Abstract
Due to the unique properties of nanoparticles (NPs), their application has been proposed as an innovative and promising enhanced oil recovery (EOR) technique. They enhance oil recovery by improving EOR mechanisms including decreasing interfacial tension (IFT), wettability alteration to water-wet, and preventing asphaltene precipitation. In this study, Fe3O4@Gelatin NPs were synthesized by a convenient and single-step method and then investigated for EOR purposes for the first time. These NPS were characterized by Transmission Electron Microscopy (TEM), X-ray diffraction (XRD), and Fourier-transform infrared spectroscopy (FTIR). Afterward, various experiments including contact angle, interfacial tension (IFT), and micromodel flooding were performed to evaluate the capability of Fe3O4@Gelatin NPs for EOR application. Based on the results of IFT measurements, the synthesized NPs caused the highest reduction in IFT of crude oil-seawater compared to Fe3O4 NPs and gelatin. This significant reduction is due to the synergistic effects of Fe3O4 NPs and gelatin in the form of a nanocomposite. According to the results from contact angle measurements, the Fe3O4@Gelatin NPs could significantly change the glass surface wettability from oil-wet to water-wet, which showed a better performance than Fe3O4 NPs. The surface modification of Fe3O4 NPs with gelatin causes to enhance the capability of these NPs for wettability alteration because the gelatin has surface active property. In the micromodel flooding tests, the seawater containing 0.06 wt. % Fe3O4@Gelatin NPs had the highest oil recovery factor (RF = 42.9%) compared to the seawater containing 0.06 wt. % Fe3O4 NPs (RF = 33.92%) and only seawater (RF = 26.57%), due to more IFT reduction and altering the surface wettability from oil-wet to strongly water-wet. Therefore, according to the results of this research, the synthesized NPs can be a good option for EOR.
Similar content being viewed by others
Introduction
Despite the recent progress made in energy production from renewable sources, oil and gas remain as the major supplier of energy in the world1. The estimates show that the energy demand in 2040 will be 30% more than in 2010, and it is evaluated that by 2040, the world’s oil consumption will reach 118 million barrels per day2.
In recent decades, we have seen a global decline in new hydrocarbon reservoir discoveries3. On the other hand, the increasing extraction from oil reservoirs and consequently drop in reservoir pressure over time, leads to a reduction in oil production4. Therefore, to increase production from oil reservoirs in order to respond to the growing energy demand in the world, it is necessary to use improved oil recovery methods5,6.
The recovery process of oil reservoirs is separated into three stages: primary, secondary, and tertiary recovery7,8. In the primary recovery stage, oil is produced by natural reservoir energy (compaction drive, gas cap drive, solution gas drive, water drive, gravity drive)9. As reservoir pressure decreases, the secondary recovery is necessary to reenergize or pressure up the reservoir. This is done by injecting external fluids (gas or water) into the reservoir10. At the end of the primary and secondary stages, a considerable volume of oil (about two-thirds of the original oil in place (OOIP)) remains in the reservoir due to unfavorable wetting conditions, heterogeneity of the reservoir rock, and oil trapping due to capillarity11. To recover some of this remaining oil, the tertiary or enhanced oil recovery (EOR) methods are used12.
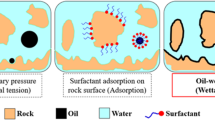
The EOR process involves thermal and non-thermal methods. Thermal techniques aim to increase the reservoir temperature in order to decrease oil viscosity to flow more easily. These methods include hot water injection, in situ combustion, and steam injection. Thermal methods are frequently used for recovery of heavy oils (American Petroleum Institute (API) gravities of lower than 20). Non-thermal methods are classified as chemical and miscible gas injection13. Among non-thermal techniques, chemical EOR techniques are one of the most prospective methods due to higher oil recovery and technical and economic feasibility14. These methods decrease the remaining oil saturation by reducing IFT between oil and water (alkaline/ surfactant/nanofluid), wettability change from oil-wet to water-wet (surfactant/nanofluid), and increment of volumetric sweep efficiency through reduction of the water–oil mobility ratio (polymer)10. Nanofluid flooding is the latest and one of the most promising chemical EOR methods15. These fluids are defined as any fluids used in oil and gas fields (exploration, drilling, completion, EOR) that contain at least one additive with a size range from 1 to 100 nm16. The EOR mechanisms of nanofluids include IFT reduction17,18, wettability alteration19,20, increased structural disjoining pressure, preventing asphaltene precipitation, pore channel plugging, and formation and stabilization of emulsions21,22. Some of these mechanisms are shown in Fig. 1.
In recent years, field tests of nanoflooding for EOR have been reported in some Colombian oil fields (TN, Cupiagua Sur, Castilla, and Chichimene) with a significant oil recovery for all fields23. Many experimental results showed that nanofluids noticeably could increase oil recovery24,25,26,27.
Fe3O4 or magnetic NPs are widely used for nanoflooding because of their interesting properties including biodegradable, biocompatible, superparamagnetic, available, and low cost28. The superparamagnetic feature causes the Fe3O4 NPs to be separated after the operation by magnetite separation. Therefore, the recyclability of Fe3O4 NPs makes the reusing of these NPs for operation, which leads to reduced operation costs29,30.
In solution form, NPs have a trend of aggregation, because of their small size. To avoid this phenomenon, surface modification of NPs is an effective way for their stabilization30. Sharma et al.31 synthesized a nanocomposite by grafting polyacrylamide (PAM) onto the surface of SiO2 NPs. The results indicated that obtained NPs had significant stability under harsh reservoir conditions (high temperature and salinity).
Sang et al.32 employed a hydrophobically associative hygroscopic zwitterionic polymer to coat the surface of silica NPs to stabilize them. They observed that the modified silica NPs were able to improve the oil recovery factor by 74.1% in a coreflooding test. Also, surface modification of NPs leads to improvement of the EOR mechanism such as wettability change to water-wet and IFT decrease. Rezvani et al.33 synthesized a nanocomposite by coating chitosan on the surface of Fe3O4 NPs. They concluded that the obtained NPs had a significant effect on IFT reduction and wettability alteration (toward more water-wet). Also, they reported that the ultimate oil recovery factor was 67.5% by using 0.03 wt. % of this nanocomposite in the seawater. Divandari et al.34 modified the surface of Fe3O4 NPs by using citric acid. They observed that the synthesized NPs caused more oil recovery factor compared to Fe3O4 and Fe2O3 NPs, due to more IFT reduction between the injection fluid and oil and wettability alteration from oil-wet to strongly water-wet.
Gelatin is an ideal candidate for surface modification of NPs to use in nanoflooding due to its special properties such as biodegradability, biocompatibility, nontoxicity, low cost, easy availability, presence of many functional groups (COOH, NH2, OH)35 and surface activity36,37,38. This natural polymer is produced by thermal denaturation or partial hydrolysis of collagen from the skin and bone of animals39. Gelatin is a mix of 18 amino acids joined by peptide bonds in a long molecular chain40,41,42. The carboxyl group of amino acid 1 is linked to the amino group of amino acid 2 by a covalent bond known as a peptide bond. The result is a C-N bond to link the two amino acids. Figure S1 (in Supplementary Information) shows a typical structure of a gelatin molecule.
Amino acids are a type of organic compound made up of an organic R group (also known as the side chain) that is specific to each amino acid, an acidic carboxyl group (COOH), and a basic amino group (NH2) (see Supplementary Fig. S2). Each molecule has an amino and carboxyl group connected to its central carbon (C) atom, known as the α-carbon. The hydrogen (H) atom and the R group are connected to the remaining two bonds of the α-carbon atom. The side chain R groups distinguish the amino acids from one another. Therefore, The properties of each amino acid are dictated by the side chain43,44.
The hydrophilicity or hydrophobicity of amino acids is determined by the hydrophathy index which was proposed in 1982 by Kyte and Doolittle45. According to this index, the gelatin consists of hydrophilic amino acids (Arginine, Lysine, Glycine, Aspartic acid, Glutamic acid, Proline, Tyrosine, Serine, Threonine, Hisitidine, and Tryptophan) and hydrophobic amino acids (Alanine, Methionine, Phenylalanine, Leucine, Valine, and Isoleucine). Therefore, this substance can act as a biosurfactant due to has hydrophilic and hydrophobic amino acids36,38. The gelatin molecule containing hydrophilic and hydrophobic amino acids is schematically shown in Supplementary Information (Fig. S3).
In this research, the Fe3O4@Gelatin NPs are synthesized by a simple one-step method that is more convenient than the multi-step method. This coating gives the Fe3O4 NPs surface active property, high stability and oxidation prevention in water. Accordingly, the objectives of this study are to: (1) synthesis of Fe3O4@Gelatin NPs; (2) characterization of these NPs; (3) feasibility test of the synthesized NPs for EOR purposes. For the first time, the application of Fe3O4@Gelatin NPs is examined for EOR. To understand the effect of the synthesized NPs on EOR, the interfacial properties such as IFT and wettability are evaluated. In the end, the micromodel flooding test is carried out to compare the obtained recovery factor by using the synthesized Fe3O4@Gelatin NPs (seawater as a carrier fluid) with Fe3O4 NPs (seawater as a carrier fluid) and only seawater (without NPs).
Experimental section
Materials
The starting reactants were Ferrous chloride tetrahydrate (FeCl2.4H2O, Sigma-Aldrich) and ferric chloride anhydrous (FeCl3, Sigma-Aldrich). Ammonium hydroxide (NH4OH 25 wt. % in water- Merck) was used to set the pH value. Gelatin (Sigma-Aldrich) was used as coating material. Excess chemical reagents were eliminated by using distilled water and ethanol (Merck).
The crude oil used in this research was provided from one of the Iranian oil reservoirs. According to the results of SARA analysis, this oil contains 38.6% saturates, 46.2% aromatics, 9.5% resins, and 5.7% asphaltenes. The seawater used in this research was sampled from the Persian Gulf. The results of seawater analysis are presented in Table 1.
Synthesis of Fe 3 O 4 and Fe 3 O 4 @Gelatin NPs
In this study, the chemical co-precipitation method was used to synthesize Fe3O4@Gelatin NPs (core/shell). The Fe3O4 and Fe3O4@Gelatin NPs were synthesized with a reported method similar to that of Sivirat and Paradee46. At first, a solution including 0.5 g of gelatin in 100 mL of distilled water was made overnight at 50 °C with constant stirring to prepare a gelatin solution. Then, the gelatin solution cooled down to 30 °C. The FeCl2.4H2O (Fe2+; 1.5 g) and FeCl3 (Fe3+; 3.0 g) were added into the gelatin solution with constant stirring at 30 °C for 1 h. After that, an ammonium hydroxide solution was slowly added to the mixture and stirred continuously until the pH of the mixture reached 10 and the resulting suspension was black. After 4 h of constant stirring at 30 °C, the suspension was centrifuged at 3000 rpm for 10 min. To eliminate excess chemical reagents, the precipitated particles were washed two times with distilled water and ethanol and then dried in an oven at 80 °C for 12 h. A proposed schematic of the interaction between Fe3O4 and gelatin is shown in Fig. 2. After sonication for 10 min, the synthesized NPs remained stable for several weeks, which is an important factor in nanoflooding. The preparation of Fe3O4 particles was done by the same procedure but without adding gelatin.
Scanning electron microscope (SEM) and transmission electron microscopy (TEM)
The SEM (Tescan Mira3) method was used to investigate the morphology and size of the synthesized Fe3O4 NPs. Due to the core/shell structure of synthesized Fe3O4@Gelatin NPs, the TEM technique was employed to study their morphology and size. The TEM Philips EM 208S was used for the TEM test.
X-ray diffraction (XRD) and fourier transform infrared spectroscopy (FT-IR)
The crystalline structures of gelatin and the synthesized Fe3O4, Fe3O4@Gelatin NPs were detected by a X-ray diffractometer (XRD Philips PW1730). A FT-IR spectrometer (Brucker Tensor 27) was employed to study the functional group of gelatin, synthesized Fe3O4 and Fe3O4@Gelatin NPs.
Contact angle test
The contact angle test was used as a common method for investigating the impact of NPs on the glass surface’s contact angle. To perform the contact angle test, six flat glass plates (3 cm × 3 cm × 5 mm) were provided. At first, the glass plates were cleaned with ethanol and then heated in an oven at 100 °C for 2 h. The surface of the glasses was coated with oil for 30 days and then the contact angle of a deionized water droplet on the surface was determined. Also, on an uncoated glass surface (cleaned glass), the contact angle was measured. The deionized water was used in every contact angle test. After that, oil-wet glasses were coated with different fluids (seawater as a carrier fluid) for 30 days and then the contact angle was determined. Actually, the contact angle between the deionized water and four surfaces coated with different fluids (seawater, 0.06 wt. % of gelatin, 0.06 wt. % of Fe3O4 NPs, and 0.06 wt. % of Fe3O4@Gelatin NPs) was measured. Four points on the glass surface were selected to obtain reliable results from contact angle measurements. All four points were measured, and the average results were calculated. Also, the contact angle tests were calculated using the DSA100 device of Kruss Company and the sessile drop technique. Figure 3(a) shows the schematic diagram of the sessile drop technique for measuring contact angles.
IFT test
To investigate the effect of Fe3O4@Gelatin NPs on IFT between oil and water, the pendant drop technique was employed for measuring IFT between crude oil and the concentration of nanofluids (0.06 wt. %) at 25 °C. The IFT test was performed by the DSA100 device of Kruss Company. The schematic diagram of the pendant drop technique for IFT calculation is shown in Fig. 3(b).
Micromodel flooding test
To observe the impact of Fe3O4@Gelatin NPs on the oil recovery, a homogeneous glass micromodel was employed to create the porous media for nanoflooding. The 2D micromodel.
pattern was created using laser technology. The micromodel was placed horizontally above a source of light. The experimental equipment included a video camera recorder (Canon SX620 HS, Japan) to carry out visualization during experiments and a high-precision, low-rate syringe pump (Fusion 1, US), which accurately regulates the flow rate of the injected fluids through micromodel, as depicted in Fig. 4.
Experimental procedure for micromodel flooding
The micromodel was washed in acetone, toluene, and hot water before the injection. After that, the micromodel was first saturated with synthetic brine (50,000 ppm NaCl), and then with crude oil for the visualization of nanoflooding to create the condition of connate water saturation. To obtain oil recovery, the synthesized NPs with a concentration of 0.06 wt. % were dispersed in the seawater and injected into the micromodel at a constant injection rate of 0.01 cc/min. To compare the impact of NPs on the oil recovery, the tests were carried out by seawater (0 wt. % of NPs). The outlet port of the micromodel was always kept open to the atmosphere, and all tests were conducted at room temperature.
Based on measurements of oil recovery efficiency, the efficiency results of tests for improving EOR were considered. The calculation formula is given in Eq. (1):
RF represents recovery factor (%), Soi is the initial oil saturation (%) before flooding and Sof is the final oil saturation (%) after flooding.
Results and discussion
SEM, TEM, XRD, and FT-IR of the synthesized NPs
SEM and TEM
The morphology and size of Fe3O4 NPs were observed by SEM image as shown in Fig. 5. The shape of Fe3O4 NPs was spherical with a size range of 20–30 nm. Figure 6 shows a TEM image of synthesized Fe3O4@Gelatin nanocomposites. As shown, the size range of synthesized nanocomposites was 40–80 nm and gelatin was well coated on the external surface of Fe3O4 NPs with a nearly spherical shape. The small size and spherical shape of synthesized NPs allow them to easily enter into the pores of reservoir rocks and consequently release residual oil47.
XRD
Figure 7 represents the XRD of Fe3O4 NPs, gelatin, and Fe3O4@Gelatin nanocomposites. As shown, the characteristic peaks of Fe3O4 crystal with the spinel structure are observed at 2θ = 30°, 35°, 43°, 53°, 57° and 63°48,49,50. Also, the characteristic peak of gelatin was indicated with a broad peak at 2θ = 19°. This peak is usually assigned to the triple-helical crystalline structure of gelatin51,52. The characteristic peaks of Fe3O4 NPs and pure gelatin are found in the XRD pattern of Fe3O4@Gelatin nanocomposites, which show the successful conformation of these synthesized nanocomposites. Moreover, decreasing intensity values of characteristic peaks of Fe3O4@Gelatin nanocomposites confirm the successful coating of gelatin onto the surface of Fe3O4 NPs53.
FT-IR
Figure 8 shows the FT-IR analysis of Fe3O4 NPs (green graph), gelatin (blue graph), and Fe3O4@Gelatin nanocomposites (red graph). In the gelatin spectrum, the characteristic peak at 3438 cm−1 is related to the stretching vibrations of the N–H bond. Also, the characteristic peaks at 1639 and 1537 cm−1 are related to amide I and amide II bonds, respectively54. In the Fe3O4 NPs spectrum, the Fe–O band is found in 564 and 476 cm−1 peaks55. The characteristic peaks of Fe3O4 NPs and gelatin can be found in the spectrum of Fe3O4@Gelatin nanocomposites, which confirm the successful coating of gelatin onto the surface of Fe3O4 NPs. Moreover, in the spectrum of Fe3O4@Gelatin nanocomposites, shifting of the N–H peak to lower wave number and decreasing broadness of its band indicates effective interaction between Fe3O4 NPs and gelatin by the electrostatic attraction53.
Wettability alteration
The contact angle between the deionized water and four surfaces coated with different fluids (seawater, 0.06 wt. % of gelatin, 0.06 wt. % of Fe3O4 NPs, and 0.06 wt. % of Fe3O4@Gelatin NPs) was measured. Figure 9 shows the wettability measurement results.
The contact angle of the deionized water on the crude oil-coated surface is greater than 90°, as shown in Fig. 9. In other words, the glass surface becomes oil-wet when it is coated with crude oil. The contact angle between the deionized water and the glass surface coated by seawater is 94°. As a result, seawater could alter the oil-wet glass surface to intermediate-wet state. During coating with seawater, the cations (Na+, K+, Ca2+, and Mg2+ in seawater) are adsorbed on negative sites of glass surface through attractive ionic interaction and consequently replacing the adsorbed oil phase, which causes wettability alteration to intermediate-wet state (Fig. 10). The impact of this mechanism on wettability change depends on the concentration of cations56.
The gelatin-coated glass surface has a contact angle of 30°. Therefore, coating the oil-wet glass surface with this substance makes a water-wet surface. The Gelatin due to has hydrophilic and hydrophobic amino acids can act as biosurfactant36,38. Hence, the suggested mechanism for the wettability change of the oil-wet glass surface by gelatin is creating capillary forces through hydrophobic interaction between its hydrophobic amino acid and the oleic phase. This force causes the oil film to remove from the glass surface and consequently alter toward water-wet conditions. Figure 11 shows the suggested mechanism for wettability modification by gelatin.
The contact angle of the deionized water on the glass surface coated by Fe3O4 NPs is 56°. In other words, the oil-wet glass surface becomes water-wet when it is coated with Fe3O4 NPs. The disjoining pressure is the main mechanism for wettability change by nanofluids (Fig. 12)57. The amount of pressure needed to disjoin fluids from the surface is because of the fluids’ adhesion force to the solid surface which is known as the disjoining pressure58. The studies demonstrate that the nanofluids decrease oil adsorption on a rock surface by creating a structural disjoining force between the oil and the rock and then through the formation of a wedge film structure on the rock surface59. During this process, The NPs in the nanofluid tend to rearrange. Actually, smaller NPs exhibit stronger repulsion forces, which cause increases in the entropy of the nanofluids; this tendency is because of the significant freedom of the NPs in the nanofluids. As a result of this action, an extra disjoining pressure is applied at that interface more so than in the bulk liquid60. Therefore, Fe3O4 NPs form a wedge film that separates the oil droplets from the glass surface by disjoining pressure, which increases oil recovery61. Also, the adsorption of some of these NPs on the glass surface creates a water-wet surface, which is preferable for EOR62.
The contact angle of deionized water on the glass surface coated by Fe3O4@Gelatin NPs is 33°. The surface modification of Fe3O4 NPs with gelatin increases their capability to alter wettability compared to only Fe3O4 NPs due to the surface activity of the gelatin.
IFT reduction
IFT reduction is one of the main mechanisms for the improvement of oil production63. Figure 13 shows the IFT value between crude oil and different fluids.
The IFT values of oil/deionized water and oil/seawater are 28.67 (mN/m) and 21.32 (mN/m), respectively. The cations (Na+, K+, Ca2+, and Mg2+) in seawater through electrostatic attractive interaction cause accumulation of carboxylic acid molecules (asphaltenes with a –COO⁻ functional groups in the oil phase) at the oil/seawater interface and consequently decrease IFT64 (Fig. 14). The large hydrocarbon skeleton and variety of polar heteroatom groups present in asphaltenes allow them to decrease the IFT of crude oil65. Therefore, these molecules can act as surface active agents because of the presence of both hydrocarbon skeleton (hydrophobic) and polar groups (hydrophilic) in them66.
Fe3O4 and Fe3O4@Gelatin NPs with a concentration of 0.06 wt. % were dispersed in seawater and then IFT between crude oil and these nanofluids was measured. The Fe3O4 NPs cause to decrease oil/seawater IFT to 12.16 (mN/m). Many researchers reported that NPs could decrease IFT due to their adsorption at the oil–water interface21,67,68. According to experimental findings, the IFT reduction process of NPs begins with the particles’ adsorption at the current oil/water interface, where they create a monolayer that replaces the current oil/water interface and operates as an interfacial particle film or a mechanical barrier (see Supplementary Fig. S4). After that, based on the distribution of particles at the interface and the surface energy of the NPs, the created monolayer reduces the interfacial energy between the two phases69.
Gelatin could decrease oil/seawater IFT to 13.81 (mN/m). The concentration of gelatin in seawater was 0.06 wt. %. This substance due to its hydrophilic and hydrophobic amino acids can act as a biosurfactant. Therefore gelatin can reduce oil/seawater IFT by adsorption on the oil/seawater interface (Fig. 15).
The synthesized Fe3O4@Gelatin NPs are able to decrease the IFT between oil/seawater to 6.59 mN/m. The synergistic effects of Fe3O4 NPs and gelatin in the form of a nanocomposite are the reason for this IFT reduction (Fig. 16). In this form, combining two IFT reduction mechanisms by Fe3O4 NPs and gelatin (as a biosurfactant) causes significant IFT reduction. The surface modification of NPs with surfactant improves their adsorption on the oil/water interface, and consequently, the oil/water IFT decreases sharply70.
Micromodel flooding
Through the flooding experiments of glass micromodels, it is feasible to see the microscopic behavior of oil and aqueous phases in porous media. Studying the displacement of two immiscible fluids has many benefits, and this approach facilitates the investigation of the EOR mechanism especially for novel EOR techniques71. Figure 17 shows the amount of remaining oil and fluids distribution in the micromodel after flooding (water cut = 100%) by different fluids. The ultimate oil recovery factor for seawater containing 0.06 wt. % Fe3O4@Gelatin NPs, seawater containing 0.06 wt. % Fe3O4 NPs and only seawater are 42.9%, 33.92%, and 26.57% respectively. Figure 17(a) is captured after seawater flooding which obviously shows that the walls of the micromodel are covered with oil and are oil-wet. Furthermore, trapped oil is clearly visible. As shown in Fig. 17(b), the porous media’s wettability was changed to water-wet, but the wettability was not significantly altered by the Fe3O4 NPs. Also, the thickness decrease of the oil film attached to the wall is visible and the amount of trapped oil has also reduced compared to seawater flooding, which results in a higher recovery factor. Figure 17(c) is taken after flooding with seawater containing 0.06 wt. % Fe3O4@Gelatin NPs. As shown, these NPs could remove oil from micromodel walls and alter wettability to strongly water-wet. In addition, the amount of trapped oil has decreased more than the previous two cases due to more IFT reduction by Fe3O4@Gelatin NPs. Therefore, flooding with these NPs provides a higher oil recovery factor compared to the previous two cases. The IFT decrease and wettability change toward water-wet leads to decreased capillary pressure and consequently releasing residual oil in the rock matrix. As a result, the microscopic sweep efficiency is improved, which leads to increased oil recovery63.
Conclusions
In this research, the Fe3O4@Gelatin NPs were synthesized by a low-cost and single-step process for EOR purposes. After that, TEM, XRD, and FTIR were used to characterize the synthesized NPs in terms of size, shape, and functional groups. Finally, contact angle, IFT, and micromodel flooding tests were done to evaluate the capability of synthesized NPs for EOR application. The main findings can be summarized as follows:
-
1-
The Fe3O4@Gelatin NPs showed the highest IFT reduction among other fluids, because of the synergistic effects of Fe3O4 NPs and gelatin in the form of a nanocomposite. The 0.06 wt. % Fe3O4@Gelatin NPs in the seawater could decrease the oil/seawater IFT to 6.59 (mN/m). (69% reduction of IFT value compared to only seawater).
-
2-
Due to the surface modification of Fe3O4 NPs with gelatin, The Fe3O4@Gelatin NPs have a greater ability to significantly change the glass surface wettability from oil-wet to water-wet (from 117° to 33°) compared to Fe3O4 NPs because the gelatin has surface active property.
-
3-
The coating surface of Fe3O4 NPs by gelatin cause to prevent oxidation in water. Also, gelatin as a biosurfactant can increase the stability of these NPs in water.
-
4-
The oil recovery factor for 0.06 wt. % Fe3O4@Gelatin NPs in the seawater is 42.9% which indicates a significant rise compared to seawater containing 0.06 wt. % Fe3O4 NPs (RF = 33.92%) and only seawater (RF = 26.57%). This considerable increase is due to more IFT decrease and wettability change to strongly water-wet by the synthesized NPs.
-
5-
According to the results of this research, the Fe3O4@Gelatin NPs could be a good candidate for EOR application due to interfacial characteristics of the oil, water, and surface have been more significantly modified.
Data availability
Data Availability the datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Barman, B. N., Cebolla, V. L., Mehrotra, A. K. & Mansfield, C. T. Petroleum and coal. Anal. Chem. 73, 2791–2803. https://doi.org/10.1021/ac010415i (2001).
Suleiman, M. Modelling and forecasting world oil demand: A regional analysis accounting for asymmetric price responses and technical progress. OPEC Energy Rev. 43, 193–216. https://doi.org/10.1111/opec.12147 (2019).
Angulo, A. & Vargas, C. A. Global distribution of the hydrocarbon Golden Zone. Mar. Pet. Geol. 144, 105832. https://doi.org/10.1016/j.marpetgeo.2022.105832 (2022).
Ghatee, A. & Zarrinpoor, N. Designing an oil supply chain network considering sustainable development paradigm and uncertainty. Chem. Eng. Res. Des. 184, 692–723. https://doi.org/10.1016/j.cherd.2022.06.026 (2022).
Alvarado, V. & Manrique, E. Enhanced oil recovery: An update review. Energies 3, 1529–1575. https://doi.org/10.3390/en3091529 (2010).
Al-Asadi, A., Arce, A., Rodil, E. & Soto, A. Enhanced oil recovery with nanofluids based on aluminum oxide and 1-dodecyl-3-methylimidazolium chloride ionic liquid. J. Mol. Liq. 363, 119798. https://doi.org/10.1016/j.molliq.2022.119798 (2022).
Pal, N., Saxena, N. & Mandal, A. Studies on the physicochemical properties of synthesized tailor-made gemini surfactants for application in enhanced oil recovery. J. Mol. Liq. 258, 211–224. https://doi.org/10.1016/j.molliq.2018.03.037 (2018).
Saxena, N., Pal, N., Dey, S. & Mandal, A. Characterizations of surfactant synthesized from palm oil and its application in enhanced oil recovery. J. Taiwan Inst. Chem. Eng. 81, 343–355. https://doi.org/10.1016/j.jtice.2017.09.014 (2017).
Hussain, S. S., Kamal, M. S. & Fogang, L. T. Effect of internal olefin on the properties of betaine-type zwitterionic surfactants for enhanced oil recovery. J. Mol. Liq. 266, 43–50. https://doi.org/10.1016/j.molliq.2018.06.031 (2018).
Sun, X., Zhang, Y., Chen, G. & Gai, Z. Application of nanoparticles in enhanced oil recovery: A critical review of recent progress. Energies 10, 345. https://doi.org/10.3390/en10030345 (2017).
Joonaki, E. & Ghanaatian, S. The application of nanofluids for enhanced oil recovery: Effects on interfacial tension and coreflooding process. Pet. Sci. Technol. 32, 2599–2607. https://doi.org/10.1080/10916466.2013.855228 (2014).
Atta, D. Y., Negash, B. M., Yekeen, N. & Habte, A. D. A state-of-the-art review on the application of natural surfactants in enhanced oil recovery. J. Mol. Liq. 321, 114888. https://doi.org/10.1016/j.molliq.2020.114888 (2021).
Thomas, S. Enhanced oil recovery-an overview. Oil Gas Sci. Technol.-Rev. de l’IFP 63, 9–19. https://doi.org/10.2516/ogst:2007060 (2008).
Levitt, D. B. & Pope, G. A. In SPE Improved Oil Recovery Conference? SPE-113845-MS (SPE).
Esfe, M. H., Hosseinizadeh, E. & Esfandeh, S. Flooding numerical simulation of heterogeneous oil reservoir using different nanoscale colloidal solutions. J. Mol. Liq. 302, 111972. https://doi.org/10.1016/j.molliq.2019.111972 (2020).
Lau, H. C., Yu, M. & Nguyen, Q. P. Nanotechnology for oilfield applications: Challenges and impact. J. Pet. Sci. Eng. 157, 1160–1169. https://doi.org/10.1016/j.petrol.2017.07.062 (2017).
Saien, J., Moghaddamnia, F. & Bamdadi, H. Interfacial tension of methylbenzene–water in the presence of hydrophilic and hydrophobic alumina nanoparticles at different temperatures. J. Chem. Eng. Data 58, 436–440. https://doi.org/10.1021/je3011584 (2013).
Moghadam, T. F. & Azizian, S. Effect of ZnO nanoparticles on the interfacial behavior of anionic surfactant at liquid/liquid interfaces. Coll. Surf. A: Physicochem. Eng. Asp. 457, 333–339. https://doi.org/10.1016/j.colsurfa.2014.06.009 (2014).
Al-Anssari, S., Barifcani, A., Wang, S., Maxim, L. & Iglauer, S. Wettability alteration of oil-wet carbonate by silica nanofluid. J. coll. Interfac. Sci. 461, 435–442. https://doi.org/10.1016/j.jcis.2015.09.051 (2016).
Roustaei, A. & Bagherzadeh, H. Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J. Pet. Explor. Prod. Technol. 5, 27–33. https://doi.org/10.1007/s13202-014-0120-3 (2015).
Roustaei, A., Saffarzadeh, S. & Mohammadi, M. An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt. J. Pet. 22, 427–433. https://doi.org/10.1016/j.ejpe.2013.06.010 (2013).
Agi, A., Junin, R. & Gbadamosi, A. Mechanism governing nanoparticle flow behaviour in porous media: Insight for enhanced oil recovery applications. Int. Nano Lett. 8, 49–77. https://doi.org/10.1007/s40089-018-0237-3 (2018).
Franco, C. A., Zabala, R. & Cortés, F. B. Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J. Pet. Sci. Eng. 157, 39–55. https://doi.org/10.1016/j.petrol.2017.07.004 (2017).
Rayeni, N. S., Imanivarnosfaderani, M., Rezaei, A. & Gomari, S. R. An experimental study of the combination of smart water and silica nanoparticles to improve the recovery of asphaltenic oil from carbonate reservoirs. J. Pet. Sci. Eng. 208, 109445. https://doi.org/10.1016/j.petrol.2021.109445 (2022).
Wahaab, F. A. et al. Electromagnetic wave-induced nanofluid-oil interfacial tension reduction for enhanced oil recovery. J. Mol. Liq. 318, 114378. https://doi.org/10.1016/j.molliq.2020.114378 (2020).
Wijayanto, T., Kurihara, M., Kurniawan, T. & Muraza, O. Experimental investigation of aluminosilicate nanoparticles for enhanced recovery of waxy crude oil. Energy Fuels 33, 6076–6082. https://doi.org/10.1021/acs.energyfuels.9b00781 (2019).
Alomair, O. A., Matar, K. M. & Alsaeed, Y. H. Experimental study of enhanced-heavy-oil recovery in Berea sandstone cores by use of nanofluids applications. SPE Reserv. Eval. Eng. 18, 387–399. https://doi.org/10.2118/171539-PA (2015).
Pereira, M. L. D. O. et al. Fe3O4 nanoparticles as surfactant carriers for enhanced oil recovery and scale prevention. ACS Appl. Nano Mater. 3, 5762–5772. https://doi.org/10.1021/acsanm.0c00939 (2020).
Zhou, K., Zhou, X., Liu, J. & Huang, Z. Application of magnetic nanoparticles in petroleum industry: A review. J. Pet. Sci. Eng. 188, 106943. https://doi.org/10.1016/j.petrol.2020.106943 (2020).
Zhang, Z., Li, H., Sui, H., He, L. & Li, X. Synthesis and application of hydrophilically-modified Fe 3 O 4 nanoparticles in oil sands separation. RSC Adv. 8, 15813–15824. https://doi.org/10.1039/C8RA01966E (2018).
Sharma, T., Iglauer, S. & Sangwai, J. S. Silica nanofluids in an oilfield polymer polyacrylamide: Interfacial properties, wettability alteration, and applications for chemical enhanced oil recovery. Ind. Eng. Chem. Res. 55, 12387–12397. https://doi.org/10.1021/acs.iecr.6b03299 (2016).
Choi, S. K., Son, H. A., Kim, H. T. & Kim, J. W. Nanofluid enhanced oil recovery using hydrophobically associative zwitterionic polymer-coated silica nanoparticles. Energy Fuels 31, 7777–7782. https://doi.org/10.1021/acs.energyfuels.7b00455 (2017).
Rezvani, H., Riazi, M., Tabaei, M., Kazemzadeh, Y. & Sharifi, M. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4@ Chitosan nanocomposites. Coll. Surf. A: Physicochem. Eng. Asp. 544, 15–27. https://doi.org/10.1016/j.colsurfa.2018.02.012 (2018).
Divandari, H., Hemmati-Sarapardeh, A., Schaffie, M. & Ranjbar, M. Integrating synthesized citric acid-coated magnetite nanoparticles with magnetic fields for enhanced oil recovery: Experimental study and mechanistic understanding. J. Pet. Sci. Eng. 174, 425–436. https://doi.org/10.1016/j.petrol.2018.11.037 (2019).
Elzoghby, A. O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Controll. Releas. 172, 1075–1091. https://doi.org/10.1016/j.jconrel.2013.09.019 (2013).
Derkatch, S., Kolotova, D., Milyaeva, O. Y. & Noskov, B. Dynamic properties of gelatin/surfactant adsorption layers. Coll. Surf. A: Physicochem. Eng. Asp. 508, 251–256. https://doi.org/10.1016/j.colsurfa.2016.08.046 (2016).
Abouzeid, F. M. Surface active properties of gelatin and their effect on the electropolishing and corrosion behavior of steel in orthophosphoric acid. Egypt. J. Pet. 25, 229–237. https://doi.org/10.1016/j.ejpe.2015.05.014 (2016).
Elbatouti, M. & Fetouh, H. A. Extraction of eco-friendly and biodegradable surfactant for inhibition of copper corrosion during acid pickling. Adsorpt. Sci. Technol. 37, 649–663. https://doi.org/10.1177/02636174198651 (2019).
Feng, X. et al. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: Influence of the nanoparticles concentration. Coll. Surf. B: Biointerfaces. 196, 111294. https://doi.org/10.1016/j.colsurfb.2020.111294 (2020).
Sultana, S., Ali, M. E. & Ahamad, M. N. U. Preparation and processing of religious and cultural foods 215–239 (Elsevier, 2018).
Gün Gök, Z. Synthesis and characterization of polyvinyl alcohol–silk sericin nanofibers containing gelatin-capped silver nanoparticles for antibacterial applications. Polym. Bull. 79, 10357–10376. https://doi.org/10.1007/s00289-022-04455-0 (2022).
Li, N., Xu, J.-Z., Yao, H., Zhu, J.-J. & Chen, H.-Y. The direct electron transfer of myoglobin based on the electron tunneling in proteins. J. Phys. Chem. B 110, 11561–11565. https://doi.org/10.1021/jp060653n (2006).
Cerf, C. & Jorissen, A. Is amino-acid homochirality due to asymmetric photolysis in space?. Space Sci. Rev. 92, 603–612. https://doi.org/10.1023/A:1005248813761 (2000).
Engelking, L. Textbook of veterinary physiological chemistry, updated 2/e (Academic Press, 2010).
Kyte, J. & Doolittle, R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Boil. 157, 105–132. https://doi.org/10.1016/0022-2836(82)90515-0 (1982).
Sirivat, A. & Paradee, N. Facile synthesis of gelatin-coated Fe3O4 nanoparticle: Effect of pH in single-step co-precipitation for cancer drug loading. Mater. Des. 181, 107942. https://doi.org/10.1016/j.matdes.2019.107942 (2019).
Gbadamosi, A. O. et al. Recent advances and prospects in polymeric nanofluids application for enhanced oil recovery. J. Ind. Eng. Chem. 66, 1–19. https://doi.org/10.1016/j.jiec.2018.05.020 (2018).
Ghoohestani, E., Samari, F., Homaei, A. & Yosuefinejad, S. A facile strategy for preparation of Fe3O4 magnetic nanoparticles using Cordia myxa leaf extract and investigating its adsorption activity in dye removal. Sci. Rep. 14, 84. https://doi.org/10.1038/s41598-023-50550-1 (2024).
Mahdavi, M., Namvar, F., Ahmad, M. B. & Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 18, 5954–5964. https://doi.org/10.3390/molecules18055954 (2013).
Loh, K.-S., Lee, Y. H., Musa, A., Salmah, A. A. & Zamri, I. Use of Fe3O4 nanoparticles for enhancement of biosensor response to the herbicide 2, 4-dichlorophenoxyacetic acid. Sensors 8, 5775–5791. https://doi.org/10.3390/s8095775 (2008).
Yakimets, I. et al. Mechanical properties with respect to water content of gelatin films in glassy state. Polymer 46, 12577–12585. https://doi.org/10.1016/j.polymer.2005.10.090 (2005).
Peña, C., De La Caba, K., Eceiza, A., Ruseckaite, R. & Mondragon, I. Enhancing water repellence and mechanical properties of gelatin films by tannin addition. Bioresour. Technol. 101, 6836–6842. https://doi.org/10.1016/j.biortech.2010.03.112 (2010).
Dorniani, D. et al. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. https://doi.org/10.2147/IJN.S35746 (2012).
Gaihre, B., Khil, M. S., Lee, D. R. & Kim, H. Y. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. Int. J. Pharm. 365, 180–189. https://doi.org/10.1016/j.ijpharm.2008.08.020 (2009).
Belikov, V., Kuregyan, A. & Ismailova, G. Standardization of magnetite. Pharm. Chem. J. 36, 333–336. https://doi.org/10.1023/A:1020845110683 (2002).
Sun, C. et al. Ion-induced oil water wettability alteration of rock surfaces. Part I: Polar interactions between oil and solid. Chem. Eng. Sci. 232, 116366. https://doi.org/10.1016/j.ces.2020.116366 (2021).
Keykhosravi, A. & Simjoo, M. Insights into stability of silica nanofluids in brine solution coupled with rock wettability alteration: An enhanced oil recovery study in oil-wet carbonates. Coll. Surf. A: Physicochem. Eng. Asp. 583, 124008. https://doi.org/10.1016/j.colsurfa.2019.124008 (2019).
Basu, S. & Sharma, M. M. In SPE Annual Technical Conference and Exhibition. (OnePetro).
Kopanichuk, I. V., Vanin, A. A. & Brodskaya, E. N. Disjoining pressure and structure of a fluid confined between nanoscale surfaces. Coll. Surf. A: Physicochem. Eng. Asp. 527, 42–48. https://doi.org/10.1016/j.colsurfa.2017.04.072 (2017).
Eltoum, H., Yang, Y.-L. & Hou, J.-R. The effect of nanoparticles on reservoir wettability alteration: A critical review. Pet. Sci. 18, 136–153. https://doi.org/10.1007/s12182-020-00496-0 (2021).
Kumar, G., Behera, U. S., Mani, E. & Sangwai, J. S. Engineering the wettability alteration of sandstone using surfactant-assisted functional silica nanofluids in low-salinity seawater for enhanced oil recovery. ACS Eng. Au 2, 421–435. https://doi.org/10.1021/acsengineeringau.2c00007 (2022).
Yuan, B. et al. Permeability reduction of berea cores owing to nanoparticle adsorption onto the pore surface: Mechanistic modeling and experimental work. Energy Fuels 31, 795–804. https://doi.org/10.1021/acs.energyfuels.6b02108 (2017).
Deng, X. et al. Investigation of the coupled effect of IFT reduction and wettability alteration for oil recovery: New insights. ACS Omega 8, 12069–12078. https://doi.org/10.1021/acsomega.2c07906 (2023).
Divandari, H., Hemmati-Sarapardeh, A., Schaffie, M. & Ranjbar, M. Integrating functionalized magnetite nanoparticles with low salinity water and surfactant solution: Interfacial tension study. Fuel 281, 118641. https://doi.org/10.1016/j.fuel.2020.118641 (2020).
Havre, T. E., Sjöblom, J. & Vindstad, J. E. Oil/water-partitioning and interfacial behavior of naphthenic acids. J. Dispers. Sci. Technol. 24, 789–801. https://doi.org/10.1081/DIS-120025547 (2003).
Yarranton, H. W., Alboudwarej, H. & Jakher, R. Investigation of asphaltene association with vapor pressure osmometry and interfacial tension measurements. Ind. Eng. Chem. Res. 39, 2916–2924. https://doi.org/10.1021/ie000073r (2000).
Murshed, S. S., Tan, S.-H. & Nguyen, N.-T. Temperature dependence of interfacial properties and viscosity of nanofluids for droplet-based microfluidics. J. Phys. D: Appl. Phys. 41, 085502. https://doi.org/10.1088/0022-3727/41/8/085502 (2008).
Alomair, O. A., Matar, K. M. & Alsaeed, Y. H. In SPE Asia Pacific oil and gas conference and exhibition. SPE-171539-MS (SPE).
Hendraningrat, L., Li, S. & Torsæter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Pet. Sci. Eng. 111, 128–138. https://doi.org/10.1016/j.petrol.2013.07.003 (2013).
Liang, T., Hou, J.-R., Qu, M., Xi, J.-X. & Raj, I. Application of nanomaterial for enhanced oil recovery. Pet. Sci. 19, 882–899. https://doi.org/10.1016/j.petsci.2021.11.011 (2022).
Li, S. & Torsæter, O. In Paper SCA2014–022 was prepared for presentation at the International Symposium of the Society of Core Analysts held in Avignon, France. 8–11.
Author information
Authors and Affiliations
Contributions
Hossein Ghalenavia: Investigation, Data curation, Methodology, Writing original draft, Abdolhossein Hemmati-Sarapardeh: Supervision, Conceptualization, Validation, Writing-Review & Editing , Mahin Schaffie: Visualization, Methodology, Validation, Writing-Review & Editing, Saeid Norouzi-Apourvaria: Validation, Methodology, Writing-Review & Editing .
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghalenavi, H., Hemmati-Sarapardeh, A., Schaffie, M. et al. Application of synthesized Fe3O4@Gelatin nanoparticles on interfacial properties and enhanced oil recovery. Sci Rep 15, 2558 (2025). https://doi.org/10.1038/s41598-024-84953-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84953-5
Keywords
This article is cited by
-
Experimental investigation of wettability alteration in sandstone rock by nanoparticles, gelatin biopolymer, salt ions, and synthesized Fe3O4/gelatin nanocomposite for EOR applications
Scientific Reports (2025)
-
Nanofluid Thermophysical Property Modeling for Enhanced Oil Recovery: A Comprehensive Review and Future Outlook for Artificial Intelligence Integration
Archives of Computational Methods in Engineering (2025)