Abstract
374 pregnant women with isolated hypothyroxinemia (IH) who were ≤ 20 weeks were included retrospectively in this study. Based on the confirmed gestational age and the use of levothyroxine (LT4), the patients were divided into the treated group (T1 group) and untreated group (C1 group) in first trimester (≤ 13+ 6 weeks), the treated group(T2 group) and untreated group (C2 group) in second trimester (14–20 weeks). Data on thyroid function and lipid indices was collected both before and after LT4 treatment. To compare the thyroid function, lipid indices and pregnancy outcomes after LT4 treatment. There was a negative correlation between FT4 levels and TC and LDL levels in the first trimester (P<0.05). FT4 and HDL levels in T1 group were significantly increased, and TSH, TC, TG and LDL levels were decreased, compared to C1 group (P < 0.05). FT4 levels in T2 group were higher than C2 group, and there was no significant difference in other indicators. The risk of spontaneous abortion, gestational diabetes mellitus (GDM) and macrosomia in T1 group was significantly decreased (P < 0.05), and there was no significant difference between T2 and C2 groups. Thus, LT4 treatment can improve the level of FT4 and lipids and reduce adverse pregnancy outcomes in women with IH in the first trimester.
Similar content being viewed by others
Introduction
Thyroid hormone (TH) is essential for maintaining normal metabolism and promoting physical growth and neurodevelopment1. Thyroid dysfunction is a common disease in pregnant women. Pregnancy with isolated hypothyroxinemia (IH) refers to pregnant woman with negative thyroid autoantibodies and normal serum thyroid stimulating hormone (TSH) levels, but free thyroxine (FT4) levels below the lower limit of the pregnancy-specific reference range2. The prevalence of IH in pregnancy ranges widely from 0.2–31%3. Pregnancy-related physiological changes cause an increasing demand for TH until the 20th week of gestation, after which it levels off until delivery. The fetus is dependent on the mother’s transplacental transfer of TH before 20 weeks of pregnancy. Notably, FT4 is the main source of FT3 in the embryonic brain because it can cross the fetal blood-brain barrier more easily than free triiodothyronine (FT3). The potential effect of IH on pregnancy outcomes and offspring development remains controversial. Most scholars believe that gestational IH is associated with adverse pregnancy outcomes such as gestational hypertensive disease (HDP), gestational diabetes mellitus (GDM), macrosomia, miscarriage, premature delivery, placental abruption, premature rupture of membranes, fetal distress4,5,6,7,8. In addition, gestational IH may increase the risk of offspring autism9,10.
During pregnancy, it is essential for pregnant women to maintain elevated blood lipid levels to support normal fetal development and provide energy for pregnancy, labor and postpartum lactation11. TH plays a crucial role in lipid metabolism. Research conducted by Karbownik et al. identified a negative correlation between FT4 concentrations in women with IH of childbearing age and levels of low-density lipoprotein (LDL) and triglycerides (TG), while demonstrating a positive correlation with high-density lipoprotein (HDL)12. Additionally, a study by Xu et al. indicated that pregnant women with IH exhibited increased levels of total cholesterol (TC), TG, and LDL during pregnancy. This study further established that treatment with levothyroxine (LT4) could elevate serum FT4 levels and improve lipid profiles in this population13. However, existing literature also suggests that pregnant women with IH do not exhibit dyslipidemia14. Currently, there is a paucity of research evaluating the effect of LT4 on lipid metabolism in pregnant women with IH.
In recent years, there have been limited and controversial literatures on whether LT4 therapy can improve adverse maternal and fetal outcomes in pregnant women with IH. The study by Li et al. found that LT4 treatment reduced the risk of miscarriage and the likelihood of the neonatal intensive care unit (NICU) admission in pregnant women with IH15. Conversely, Casey et al. showed that LT4 intervention did not improve adverse pregnancy outcomes and offspring cognition16. Despite these conflicting findings, domestic and international guidelines vary in their recommendations for the management of IH during pregnancy. In 2014, the European Thyroid Association (ETA) recommended LT4 treatment for women with IH during the first trimester17, while the 2017 American Thyroid Association (ATA) did not recommend intervention18. The Chinese Guidelines for the Diagnosis and Treatment of Thyroid Diseases in Pregnancy and Postpartum (2nd edition) published in 2019 and the recent 2022 Guidelines for the Prevention and Management of Thyroid Diseases during pregnancy did not definitively recommend or oppose the use of LT4 in the first trimester for pregnant women with IH2,19.
Therefore, the objective of this study was to investigate the effect of LT4 treatment on blood lipid levels and pregnancy outcomes in pregnant women with IH during the first half of pregnancy.
Materials and methods
Research target
This is a retrospective study. Clinical data were collected from pregnant women diagnosed with IH during the first half of pregnancy who delivered at the Third Affiliated Hospital of Zhengzhou University between January 2021 and October 2023.
The inclusion criteria were established as follows: (1) Participants were required to be between the age of 18 and 35. (2) The thyroid function levels of the pregnant women must have met the diagnostic criteria outlined in the 2019 Guidelines for the Diagnosis and Treatment of Thyroid Diseases in Pregnancy and Postpartum (2nd edition)2, as well as the reference-range criteria established by the Department of Clinical Laboratory of the Third Affiliated Hospital of Zhengzhou University [TSH: 0.27-4.2mIU/L, FT4 < 12.3pmol/L, thyroid peroxidase antibody(TPOAb)<34 IU/ml, thyroglobulin antibody (TgAb)<115 IU/ml].(3) The gestational age of pregnant women diagnosed with IH was less than 20 weeks.
The exclusion criteria were delineated as follows: (1) Patients with multiple pregnancies. (2) Patients who conceived through assisted reproductive technology. (3) Patients with a history of hyperthyroidism, Hashimoto’s thyroiditis, subacute thyroiditis, clinical hypothyroidism, subclinical hypothyroidism, prior thyroid surgery, hypertension, diabetes mellitus, cardiovascular disease, autoimmune disease or psychiatric disease prior to enrollment.(4) Previous use of drugs that may affect blood lipids or thyroid function; (5) Patients with incomplete data.
In this study, a cohort of 374 pregnant women with IH during the first half of pregnancy was identified through electronic medical records. These women were classified into different groups according to their gestation week and whether or not they received levothyroxine (LT4) treatment. Specifically, the groups included the first trimester (gestational age ≤ 13+ 6 weeks ) treated group (80 patients, T1 group), the first trimester untreated group (98 patients, C1 group), the second trimester (gestational age 14–20 week) treated group (95 patients, T2 group), and the second trimester untreated group (101 patients, C2 group). In the treatment groups, LT4 therapy was initiated upon diagnosis, with dosage adjustments made based on FT4 and TSH levels. Treatment was sustained throughout the entirety of the pregnancy until delivery.
Statement. (1) This study was approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (Approval No. 2023-041-01). The need for informed consent was waived by the ethics committee of The Third Affiliated Hospital of Zhengzhou University. (2) All methods were carried out in accordance with relevant guidelines and regulations.
Method
-
1.
The age, pre-pregnancy body mass index (BMI), confirmed gestational week, timing of cesarean section, serum fasting blood glucose (FPG) and glycosylated hemoglobin (HbA1c) levels of pregnant women with IH were collected by consulting through the electronic medical record system.
-
2.
Thyroid function indexes and blood lipid indexes, including FT4, TSH, TC, TG, HDL and LDL were collected in pregnant women with IH before and after LT4 treatment. These metabolic indicators were monitored with a minimum interval of four weeks.
-
3.
Maternal and neonatal outcomes were collected, including miscarriage, GDM, premature rupture of membranes (PROM), HDP, placental abruption, cesarean section, postpartum hemorrhage, gestational week of delivery, neonatal birth weight (BW), length, 1-minute and 5-minute Apgar scores, preterm labor, fetal distress, macrosomia, neonatal asphyxia, and admission to NICU.
Main instruments and testing methods
LDL and HDL levels were determined by a uniform phase assay (Beckman Coulter automatic biochemical analyzer, Beckman Coulter Experimental System, Suzhou), and TC and TG levels were determined by an enzymatic method (Beckman Coulter automatic biochemical analyzer, Beckman Coulter Experimental System, Suzhou). TSH, FT4, TPOAb and TgAb levels were measured by an electroluminescence immunoassay (Roche, Shanghai, China). Thyroid function indices and blood lipid indices were detected by the same instrument and detection method before and after LT4 treatment.
Statistical analysis
Statistical analysis was conducted using SPSS version 26.0. The Kolmogorov-Smirnov (K-S) and Shapiro-Wilk (S-W) tests were employed to assess the normality of the measurement data. For data that conformed to a normal distribution, results were presented as mean ± standard deviation, and comparisons between groups were performed using the independent samples t-test. Conversely, for data that did not meet the criteria for normal distribution, results were reported as median (25th percentile, 75th percentile), and the rank sum test was utilized for group comparisons. Categorical data were expressed in terms of frequency and percentage (%), with the chi-square test or Fisher’s exact test applied for intergroup comparisons. Spearman correlation analysis was used to investigate the relationship between FT4 and TC, TG, LDL and HDL. Two-sided test. P < 0.05 was established to determine statistical significance.
Result
General characteristics and baseline serological indicators of pregnant women with IH in the first and second trimesters
There were no significant differences in the confirmed gestational week, age, pre-pregnancy BMI, cesarean section time, the levels of FPG, HbA1c, FT4, TSH, TC, TG, HDL and LDL at diagnosis between the T1 and C1 groups (P > 0.05) (Table 1). Similarly, no significant differences were found in these parameters between the T2 and C2 groups (P > 0.05) (Table 2).
Correlation analysis of serum FT4 and blood lipid indexes in pregnant women with IH at the time of diagnosis
As shown in Table 3, Spearman correlation analysis showed that FT4 level in pregnant women with IH at the time of diagnosis were negatively correlated with TC (ρ=-0.369,P = 0.004) and LDL (ρ=-0.326,P = 0.011) levels, and had no correlation with TG and HDL in the first trimester(P>0.05). There was no correlation between FT4 levels and TC, TG, LDL and HDL in pregnant women with IH in the second trimester(P>0.05).
Thyroid function and lipid indexes of pregnant women with IH after LT4 treatment in the first trimester
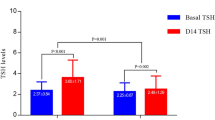
The levels of FT4 and HDL in T1 group after LT4 treatment were higher than C1 group, and the levels of TSH, TC, TG and LDL were lower than C1 group(P < 0.05) (Table 4).
Thyroid function and lipid indexes of pregnant women with IH after LT4 treatment in the second trimester
FT4 levels in T2 group were higher than C2 group (P < 0.05), and there was no significant difference in TSH, TC, TG, LDL and HDL levels between T2 and C2 group(P>0.05) (Table 5).
Pregnancy outcomes of pregnant women with IH in the first and second trimesters
The incidence of miscarriage, GDM and macrosomia in T1 group was lower than that in C1 group, and the difference was statistically significant (P < 0.05). There was no statistically significant difference in the incidence of pregnancy outcomes such as HDP, cesarean section, postpartum hemorrhage, fetal distress, premature delivery and NICU transfer rate between C1 and T1 groups (P>0.05) (Table 6). There was no significant difference in pregnancy outcomes between T2 and C2 groups (P>0.05) (Table 7).
Discussion
Normal TH levels facilitate a dynamic equilibrium between the synthesis and catabolism of serum lipids in pregnant women. Studies suggest that IH during pregnancy may be associated with adverse perinatal outcomes5. However, there is limited research on the treatment of IH in pregnancy Casey et al. conducted a randomized study involving patients diagnosed with hypothyroxinemia, assigning them to receive either levothyroxine replacement therapy or a placebo. The objective of the study was to examine the relationship between iodine status in pregnant women with hypothyroxinemia during the first trimester and various behavioral and neurodevelopmental indicators in children under the age of five. The findings indicated that irrespective of the maternal iodine levels, treatment with levothyroxine did not demonstrate a significant association with neurodevelopmental or behavioral outcomes in the offspring20. This study retrospectively analyzed the thyroid function indexes, lipid indexes and maternal and infant outcomes of pregnant women with IH treated with LT4 at different gestational weeks, and aimed to investigate the effect of LT4 treatment on lipid metabolism and pregnancy outcomes of pregnant women with IH.
We first analyzed the correlation between FT4 levels and blood lipid indexes in pregnant women with IH in the first and second trimesters. Our findings indicated that serum FT4 levels in the first trimester were negatively correlated with TC and LDL levels, and had no significant correlation with TG and HDL levels. There was no correlation between serum FT4 level and lipid indexes in the second trimester. The study conducted by Liu et al. also indicated a negative correlation between FT4 levels and TC in pregnant women with IH during the first trimester, which aligns with our findings21. These results imply that pregnant women with IH may experience dyslipidemia. TH plays a key role in the process of cholesterol synthesis and catabolism. In instances of TH deficiency, although the synthesis rate of TC may decline, the rates of TC degradation and excretion decrease even more markedly, resulting in a significant increase in TC levels.
Secondly, we analyzed the thyroid function and lipid indexes of pregnant women with IH after LT4 treatment, and the results showed that the FT4 levels of pregnant women with LT4 treatment in the first trimester group were higher, and the TSH levels were lower, compared to the untreated group. This suggests that oral administration of LT4 may enhance the body’s thyroxine (T4) level, consequently increasing FT4 concentration. Furthermore, T4 undergoes deiodination to form triiodothyronine (T3), which exerts a negative feedback effect on the hypothalamic-pituitary-thyroid axis, thereby reducing TSH synthesis and secretion22. We also found that the levels of TC, TG and LDL in the first trimester treated group were lower than the untreated group, and the level of HDL was higher. Current literature on LT4 treatment for IH during early pregnancy is limited. Zhao et al. reported that LT4 treatment in non-pregnant women with hypothyroidism resulted in decreased LDL level23. Similarly, Yang et al. observed that LT4 administration in pregnant women with subclinical hypothyroidism during the first trimester led to improvements in LDL and TC levels24. We speculate that LT4 supplementation may influence lipid metabolism through several mechanisms: (1) LT4 supplementation may increase the expression of cholesterol 7α-hydroxylase, promoting the conversion of TC to bile acid, reducing the level of serum TC25. (2) FT4 may bind to the corresponding sites in LDL, inhibiting the formation of oxidized LDL (ox-LDL), which is not recognized by LDL receptors, leading to cellular accumulation of LDL26. The supplementation of LT4 can stimulate the expression of LDL receptor and inhibit the oxidation of LDL, so that it can be easily recognized and cleared by LDL receptor, and then reduce the serum LDL levels. (3) LT4 can increase the basal metabolic rate, up-regulate the activity of lipoprotein esterase, and promote the hydrolysis of TG27. (4) Supplementation with LT4 reduces the concentration of cholesterol ester transfer protein (CETP)28, potentially resulting in increased HDL levels in plasma. Therefore, we believe that LT4 treatment can improve serum FT4 and lipid levels in pregnant women with IH in the first trimester.
The result of the second trimester showed that the level of FT4 in the treated group was higher than that in the untreated group, and the levels of TSH and lipids had no significant alterations. Xu et al. ‘s study which included 77 pregnant women with IH in the treated group and 87 patients in the untreated group found that after LT4 supplementation, FT4, TSH, TC and LDL were significantly improved compared with the untreated group, but the changes of TG and HDL were not significantly difference13. This is not exactly consistent with our results, potentially due to variations in gestational age. Research indicates that FT4 levels in healthy pregnant women typically rise during the first trimester, gradually decline in the second and third trimesters, and revert to non-pregnant levels around 20 weeks of gestation. Conversely, TSH levels generally decrease during the first trimester and progressively increase after the second trimester2. We tested that supplementation of LT4 in the second trimester increased the level of T4 in vivo and directly increased the level of FT4, but indirectly acted on the regulation of TSH, so the change of TSH in the second trimester after LT4 treatment was not significant. The study has shown that after LT4 treatment in patients with clinical or subclinical hypothyroidism, TSH level returns to normal, but TC and LDL levels are still higher than those in the general population29. It may be due to the persistence of subnormal levels of triiodothyronine (T3)30. In addition, blood lipids during pregnancy will gradually increase with the progress of pregnancy, reaching a peak in the third trimester31. Therefore, LT4 supplementation alone may not be sufficient to alter lipid levels in the second trimester.
We further compared maternal and infant outcomes after treatment. This study found that LT4 treatment reduced the risk of miscarriage in the first trimester. In a non-randomized intervention study by Li et al., involving 964 pregnant women with IH in the first trimester, it was demonstrated that LT4 treatment markedly decreased the miscarriage rate15. This is consistent with our results. A Spanish study showed that in women with IH with FT4 levels < 7.5 pg/mL, LT4 treatment given prior to 9 weeks of pregnancy reduced the risk of miscarriage by over 40% before 12 weeks and between 14 and 40% before 18 weeks32, which is consistent with our findings. The study had shown that ox-LDL level increase and HDL level decrease in pregnant women with spontaneous abortion33. ox-LDL can promote the secretion of inflammatory cytokines, leading to vascular endothelial cell damage26. HDL can promote the catabolism of cholesterol and is a protective factor of blood vessels. Consequently, LT4 supplementation may contribute to a reduction in LDL and ox-LDL levels in pregnant women with IH during the first trimester, while simultaneously increasing HDL levels. This may help to mitigate damage to placental vascular endothelial cells, thereby enhancing fetal blood supply and decreasing the incidence of miscarriage. Moreover, LT4 supplementation may also play a role in regulating maternal immune tolerance, facilitating embryo implantation and placental development, and further reducing the likelihood of miscarriage by promoting the secretion of progesterone, human chorionic gonadotropin, vascular endothelial growth factor, and placental growth factor from trophoblastic cells of the placenta34.
The findings of this study showed that LT4 treatment reduced the risk of GDM in pregnant women with IH in the first trimester. Currently, there is a paucity of research examining the effects of LT4 therapy on GDM and macrosomia in this population. Notably, Sitoris’s study found that LT4 treatment reduced the prevalence of GDM in subclinical hypothyroidism during pregnancy35. The studies had revealed a significant correlation between the incidence of GDM and elevated TG level in early pregnancy, suggesting that lipid metabolism disorders may contribute to or exacerbate insulin resistance36,37. LT4 supplementation may reduce insulin resistance by indirectly lowering TG levels, and increase T3 levels in vivo. T3 promotes the secretion of glucagon-like peptide-1 and insulin by inhibiting the intestinal farnesol X receptor signaling pathway, and reduces the occurrence of GDM38. Furthermore, our study found that LT4 treatment reduces the risk of macrosomia in women with IH in the first trimester. A prospective intervention study conducted by Gong et al. reported that LT4 treatment did not improve adverse pregnancy outcomes in women with IH during the first trimester and noted an increased risk of macrosomia in those with IH during the second trimester, although no intervention was performed on this latter group39. This finding contradicts our results. A study had shown that the use of LT4 in pregnant women with hypothyroidism can reduce the risk of macrosomia40. Supplementation of LT4 may reduce the occurrence of macrosomia by decreasing maternal plasma TG levels, decreasing the activity of placental lipase, and limiting the entry of free fatty acids into the fetus41.
In summary, this study retrospectively analyzed the effect of LT4 treatment on thyroid function, lipid levels and pregnancy outcomes in pregnant women with IH. Subgroup analysis based on gestational age showed that LT4 treatment could improve the serum lipid levels and adverse pregnancy outcomes of pregnant women in the first trimester to a certain degree. However, several limitations must be acknowledged: Firstly, this study is retrospective. Secondly, the sample size of this study is relatively small, which may cause some bias into the results. In addition, the study did not assess the cognitive development of the offspring of women with IH, highlighting an area for future investigation. In the future, multicenter, large sample, and randomized intervention studies are still needed to further determine whether LT4 therapy can improve pregnancy outcomes and offspring neurodevelopment in pregnant women with IH.
Data availability
The data are not publicly available due to their containing information that could compromise the privacy of research participants. The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
References
Lee, S. Y. & Pearce, E. N. Assessment and treatment of thyroid disorders in pregnancy and the postpartum period. Nat. Reviews Endocrinol. 18, 158–171 (2022).
Ad Hoc Writing Committee for Guidelines on, D. et al. Guideline on diagnosis and management of thyroid diseases during pregnancy and postpartum (2nd edition). Chinese Journal of Endocrinology and Metabolism 35, 636–665 et al. (2019).
Peng, C. C., Lee, S. Y. & Pearce, E. N. Isolated maternal hypothyroxinemia: adverse maternofetal outcomes but uncertainty about treatment remains. Thyroid 33, 535–537 (2023).
Derakhshan, A. et al. Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 8, 501–510 (2020).
Han, Y. et al. A systematic review and Meta-analysis examining the risk of adverse pregnancy and neonatal outcomes in women with isolated hypothyroxinemia in pregnancy. Thyroid 33, 603–614 (2023).
Liu, Y. et al. The interactive effect of Prepregnancy Overweight/Obesity and isolated maternal hypothyroxinemia on Macrosomia. J. Clin. Endocrinol. Metabolism. 106, e2639–e2646 (2021).
Yang, A. L. & McNabb-Baltar, J. Hypertriglyceridemia and acute pancreatitis. Pancreatology 20, 795–800 (2020).
Korevaar, T. I. M. et al. Association of thyroid function test abnormalities and thyroid autoimmunity with Preterm Birth: a systematic review and Meta-analysis. Jama-journal Am. Med. Association. 322, 632–641 (2019).
Levie, D. et al. Thyroid function in early pregnancy, child IQ, and autistic traits: a Meta-analysis of individual participant data. J. Clin. Endocrinol. Metabolism. 103, 2967–2979 (2018).
Han, Y., Liu, A., Gong, X. & Shan, Z. Gestational hypothyroxinemia and offspring autism. Chin. J. Endocrinol. Metabolism. 38, 834–838 (2022).
Ternushchak, T. & Tovt-Korshynska, M. Lipid profiles and the prevalence of dyslipidemia in pregnancy. Atherosclerosis 355, 115 (2022).
Karbownik-Lewińska, M., Stępniak, J. & Lewiński, A. Potential Risk Factors for Isolated Hypothyroxinemia in Women of Childbearing Age-Results from Retrospective Analysis. Journal of clinical medicine 10, null (2021).
Xu, Y., Zhao, Y., Xu, X., Yan, Q. & Yang, L. Serum lipid profile in relation to free thyroxine and the effect of levothyroxine treatment on lipids in patients with isolated hypothyroxinemia during pregnancy: a single-center retrospective study. Lipids Health Dis. 21, 142 (2022).
Zheng, L. X. et al. Effect of hypothyroidism of pregnant women during the pregnancy on maternal glucolipid metabolism and the pregnancy outcomes after intervention by levothyrocine. Chin. J. Family Plann. 28, 1827–1831 (2020).
Li, G. et al. Effect of levothyroxine on pregnancy outcomes in pregnant women with hypothyroxinemia: an interventional study. Front. Endocrinol. 13, 874975 (2022).
Casey, B. M. et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N. Engl. J. Med. 376, 815–825 (2017).
Lazarus, J. et al. European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. European Thyroid Journal 3, 76–94 (2014). (2014).
Alexander, E. K. et al. 2017 guidelines of the American thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the Postpartum. Thyroid 27, 315–389 (2017).
Writing Committee for Guidelines for. Guidelines for prevention and management of thyroid diseases during pregnancy and perinatal period. Chin. J. Endocrinol. Metabolism. 38, 539–551 (2022).
Casey, B. M. et al. Association of Mild Iodine Insufficiency during pregnancy with child neurodevelopment in patients with subclinical hypothyroidism or hypothyroxinemia. Am. J. Perinatol. 41, e3326–e3332 (2024).
Liu, W. Y. et al. Associations of thyroid function tests with lipid levels and adverse pregnancy outcomes during the First Trimester. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 15, 973–981 (2022).
Moog, N. K. et al. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342, 68–100 (2017).
Zhao, M. et al. A Worthy Finding: decrease in total cholesterol and Low-Density Lipoprotein Cholesterol in treated mild subclinical hypothyroidism. Thyroid 26, 1019–1029 (2016).
Yang, Y. et al. Significance of levothyroxine treatment on serum lipid in pregnant women with subclinical hypothyroidism. BMC Pregnancy Childbirth. 22, 623 (2022).
Lammel Lindemann, J. A., Angajala, A., Engler, D. A., Webb, P. & Ayers, S. D. Thyroid hormone induction of human cholesterol 7 alpha-hydroxylase (Cyp7a1) in vitro. Mol. Cell. Endocrinol. 388, 32–40 (2014).
Kumar Singh, N., Suri, A., Kumari, M. & Kaushik, P. A study on serum homocysteine and oxidized LDL as markers of cardiovascular risk in patients with overt hypothyroidism. Horm. Mol. Biol Clin. Investig. 43, 329–335 (2022).
Xu, D., Liang, C., Chen, L., Wu, X. D. & He, J. Study on the dynamic variations and influencing factors of serum lipid levels during pregnancy and postpartum. Zhonghua Fu Chan Ke Za Zhi. 53, 227–233. https://doi.org/10.3760/cma.j.issn.0529-567x.2018.04.004 (2018).
Ritter, M. J., Amano, I. & Hollenberg, A. N. Thyroid hormone signaling and the liver. Hepatology 72, 742–752 (2020).
Bianco, A. C. & Taylor, P. Levothyroxine treatment and cholesterol in hypothyroidism. Nat. Reviews Endocrinol. 16, 193–194 (2020).
Duntas, L. H. & Brenta, G. A Renewed Focus on the Association between Thyroid Hormones and lipid metabolism. Front. Endocrinol. 9, 511 (2018).
Yuan, X. X. et al. Prevalence of dyslipidemia in pregnancy and early predictive value of blood lipid levels. Chin. Gen. Practic. 27, 670–678 (2024).
Runkle, I. et al. Early Levothyroxine Treatment for subclinical hypothyroidism or hypothyroxinemia in pregnancy: the St Carlos Gestational and thyroid protocol. Front. Endocrinol. 12, 743057 (2021).
Tulppala, M., Ailus, K., Palosuo, T. & Ylikorkala, O. Antibodies to oxidized low-density lipoprotein and to cardiolipin in nonpregnant and pregnant women with habitual abortion. Fertil. Steril. 64, 947–950 (1995).
Silva, J. F., Ocarino, N. M. & Serakides, R. Placental angiogenic and hormonal factors are affected by thyroid hormones in rats. Pathol. Res. Pract. 211, 226–234 (2015).
Sitoris, G. et al. Impact of thyroid hormone treatment on maternal pregnancy outcomes in women with subclinical hypothyroidism without TPOAb: a retrospective cross-sectional study. Thyroid Res. 16, 29 (2023).
Wani, K. et al. Early-pregnancy metabolic syndrome and subsequent incidence in gestational diabetes Mellitus in Arab women. Front. Endocrinol. 11, 98 (2020).
Zhu, H. et al. High serum triglyceride levels in the early first trimester of pregnancy are associated with gestational diabetes mellitus: a prospective cohort study. J. Diabetes Invest. 11, 1635–1642 (2020).
Yan, Y. et al. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nat. Commun. 13, 6408 (2022).
Gong, X. et al. The impact of isolated maternal hypothyroxinemia during the first and second trimester of gestation on pregnancy outcomes: an intervention and prospective cohort study in China. J. Endocrinol. Investig. 42, 599–607 (2019).
Ma, L. et al. The effects of screening and intervention of subclinical hypothyroidism on pregnancy outcomes: a prospective multicenter single-blind, randomized, controlled study of thyroid function screening test during pregnancy. J. Maternal-Fetal Neonatal Med. 29, 1391–1394 (2016).
Wu, W. et al. Triglycerides mediate the relationship between maternal free thyroxine and Birth Weight: a prospective cohort study from China. Thyroid 33, 615–624 (2023).
Author information
Authors and Affiliations
Contributions
Y.X. conceived and designed research. D.L., X.T., L.H., C.Z., Y.W., P.L. collected data . D.L., X.T., L.H., M.Z., L.H. analyzed data. D.L. drafted manuscript. D.L., Y.X., J.L., Z.S., Y.B. edited and revised manuscript. All authors approved final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, D., Xu, Y., Li, J. et al. Effect of levothyroxine treatment on serum lipids and pregnancy outcomes in pregnant women with isolated hypothyroxinemia. Sci Rep 15, 11601 (2025). https://doi.org/10.1038/s41598-024-84866-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84866-3