosynthesis as well as causing imbalances on the activity of antioxidant enzymes. In addition, Geurden et al.24 suggested that these compounds can work directly (affecting larval establishment, larval motility, mortality, decreasing faecal egg output, impairing worm development, and decreasing egg hatchability from faeces) or indirectly (balancing antioxidant blood levels, improving the nutritional status, and boosting the immune system of parasitized animals). In agreement Bedasa et al.33aver that phytochemicals are amplified by the prohibition of DNA polymerase and prevent the translation of DNA as well as protein synthesis, cell proliferation and elongation in their target organisms. It has been confirmed that thiophenes in wormwood plays a more important role in nematicidal activity than other compounds29 hence we believe they had a direct effect on worms in the current study.
Chemical dewormers’ use builds resistance within parasite populations over time34. The single most important measure to slow the development of resistance is to reduce the exposure of worms to treatments by only using dewormers when needed (Hansen and Perry, 1994). However current methods promote the use of medicinal plants, as shown in this study. We therefore advocate that farmers desist from ‘just in case’ deworming practices and employ evident based management protocols like FAMACHA in internal parasite control. The use of natural plants with phytochemical properties inevitably reduces anthelmintic resistance (AR), however the promotion of this technology is not finding adequate support throughout the world, probably due to business’interest protectionism. We believe there is enough evidence to support the use of medicinal plants in the fight against AR. Recent studies have shown beyond any reasonable doubt the futility of relying on commercial drugs in the control of both internal35,36,37and external parasites38,39,40,41 in livestock production. Specifically, H. contortushave shown resistance against a number of commercial drugs35,36,42. Nonetheless our current results suggest that A. herb-alba was effective against H. contortus in beef cattle production and research to identify the active ingredient are necessary before it can be tested against other GIN of ruminants, such as Eimeria and Fasciola.
The effects of internal parasites are dependent upon geographic and climatic locations. According to Arsenopoulos et al.42, a high temperature-humidity index promote parasitism in cattle. In agreement, Pfukenyi et al.23 noted that the prevalence of helminths eggs is related to the climatic conditions (temperature and rainfall), such that during the wet months (December to March), conditions are more favourable for the development and survival of the pre-parasitic stages, leading to increased availability of infective larvae on the pasture. Again the same authors concluded that a higher prevalence of helminths was related to altitude, similar to Zvinorova et al.22. Gastrointestinal parasitism is associated with economic losses, lowered productivity, reduced animal performance, mortality and morbidity22. Parasitic helminths represent one of the most pervasive challenges to livestock, and their intensity and distribution will be influenced by climate change, since survival and development of free-living stages is chiefly affected by temperature and moisture33. In addition, GIN increase the demand for amino acids by the host, no wonder most of the affected animals are emaciated. To counteract GIN infections, pasture management is vital22 and it has been a rule of thumb that forage height should be maintained at least 10 cm. Most parasite larvae migrate in water droplets on grass, but usually to heights no greater than 7.6 cm. Alternating or co-grazing of pastures between cattle and small ruminants is also recommended, as they do not share the same parasites, however, this is not true for H. contortus. High stocking rates are also known to ultimately increase parasitic loads on pasture22. Furthermore, over-grazing, forces animals to feed close to the soil, where worm larvae live. It is unlikely in the current modus operandothat natural products can totally substitute synthetic anthelmintic, however they can reduce dependency on commercial products, improve meat quality acceptability by the growing number of consumers demanding organic products43, as well as reduce the environmental impacts associated with pharmaceutical products38.
Conclusion
Artemisia herba-alba affected the overall in-vitro longevity of H. contortus and is a potential medicinal plant for the control of GIN, as well as a promising substitute for synthetic anthelmintics in the era of AR. It is recommended that small holder farmers should incorporate worm wood extracts into their feeding regimes and that an in-vivo study with infested animal’s is required.
Data availability
Data available on request from the corresponding author.
References
Papadopoulos, E. Haemonchus contortus Parasitism in Intensively Managed Cross-Limousin Beef Calves: Effects on Feed Conversion and Carcass Characteristics and Potential Associations with Climatic Conditions. ; (2022).
Goel, V., Singla, L. & Das, Choudhury, D. Cuminaldehyde induces oxidative stress-mediated physical damage and death of Haemonchus contortus. Biomed Pharmacother [Internet]. ;130(May 2020):110411. (2020). Available from: https://doi.org/10.1016/j.biopha.2020.110411
Tan, T. K. et al. Co-infection of Haemonchus Contortus and Trichostrongylus spp. among livestock in Malaysia as revealed by amplification and sequencing of the internal transcribed spacer II DNA region. BMC Vet. Res. 10, 1–7 (2014).
Mcrae, K. M., Stear, M. J., Good, B. & Keane, O. M. The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunol. 37 (12), 605–613 (2015).
McKay-Demeler, J. & Playford, M. Evaluating methods for conducting faecal egg count reduction tests in sheep using McMaster technique, Mini-FLOTAC and pooled counts. Aust Wool Innov Ltd [Internet]. ; (2021). Available from: https://www.wool.com/globalassets/wool/sheep/research-publications/welfare/flystrike-control/awi-fecrt-final-report-05mar2021mp-rev15.3.21.pdf
Mavrot, F., Hertzberg, H. & Torgerson, P. Effect of gastro-intestinal nematode infection on sheep performance: a systematic review and meta-analysis. Parasites Vectors. 8 (1), 1–11 (2015).
Jain, D. & Basniwal, P. K. Tapentadol, a novel analgesic : Review of recent trends in synthesis, related substances, analytical methods, pharmacodynamics and pharmacokinetics. Bull Fac Pharmacy, Cairo Univ [Internet]. ;51(2):283–9. (2013). Available from: https://doi.org/10.1016/j.bfopcu.2013.04.003
Vargason, A. M., Anselmo, A. C. & Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 5 (9), 951–967 (2021).
Vargason, A. M., Anselmo, A. C. & Mitragotri, S. technologies. Nat Biomed Eng [Internet]. ;5(September). (2021). Available from: https://doi.org/10.1038/s41551-021-00698-w
Prichard, R. K. & Geary, T. G. IJP: Drugs and Drug Resistance Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. IJP Drugs Drug Resist [Internet]. ;10(June):69–83. (2019). Available from: https://doi.org/10.1016/j.ijpddr.2019.06.002
Nyagumbo, E. et al. Medicinal plants used for the management of respiratory diseases in Zimbabwe: Review and perspectives potential management of COVID-19. Phys Chem Earth [Internet]. ;128(September):103232. (2022). Available from: https://doi.org/10.1016/j.pce.2022.103232
Niloofar, H., Raheleh, B., Roshanak, S. & Jamshid, J. European Journal of Obstetrics & Gynecology and Reproductive Biology Evaluation of the safety and efficacy of wormwood vaginal gel in improving sexual function and sexual satisfaction in women of reproductive age: A randomized, triple-blinds, placebo-c. Eur J Obstet Gynecol Reprod Biol [Internet]. ;280(July 2022):1–6. (2023). Available from: https://doi.org/10.1016/j.ejogrb.2022.11.002
Krebs, S., Omer, T. N. & Omer, B. Phytomedicine Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn ’ s disease – A controlled clinical trial. Phytomedicine [Internet]. ;17(5):305–9. (2010). Available from: https://doi.org/10.1016/j.phymed.2009.10.013
Sotenjwa, V. Z., Chen, W., Veale, C. G. L. & Anokwuru, C. P. Fitoterapia Chemotypic variation of non-volatile constituents of Artemisia afra (African wormwood) from South Africa. Fitoterapia [Internet]. ;147(September):104740. (2020). Available from: https://doi.org/10.1016/j.fitote.2020.104740
Prichard, R. K. & Geary, T. G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int J Parasitol Drugs Drug Resist [Internet]. ;10(April):69–83. (2019). Available from: https://doi.org/10.1016/j.ijpddr.2019.06.002
Seddiek, S. A., Ali, M. M., Khater, H. F. & El-shorbagy, M. M. Anthelmintic activity of the white wormwood, Artemisia herba-alba against Heterakis Gallinarum infecting Turkey poults. ;5(16):3946–3957. (2013).
Beshay, E. V. N. Therapeutic efficacy of Artemisia absinthium against Hymenolepis Nana: in vitro and in vivo studies in comparison with the anthelmintic praziquantel. ;298–308. (2018).
Coles, G. C. et al. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 136 (3–4), 167–185 (2006).
Tariq, K. A., Chishti, M. Z., Ahmad, F. & Shawl, A. S. Veterinary parasitology anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. ;160:83–88. (2009).
Sanders, J. et al. A new paraprobiotic-based treatment for control of Haemonchus contortus in sheep. Int J Parasitol Drugs Drug Resist [Internet]. ;14(November):230–6. (2020). Available from: https://doi.org/10.1016/j.ijpddr.2020.11.004
Article, R. et al. A review of the epidemiology and control of gastrointestinal nematode infections in cattle in. ;1–12. (2007).
Zvinorova, P. I. et al. Prevalence and risk factors of gastrointestinal parasitic infections in goats in low-input low-output farming systems in Zimbabwe. Small Rumin Res [Internet]. ;143:75–83. (2016). Available from: https://doi.org/10.1016/j.smallrumres.2016.09.005
Pfukenyi, D. M. & Mukaratirwa, S. A review of the epidemiology and control of gastrointestinal nematode infections in cattle in Zimbabwe. Onderstepoort J. Vet. Res. 80 (1), 1–12 (2013).
Geurden, T. et al. Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int J Parasitol Drugs Drug Resist [Internet]. ;5(3):163–71. (2015). Available from: https://doi.org/10.1016/j.ijpddr.2015.08.001
Wondimu, A. & Bayu, Y. Anthelmintic Drug Resistance of Gastrointestinal Nematodes of Naturally Infected Goats in Haramaya, Ethiopia. J Parasitol Res. ;2022. (2022).
Aguerre, S. et al. Resistance to gastrointestinal nematodes in dairy sheep: Genetic variability and relevance of artificial infection of nucleus rams to select for resistant ewes on farms. Vet Parasitol [Internet]. ;256(April):16–23. (2018). Available from: https://doi.org/10.1016/j.vetpar.2018.04.004
Kim, S. C., Adesogan, A. T., Shin, J. H., Lee, M. D. & Ko, Y. D. The effects of increasing the level of dietary wormwood (Artemisia Montana Pampan) on intake, digestibility, N balance and ruminal fermentation characteristics in sheep. ;100:261–269. (2006).
Chu, G. M. & Song, Y. M. Effect of dietary addition of wormwood (Artemisia montana Pampan) on performance of fattening pigs and selected hematological and immunological indices. Livest Sci [Internet]. ;147(1–3):188–91. (2012). Available from: https://doi.org/10.1016/j.livsci.2012.03.012
Liu, T., Wu, H., Wu, H. & Zhang, J. Industrial Crops & Products Wormwood (Artemisia absinthium L.) as a promising nematicidal and antifungal agent : Chemical composition, comparison of extraction techniques and bioassay-guided isolation. Ind Crop Prod [Internet]. ;133(March):295–303. (2019). Available from: https://doi.org/10.1016/j.indcrop.2019.03.039
Hassan, A. A. et al. Antileishmanial Activities of Medicinal Herbs and Phytochemicals In Vitro and In Vivo: An Update for the Years 2015 to 2021. ; (2022).
Bisht, D., Kumar, D., Kumar, D., Dua, K. & Chellappan, D. K. Phytochemistry and Pharmacological Activity of the Genus artemisiaVol. 44439–474 (Pharmaceutical Society of Korea, 2021). Archives of Pharmacal Research.
Bisht, D., Kumar, D. & Archives of Pharmacal Research. Phytochemistry and pharmacological activity of the genus artemisia [Internet]. Vol. 44,. Pharmaceutical Society of Korea; 439–474 p. (2021). Available from: https://doi.org/10.1007/s12272-021-01328-4
Duguma, A. Journal of Veterinary V Eterinary Science & T echnology status of Helminthes Parasites of Cattle in dairy farms of Holleta. ;7(5). (2016).
Assan, N. & Assan, N. Genetic Improvement and Utilization of Indigenous Cattle Breeds for Beef Production in Zimbabwe : Past, Present and Future Prospects. A Review article Genetic improvement and utilization of indigenous cattle breeds for beef productio. 2014;(January 2012). (2012).
Sanders, J. et al. A new paraprobiotic-based treatment for control of Haemonchus contortus in sheep. Int. J. Parasitol. Drugs Drug Resist. 14 (September), 230–236 (2020).
Baltrušis, P., Halvarsson, P. & Höglund, J. Exploring benzimidazole resistance in Haemonchus contortus by next generation sequencing and droplet digital PCR. Int J Parasitol Drugs Drug Resist [Internet]. ;8(3):411–9. (2018). Available from: https://doi.org/10.1016/j.ijpddr.2018.09.003
Adduci, I. et al. Haemonchosis in Sheep and Goats, Control Strategies and Development of vaccines against Haemonchus Contortus. Animals 12 (18), 1–20 (2022).
Adenubi, O. T., McGaw, L. J., Eloff, J. N. & Naidoo, V. In vitro bioassays used in evaluating plant extracts for tick repellent and acaricidal properties: a critical review. Vet. Parasitol. 254 (May 2017), 160–171 (2018).
Elmhalli, F. et al. Veterinary parasitology: Regional studies and reports Acaricidal activity against Ixodes ricinus nymphs of essential oils from the Libyan plants Artemisia herba Alba, Origanum majorana and Juniperus phoenicea. ;24(March). (2021).
Sanhokwe, M., Mupangwa, J., Masika, P. J., Maphosa, V. & Muchenje, V. Medicinal plants used to control internal and external parasites in goats. Onderstepoort J. Vet. Res. 83 (1), 1–7 (2016).
van Wyk, R. D. J., Baron, S. & Maritz-Olivier, C. An integrative approach to understanding pyrethroid resistance in Rhipicephalus microplus and R. decoloratus ticks. Ticks Tick Borne Dis [Internet]. ;7(4):586–94. (2016). Available from: https://doi.org/10.1016/j.ttbdis.2016.01.007
Arsenopoulos, K. V., Katsarou, E. I., Mendoza Roldan, J. A., Fthenakis, G. C. & Papadopoulos, E. Haemonchus Contortus Parasitism in intensively managed Cross-limousin Beef calves: effects on feed Conversion and carcass characteristics and potential associations with climatic conditions. Pathogens ;11(9). (2022).
Chikwanha, O. C., Vahmani, P., Muchenje, V., Dugan, M. E. R. & Mapiye, C. Nutritional enhancement of sheep meat fatty acid profile for human health and wellbeing. ; (2017).
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, Formal analysis and investigation, Writing - review and editing, Supervision: [Washaya Soul] Writing - original draft preparation: [Hoshiki Annamore]; Supervision: [Nyamushamba Godfrey B]. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hoshiki, A., Soul, W. & Bernard, N.G. The anthelmintic activity of the white wormwood (Artemisia herba Alba) against Haemonchus contortus in beef cattle. Sci Rep 15, 637 (2025). https://doi.org/10.1038/s41598-024-84656-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84656-x