Abstract
Macular degeneration is a leading cause of irreversible vision loss, significantly impacting quality of life. To enhance clinical practice and reduce the risk of drug-related macular degeneration, we analyzed drug-related trends using real-world data. Disproportionality analysis of adverse event reports from the FDA Adverse Event Reporting System (FAERS, 2004–2023) identified 67,683 cases involving 1402 drugs. Among these, 42 drugs were linked to significant risks, including treatments for breast cancer (tamoxifen, raloxifene, anastrozole, letrozole) and diabetes (insulin lispro, insulin human). The BCPNN algorithm revealed that 45.2% (19/42) of these drugs had the strongest associations with macular degeneration, with pentosan polysulfate sodium, travoprost, and tolterodine being the highest-risk drugs. Lifitegrast, nicotine, and travoprost were associated with the shortest onset times for ocular adverse events. Among drug classes, glucocorticosteroids were linked to the most rapid onset of ocular side effects (P < 0.001), typically occurring within two months compared to other drugs. Drug-related macular degeneration was more common in women (70.4%) and predominantly affected those aged 60–80. The incidence of drug-related macular degeneration has steadily increased in recent years. This study offers valuable pharmacovigilance insights, highlighting drugs and demographic factors linked to macular degeneration.
Similar content being viewed by others
Introduction
Macular degeneration is an eye disease marked by structural or functional damage to the macula1. This damage leads to a progressive loss of central vision, significantly impairing fine visual tasks such as reading, driving, and facial recognition2. Approximately 20 million people in the United States are affected by macular degeneration, while the global count is close to 196 million3. In 2020, it ranked as the fourth leading cause of blindness worldwide4.
Multiple risk factors contribute to the development of macular degeneration, including age, smoking, and sun exposure5. Long-term use of certain medications has also been associated with damage to the macular region. For instance, pentosan polysulfate sodium (PPS) has been linked to a specific type of macular degeneration, particularly impacting the retinal pigment epithelium (RPE) and its interface with photoreceptors. The toxicity of PPS is significantly dose-dependent, and macular degeneration may continue to progress even after the drug is discontinued6. Given the potential link between drug use and macular pathology, investigating adverse events from large, real-world databases is essential1,7. Identifying drugs associated with macular degeneration is also key to guiding diagnosis and treatment in clinical practice.
The FDA Adverse Event Reporting System (FAERS) is a publicly accessible database that collects reports of adverse drug events to assist the FDA in post-market safety monitoring of drugs and therapeutic products8. Drug-related macular degeneration is a serious adverse reaction that can occur during drug use. Through disproportionality analysis of the FAERS database, the risk of various drugs inducing macular degeneration can be quantified9.
In this study, we analyzed all adverse event reports related to macular degeneration in the FAERS database, specifically evaluating the risk of macular degeneration and the onset times of associated ocular adverse reactions for these drugs. Our goal is to characterize the risk profiles of various drugs that may lead to macular degeneration, providing valuable insights for clinical decision-making and helping to minimize drug-related macular degeneration.
Methods
Data source
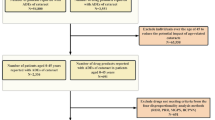
Data were collected from the FAERS database, covering the period from January 2004 to December 2023. To ensure data rigor, only reports from healthcare professionals, specifically physicians and pharmacists (code = MD or PH), were selected. These reports were used to assess the prevalence of drug-related adverse reactions and the use of drugs associated with macular degeneration. Adverse event data from the FAERS database were downloaded from the FDA website (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). The database primarily includes all spontaneously reported adverse events from global healthcare professionals, pharmaceutical manufacturers, and drug users. It consists of seven datasets: patient demographics and administrative information (DEMO), drug and biologic information (DRUG), adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), start and end dates of drug therapy (THER), and indications for drug use and diagnosis (INDI)9. Between January 2004 and December 2023, a total of 206,298,811 raw data entries were collected. After removing duplicates, 173,796,909 entries remained. Among these, 67,683 entries reported adverse events related to macular degeneration, affecting 9,693 subjects. A total of 1402 drugs were associated with macular degeneration adverse reactions. Due to the occurrence of duplicate drug names across commercial brands, drugs with fewer than 3 reported cases were excluded, resulting in 470 unique drugs being retained after duplication removal. The data-cleaning process is illustrated in Fig. 1. As FAERS is a publicly accessible, fully anonymized dataset that does not include personally identifiable information, the study adheres to ethical guidelines, and no IRB approval was required, in line with FDA policies ensuring the privacy and confidentiality of the data10.
Flowchart of patient selection and data cleaning for drug-related macular degeneration in the FAERS database. The background map and flowchart were generated using Microsoft Visio 2021 (https://www.microsoft.com/visio) to illustrate the patient selection and data cleaning methodology for analyzing drug-related macular degeneration cases within the FAERS database.
Identification of ADRs
The definition of adverse drug reactions (ADRs) in this study is based on version 20.0 of the Medical Dictionary for Regulatory Activities (MedDRA, http://www.meddra.org/)11. Adverse events were encoded using MedDRA® preferred terms (PTs), and standardized MedDRA® queries were applied to identify PTs related to macular degeneration. This study specifically utilized PTs with a ‘narrow’ scope12.
Statistical analysis
Signal detection was conducted using four disproportionality methods: the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and multi-item gamma Poisson shrinker (MGPS)13,14,15,16. These methods identify potential positive signals by comparing the frequency of target events and drugs against all other events and drugs using a fourfold table calculation, with brief definitions provided in Tables 1 and 2 for clarity. The criteria for positive signals were as follows: (1) ROR: a ≥ 3 and 95% CI lower > 1; (2) PRR: a ≥ 3 and 95% CI lower > 1; (3) BCPNN: IC025 > 0; and (4) MGPS: EBGM05 > 2 and a > 0. In this study, a drug was considered a positive signal only if it met the criteria across all four methods, suggesting a potential association between the drug and the event. Subsequently, the BCPNN algorithm was used to stratify the risk of macular degeneration associated with different drugs. Further analysis was conducted on the onset times of eye-related adverse reactions for drugs identified as positive signals. These drugs were categorized into quartiles based on the onset times of these adverse reactions, and differences in onset times among the various drugs were compared. The DrugBank database was used to identify and categorize the trade and generic names of the drugs.17 Statistical analyses were conducted using SPSS (version 26.0; IBM, US), GraphPad Prism (version 10.1.2), Microsoft Excel 2019, and R (version 4.2.2), with P values < 0.05 considered statistically significant. For R-based analysis, the main packages used were ggplot2 (version 3.4.4), ggrepel (version 0.9.4), dplyr (version 1.1.4), and DescTools (version 0.99.52).
Result
Subject descriptive analysis
A total of 9693 subjects who reported adverse events related to macular degeneration were included in this study. The mean age of the cohort was 72.5 ± 14.3 years, with a predominance of female patients (70.4%) compared to male patients (23.1%). This gender disparity was observed consistently across all age groups. The majority of adverse events were reported within the 60–80 age range (Fig. 2A). Between 2004 and 2023, the FAERS database recorded a steady increase in the number of macular degeneration cases, culminating in a peak in 2023 (Fig. 2B). In terms of the occupational distribution of reporters, consumers represented the largest group, comprising 5,888 individuals (61%), followed by physicians at 1,663 individuals (17%) (Fig. 2C). The most frequently reported route of drug administration associated with adverse events was oral administration (28%), followed by subcutaneous injection (14%) and respiratory administration (6%) (Fig. 2D). Regarding patient outcomes, "Other serious conditions" accounted for the majority of cases (80.1%), with hospitalization being the second most common outcome (13.0%) (Fig. 2E). Geographically, the United States reported the highest number of cases (6,471; 66.8%), followed by Canada (12.4%), Germany (3.2%), and the United Kingdom (1.8%) (Fig. 2F,G). Comprehensive demographic details are provided in Table 3. Additionally, we conducted subgroup analyses by country, dividing cases into U.S. and non-U.S. groups, and found similar demographic patterns as described above (refer to Supplementary Material Table 1).
Distribution of baseline data for patients reporting adverse events of macular degeneration in the FAERS database. (A) A pyramid of the age distribution of patients reporting adverse events of macular degeneration by gender. (B) The temporal distribution of macular degeneration adverse event reports. (C) The distribution of occupations among patients with macular degeneration adverse events. (D) The distribution of routes of administration for patients with macular degeneration adverse events. (E) A histogram of the distribution of outcomes in patients with macular degeneration adverse events. (F) A histogram of the distribution of macular degeneration adverse event reports by country. (G) A heat map of the distribution of reported adverse events of macular degeneration by country.
Drug screening and disproportionality analysis
During the screening of 1402 drugs, those with fewer than three reported cases were excluded. A disproportionality analysis of the remaining 470 drugs, each with three or more reported cases of drug-related macular degeneration, identified 66 drugs with positive signals. The DrugBank database was then used to identify the brand names, generic names, and mechanisms of action for these 66 drugs. After excluding drugs with therapeutic effects on macular degeneration and consolidating those with the same generic name, the list was narrowed to 42 drugs, and their signal values were recalculated for further analysis. These 42 drugs were categorized by mechanism of action as follows: insulin and insulin analogs (4;9.5%), Phosphodiesterase type 5 inhibitors (PDE5 inhibitors) (3;7.1%), glucocorticosteroids (3;7.1%), bisphosphonates (3;7.1%), aromatase inhibitors (3;7.1%), selective estrogen receptor modulators (2;4.8%), prostaglandin analogs (2;4.8%), calcium channel blockers (2;4.8%), anticholinergic agents (2;4.8%), and 18 other drugs classified as "other medications," which collectively accounted for 42.9% of the total (Fig. 3A).
Detection of positive signaling drugs via disproportionate analysis and their purpose-based classification. (A) A Venn diagram illustrating the detection of positive signal drugs through four distinct disproportionality analysis methods and their subsequent classification. (B) A heatmap categorizing these positive signal drugs based on their mechanisms of action; darker colors represent higher signal values, indicating a greater risk of drug-related macular degeneration. ROR reporting odds ratio, PRR proportional reported ratio, BCPNN Bayesian confidence propagation neural network, MGPS multi-item gamma poisson shrinker.
Drug classification by therapeutic purpose
The drugs associated with macular degeneration were classified according to their mechanisms of action as follows: selective estrogen receptor modulators (SERMs) included tamoxifen (ROR = 16.92) and raloxifene (ROR = 7.82); prostaglandin analogs included travoprost (ROR = 27.99) and latanoprost (ROR = 17.55); PDE5 inhibitors included vardenafil (ROR = 22.20) and tadalafil (ROR = 17.55); insulin and insulin analogs included insulin lispro (ROR = 15.44) and insulin human (ROR = 9.48); glucocorticosteroids included fluticasone furoate (ROR = 27.53) and budesonide (ROR = 19.05); calcium channel blockers included amlodipine (ROR = 10.69) and exforge (ROR = 6.46); bisphosphonates included risedronate (ROR = 7.34) and ibandronate (ROR = 5.34); aromatase inhibitors included anastrozole (ROR = 16.29) and letrozole (ROR = 14.40); anticholinergic agents included tolterodine (ROR = 27.80) and tiotropium (ROR = 5.00); and other medications included pentosan polysulfate sodium (ROR = 54.93) and simbrinza (ROR = 15.07) (Fig. 3B and Table 4). To provide further insights, the most commonly reported dosages for these drugs in the FAERS database are summarized in Supplementary Table 2.
Drug classification by risk degree
We applied the BCPNN algorithm to assess the risk of drug-related macular degeneration, categorizing risk levels based on BCPNN values: values between 0 and 1.5 were classified as low risk, values between 1.5 and 3 as moderate risk, and values above 3 as high risk18. Using these criteria, we evaluated the risk of drug-related macular degeneration for 42 medications. The four drugs identified with the highest risk were pentosan polysulfate sodium (BCPNN = 4.09), travoprost (BCPNN = 3.13), tolterodine (BCPNN = 3.12), and fluticasone furoate (BCPNN = 3.11) (Fig. 4A).
Distribution of drug risk levels and time-to-onset of ocular adverse reactions for macular degeneration-associated drugs, ordered by descending risk and onset time. (A) The risk levels of positive drugs assessed using the BCPNN algorithm. (B) The median induction time for drug-related macular degeneration. BCPNN Bayesian confidence propagation neural network.
Drug classification by duration of adverse reactions
We analyzed the time to onset of ocular adverse effects for 42 drugs potentially linked to drug-related macular degeneration. The six drugs with the shortest time to onset arelifitegrast, nicotine, travoprost, budesonide, simbrinza, and rotigotine, which typically induced ocular adverse effects within one month of initiation. In contrast, the four drugs with the longest time to onset are conjugated estrogens, human insulin, levothyroxine, and insulin lispro, whichgenerally required over three years of use before ocular adverse effects manifested (Fig. 4B and Table 5). If categorized by the mechanism of action, glucocorticosteroids demonstrated the shortest time to onset of ocular adverse effects, followed by anticholinergic agents. In contrast, SERMs, along with insulin and its analogs, showed the longest onset times. The overall comparison across all ten categories was highly statistically significant (P < 0.0001) (Fig. 5A,B). Further analysis based on the ATC classification system revealed that respiratory system medications had the shortest time to onset of ocular adverse effects, followed by ophthalmic medications. In contrast, urogenital system and sex hormone medications, as well as diabetes medications, exhibited the longest onset times. The overall comparison of onset times across all eight categories was statistically significant (P < 0.0001) (Fig. 6A,B).
Cumulative risk curve illustrating the timeline of ocular adverse reactions categorized by ATC classification. Figure 5A and (A) depict cumulative risk timelines for drug-induced reactions across various drug classifications. Figure 5B and (B) illustrate violin plots showing time differences in drug-induced reactions across various drug classifications. Horizontal lines above the plots indicate statistically significant differences between groups (P < 0.05).
Discussion
Macular degeneration significantly impairs central vision, greatly impacting patients’ quality of life. Although treatment strategies for macular degeneration have advanced in recent years, effective methods to prevent irreversible photoreceptor degeneration remain elusive19,20. Given that drug use is a recognized risk factor for macular degeneration, rational drug decision-making is crucial for prevention. In this study, we systematically analyzed drug-related adverse events associated with macular degeneration reported in the FAERS database since its inception in January 2004. Our findings showed that drug-related macular degeneration cases were predominantly reported among elderly women in Western countries, with a notable increase in reports over time. Using four disproportionality analysis methods, we identified 42 drugs with positive signals for drug-related macular degeneration. These drugs, which span various mechanisms of action, exhibited significant differences in their associated risk of inducing macular degeneration. Particularly noteworthy were categories such as SERMs, insulin and its analogs, and glucocorticoids, underscoring the need for careful consideration in clinical settings. Further analysis revealed significant differences between drug categories in terms of the risk of macular degeneration and the timing of ocular adverse events. These findings provide valuable real-world data and theoretical support for clinical decision-making, guiding the rational use of medications to reduce the risk of macular degeneration and offering important insights for future research.
Aromatase inhibitors (AIs) are frequently employed in clinical settings to manage estrogen-dependent breast cancer by blocking the conversion of androgens to estrogens, effectively reducing estrogen levels21,22. Similarly, SERMs target this cancer type by competing with estrogen and modulating estrogen receptor (ER) activity via alterations in associated cofactors22,23. Previous studies have shown that prolonged exposure to both endogenous and exogenous estrogen is associated with a reduced risk of developing macular degeneration later in life, suggesting a potential protective role of estrogen in preserving macular health24. However, the use of AIs and SERMs may counteract its protective effects. For instance, tamoxifen, a widely used SERM, has been linked to an elevated risk of macular degeneration, possibly due to its reduction of macular pigment concentration, which may be related to diminished estrogen activity25. Our study corroborates this association by revealing a significantly higher incidence of drug-related macular degeneration in female patients over the age of 55 compared to male counterparts following the use of these medications. This finding underscores the potential impact of reduced estrogen activity, particularly in increasing the risk of macular degeneration. Considering that endocrine therapy for breast cancer is usually prolonged, often exceeding five years to effectively lower the risk of late recurrence, vigilant monitoring of potential ocular toxicity associated with these treatments is crucial26. The median onset of ocular adverse effects from AIs and SERMs is relatively brief, at 436.27 days and 633.07 days, respectively. Therefore, regular ophthalmologic assessments are essential during the prolonged use of these medications to enable timely intervention and mitigate potential risks. Future research should aim to elucidate the complex interplay between estrogen, pharmacotherapy, and eye health. This could facilitate the development of strategies that not only optimize breast cancer treatment but also preserve the health of macula, paving the way for more personalized and comprehensive therapeutic approaches.
Prostaglandin analogs, commonly prescribed for glaucoma, have traditionally been associated with side effects such as eyelash growth and conjunctival hyperemia27,28. However, emerging evidence suggests a potential link between these drugs and macular degeneration. The proposed mechanism may involve the reduction of intraocular pressure, leading to changes in ocular perfusion pressure (OPP) that impact choroidal blood flow and, subsequently, the function of the RPE. RPE dysfunction can cause the accumulation of metabolic waste, such as lipofuscin, and oxidative damage, accelerating degeneration within the macular region29,30. In our analysis, we compared the risk of macular degeneration between glaucoma and non-glaucoma patients using prostaglandin analogs, finding a significantly higher risk in non-glaucoma patients. This observation suggests that prostaglandin analogs may indirectly affect RPE health by modulating OPP, potentially promoting the onset and progression of macular degeneration. Since OPP is influenced not only by intraocular pressure but also by systemic blood pressure, future studies should account for systemic blood pressure variables to further clarify the potential risks associated with prostaglandin analogs in macular degeneration and support individualized treatment approaches. Similarly, inhaled corticosteroids (ICS), such as fluticasone furoate and budesonide, have also shown potential associations with macular degeneration. Although previous studies primarily noted ocular side effects like elevated intraocular pressure and posterior subcapsular cataracts31,32, our findings reveal a significant association between ICS use in chronic obstructive pulmonary disease (COPD) treatment and macular degeneration risk, even when administered at standard recommended doses33. Further subgroup analyses revealed a significantly higher risk of macular degeneration in patients using corticosteroids for non-approved indications compared to those using corticosteroids for approved indications. This suggests that corticosteroids may independently contribute to the risk of macular degeneration in patients using them for non-approved conditions, independent of the underlying diseases for which they are prescribed. We hypothesize that ICS may impair RPE function indirectly by influencing intraocular pressure, altering OPP, or modulating inflammatory levels, ultimately contributing to macular degeneration progression. Likewise, PDE5 inhibitors, primarily prescribed for erectile dysfunction, have been previously linked to side effects like color vision impairment34. Our study suggests that these drugs may also play a role in the development of macular degeneration, potentially leading to vision loss. The underlying mechanism may involve cross-reactivity between PDE5 inhibitors and phosphodiesterase type 6 (PDE6) in the retina35. This study is the first to establish a possible correlation between these medications and macular degeneration, underscoring the necessity for further validation through larger-scale studies. Additionally, heightened attention should be directed toward the ocular adverse effects of these medications, particularly glucocorticosteroids, which have the shortest median onset time for ocular complications, at just 58.08 days. Consequently, vigilant monitoring of their short-term effects is essential.
Additionally, a 10-year retrospective study has demonstrated an increased risk of macular degeneration in patients using the second-generation calcium channel blocker amlodipine. (CCBs) 36 This finding aligns with our analysis. Further subgroup analyses revealed that hypertensive patients using CCBs exhibited a significantly higher relative risk (ROR) of macular degeneration compared to their non-hypertensive counterparts. This suggests that hypertension may amplify the adverse effects of CCBs, thereby exacerbating the risk of macular degeneration. Consequently, hypertensive patients appear to face an elevated drug-related risk, with hypertension potentially acting as a modulating factor in this association. Regarding anticholinergic agents, a multicenter study reported that prolonged use and higher anticholinergic burden were significantly associated with an increased risk of macular degeneration37. Furthermore, a case–control study found that bisphosphonate users had a 1.99-fold higher risk of developing macular degeneration compared to non-users, with those having higher medication possession ratios (MPR) facing an even greater risk. These findings corroborate our real-world data, reinforcing the association between these drugs and macular degeneration38,39. Moreover, real-world studies leveraging the FAERS database have further validated the link between these medications and macular degeneration. Notably, these studies also confirmed the detrimental effects of hydroxychloroquine, commonly prescribed for systemic lupus erythematosus, as well as the harmful impact of nicotine from tobacco use on the development of macular degeneration7,40,41. Therefore, when making drug-related decisions, it is crucial to carefully consider the potential risks of macular degeneration associated with these medications.
Notably, earlier cohort studies have suggested that insulin and its analogs may be linked to a reduced risk of macular degeneration. For example, patients with untreated type 2 diabetes (T2D) face over a 30% higher risk of developing macular degeneration compared to those receiving treatment, while insulin users show a decreased lifetime risk of macular degeneration42. Animal studies further support this by demonstrating that systemic insulin administration can delay cone cell death and retinal pigment degeneration (RP) in mice, suggesting a protective role of insulin against macular degeneration43. However, our study presents a contrasting finding, suggesting that insulin and its analogs may be associated with an increased risk of macular degeneration. Further analysis revealed significant differences between our subgroups: non-diabetic patients using insulin and its analogs exhibited a notably higher risk of macular degeneration compared to their diabetic counterparts. This discrepancy may be due to insulin’s well-known side effect, hypoglycemia, which occurs when insulin levels surpass physiological requirements.44 For instance, in retinal models of hypoglycemic chickens, spontaneous spreading depression (SD) has been observed, potentially leading to irreversible macular degeneration45. Long-term insulin use may exacerbate this risk46. Our study revealed that the median time to ocular adverse effects associated with insulin and its analogs was 475.66 days, reinforcing the idea that prolonged insulin use could lead to hypoglycemia and subsequent macular damage. The International Diabetes Federation (IDF) predicts that by 2045, the global population of diabetes patients will reach 700 million47. In this context, the rational use of insulin and its analogs to minimize adverse effects, such as macular degeneration, poses a considerable challenge in clinical practice. Therefore, future research should aim to optimize insulin therapy by balancing its therapeutic effects with potential ocular side effects, ultimately providing safer and more effective treatment strategies for diabetes patients.
In summary, this study provides a comprehensive overview of the potential risks associated with drug-related macular degeneration, emphasizing the time frames in which ocular adverse reactions may occur. To minimize these risks in clinical practice, careful consideration of drug selection, dosage, and treatment duration is crucial. Although disproportionality analysis is valuable for identifying potential drug-event associations, it cannot establish causality. The observational nature of the FAERS data, combined with the lack of randomization, limits our ability to draw definitive causal conclusions. Moreover, confounding factors, including patient demographics, disease severity, treatment duration, comorbidities, and concomitant medications, cannot be fully controlled in this observational setting, further complicating the interpretation of results.The voluntary nature of FAERS reporting also introduces biases, including underreporting and overreporting, which affect data accuracy and the generalizability of findings. Moreover, reports often lack key demographic details, complicating the assessment of risk for specific subgroups. The inability to determine the total number of patients exposed to a drug further prevents accurate incidence calculations, which are essential for assessing the actual risk48,49. To address these limitations, future research should prioritize well-designed prospective cohort studies and randomized controlled trials (RCTs) to establish stronger causal links between medications and macular degeneration. These studies would offer more precise risk assessments, particularly in the context of underlying diseases, and clarify how medications interact with pre-existing conditions to influence the risk profile for macular degeneration. Additionally, integrating complementary data sources, such as electronic health records (EHRs) and healthcare claims data, could mitigate biases inherent in spontaneous reporting systems and provide a more comprehensive understanding of drug dosages, treatment durations, and their real-world applicability. Large-scale clinical trials will be essential for validating these findings, elucidating the mechanisms underlying drug-related macular degeneration, and refining clinical guidelines. Ultimately, these efforts will ensure that treatment practices are based on the most reliable evidence, improving patient safety and outcomes.
Conclusion
Collectively, this study leveraged real-world adverse drug reaction data and employed disproportionality analysis to identify 42 drugs potentially associated with drug-related macular degeneration. The identified drugs were systematically categorized by their mechanisms of action, and the duration of associated ocular adverse reactions was thoroughly evaluated. The findings reveal the epidemiological characteristics of drug-related macular degeneration and highlight potential high-risk medications. This research provides robust data to inform clinical decision-making, aiming to mitigate the risk of such adverse reactions. Importantly, this study elucidates the complex relationship between drug use and macular degeneration, underscores the importance of understanding drug safety, and calls for heightened vigilance regarding the newly identified high-risk medications.
Data availability
The datasets used in this study are publicly available from the FDA Adverse Event Reporting System (FAERS). FAERS provides open access to its data without the requirement for accession numbers or user accounts. Data can be directly accessed and downloaded from the FAERS Public Dashboard (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html).
References
Khan, M. J., Papakostas, T., Kovacs, K. & Gupta, M. P. Drug-induced maculopathy. Curr. Opin. Ophthalmol. 31, 563 (2020).
Guymer, R. H. & Campbell, T. G. Age-related macular degeneration. Lancet 401, 1459–1472 (2023).
Fleckenstein, M., Schmitz-Valckenberg, S. & Chakravarthy, U. Age-related macular degeneration: A review. JAMA 331, 147–157 (2024).
GBD 2019 Blindness and Vision Impairment Collaborators & Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob. Health 9, e144–e160 (2021).
Heesterbeek, T. J., Lorés-Motta, L., Hoyng, C. B., Lechanteur, Y. T. E. & den Hollander, A. I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 40, 140–170 (2020).
Lindeke-Myers, A., Hanif, A. M. & Jain, N. Pentosan polysulfate maculopathy. Surv. Ophthalmol. 67, 83–96 (2022).
Stahl, A. The diagnosis and treatment of age-related macular degeneration. Dtsch. Arztebl. Int. 117, 513–520 (2020).
Yin, Y., Shu, Y., Zhu, J., Li, F. & Li, J. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci. Rep. 12, 19555 (2022).
Sakaeda, T., Tamon, A., Kadoyama, K. & Okuno, Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 10, 796–803 (2013).
Moreland-Head, L. N., Coons, J. C., Seybert, A. L., Gray, M. P. & Kane-Gill, S. L. Use of disproportionality analysis to identify previously unknown drug-associated causes of cardiac arrhythmias using the food and drug administration Adverse event reporting system (FAERS) database. J. Cardiovasc. Pharmacol. Ther. 26, 341–348 (2021).
Brown, E. G., Wood, L. & Wood, S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 20, 109–117 (1999).
Mozzicato, P. Standardised MedDRA queries: Their role in signal detection. Drug Saf. 30, 617–619 (2007).
Zhou, S. et al. Drug-induced fall risk in older patients: A pharmacovigilance study of FDA adverse event reporting system database. Front. Pharmacol. 13, 1044744 (2022).
Khouri, C. et al. A meta-epidemiological study found lack of transparency and poor reporting of disproportionality analyses for signal detection in pharmacovigilance databases. J. Clin. Epidemiol. 139, 191–198 (2021).
A, B. Bayesian confidence propagation neural network. Drug Safety. 30, (2007).
Berlin, C. et al. Are all quantitative postmarketing signal detection methods equal? Performance characteristics of logistic regression and Multi-item Gamma Poisson Shrinker. Pharmacoepidemiol. Drug Saf. 21, 622–630 (2012).
Wishart, D. S. et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2018).
Zhao, H. et al. Sodium-glucose co-transporter-2 inhibitor (SGLT2i) treatment and risk of osteomyelitis: A pharmacovigilance study of the FAERS database. Front. Pharmacol. 14, 1110575 (2023).
Fleckenstein, M. et al. Age-related macular degeneration. Nat. Rev. Dis. Primers 7, 31 (2021).
Al-Zamil, W. M. & Yassin, S. A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 12, 1313–1330 (2017).
Kharb, R., Haider, K., Neha, K. & Yar, M. S. Aromatase inhibitors: Role in postmenopausal breast cancer. Arch. Pharm. (Weinheim) 353, e2000081 (2020).
Saatci, O., Huynh-Dam, K.-T. & Sahin, O. Endocrine resistance in breast cancer: From molecular mechanisms to therapeutic strategies. J. Mol. Med. (Berl.) 99, 1691–1710 (2021).
Patel, H. K. & Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 186, 1–24 (2018).
Nuzzi, R., Scalabrin, S., Becco, A. & Panzica, G. Gonadal hormones and retinal disorders: A review. Front. Endocrinol. (Lausanne) 9, 66 (2018).
Lim, I.-L. et al. Dosage-dependent reduction of macular pigment optical density in female breast cancer patients receiving tamoxifen adjuvant therapy. Breast 39, 117–122 (2018).
Bekes, I. & Huober, J. Extended adjuvant endocrine therapy in early breast cancer patients-review and perspectives. Cancers (Basel) 15, 4190 (2023).
Zhou, L., Zhan, W. & Wei, X. Clinical pharmacology and pharmacogenetics of prostaglandin analogues in glaucoma. Front. Pharmacol. 13, 1015338 (2022).
Makri, O. E., Georgalas, I. & Georgakopoulos, C. D. Drug-induced macular edema. Drugs 73, 789–802 (2013).
Yun, C. et al. Ocular perfusion pressure and choroidal thickness in early age-related macular degeneration patients with reticular pseudodrusen. Invest. Ophthalmol. Vis. Sci. 57, 6604–6609 (2016).
Ambati, J. & Fowler, B. J. Mechanisms of age-related macular degeneration. Neuron 75, 26–39 (2012).
Mohd Zain, A. et al. The relationship between long-term use of intranasal corticosteroid and intraocular pressure. J. Glaucoma 28, 321–324 (2019).
Carr, W. W. & Szefler, S. J. Inhaled corticosteroids: Ocular safety and the hypothalamic-pituitary-adrenal axis. Ann. Allergy Asthma Immunol. 117, 589–594 (2016).
Agustí, A. et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur. Respir. J. 61, 2300239 (2023).
Kerr, N. M. & Danesh-Meyer, H. V. Phosphodiesterase inhibitors and the eye. Clin. Exp. Ophthalmol. 37, 514–523 (2009).
Andersson, K.-E. PDE5 inhibitors—Pharmacology and clinical applications 20 years after sildenafil discovery. Br. J. Pharmacol. 175, 2554–2565 (2018).
Loukovaara, S., Auvinen, A. & Haukka, J. Associations between systemic medications and development of wet age-related macular degeneration. Acta Ophthalmol. 100, 572–582 (2022).
Aldebert, G. et al. Association of anticholinergic drug use with risk for late age-related macular degeneration. JAMA Ophthalmol. 136, 770–778 (2018).
Mammo, Z., Guo, M., Maberley, D., Matsubara, J. & Etminan, M. Oral bisphosphonates and risk of wet age-related macular degeneration. Am. J. Ophthalmol. 168, 62–67 (2016).
Garriga, C. et al. Oral bisphosphonate use and age-related macular degeneration: Retrospective cohort and nested case-control study. Ann. N. Y. Acad. Sci. 1415, 34–46 (2018).
Hasan, H., Lotery, A., Price, E. J. & Smith, G. T. An objective method of diagnosing hydroxychloroquine maculopathy. Eye (London) 35, 1922–1929 (2021).
Fernandez-Ruiz, R. et al. Discontinuation of hydroxychloroquine in older patients with systemic lupus erythematosus: A multicenter retrospective study. Arthritis Res. Ther. 22, 191 (2020).
Vergroesen, J. E. et al. Association of diabetes medication with open-angle glaucoma, age-related macular degeneration, and cataract in the rotterdam study. JAMA Ophthalmol. 140, 674–681 (2022).
Rajala, A., Dighe, R., Agbaga, M.-P., Anderson, R. E. & Rajala, R. V. S. Insulin receptor signaling in cones. J. Biol. Chem. 288, 19503–19515 (2013).
McCall, A. L. Insulin therapy and hypoglycemia. Endocrinol. Metab. Clin. N. Am. 41, 57–87 (2012).
Yu, Y. et al. Reentrant spiral waves of spreading depression cause macular degeneration in hypoglycemic chicken retina. Proc. Natl. Acad. Sci. USA 109, 2585–2589 (2012).
Little, S. A. et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: A multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 37, 2114–2122 (2014).
Teo, Z. L. et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 128, 1580–1591 (2021).
Yang, Y. et al. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for venetoclax. PLoS One 17, e0278725 (2022).
Montastruc, J.-L. et al. Pharmacovigilance for evaluating adverse drug reactions: Value, organization, and methods. Joint Bone Spine 73, 629–632 (2006).
Funding
This study was supported by the Fundamental Research Funds for Xiamen University (Grant no. 20720220056), the Natural Science Youth Foundation of Xiamen City (Grant no. 3502Z202372007) and the Natural Science Foundation of Fujian Province (Grant no. 2023J011585).
Author information
Authors and Affiliations
Contributions
Xiaodong Chen and Qian Chen conceived the research idea. Xiaodong Chen, Shinan Wu, Shaopan Wang, Chaofeng Yu conducted data cleaning and literature review. Xiaodong Chen, Zihan Guo, Shiya Huang, Peixin Cai and Yanliang Miao contributed to drafting and critically revising the work for intellectual content. Xiaodong Chen conducted the analysis and created the figures and tables. Shiying Li, and Qian Chen provided a critical review of the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The data source for this study is the public database FAERS database, which does not contain ethics. Previous studies of the database did not require ethical approval. Therefore, this study does not require the approval of the Ethics Committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, X., Wu, S., Wang, S. et al. Real world pharmacovigilance assessment of drug related macular degeneration risks. Sci Rep 15, 1220 (2025). https://doi.org/10.1038/s41598-024-84679-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84679-4
Keywords
This article is cited by
-
Pharmacovigilance of drug-induced cataract using the FDA Adverse Event Reporting System
Scientific Reports (2025)
-
Pharmacovigilance study of drug-induced eye movement disorder based on FDA adverse event reports from 2004 to 2024
Scientific Reports (2025)