Abstract
mRNA and adenoviral vector vaccine platforms were used for the primary series of COVID-19 vaccines in many countries. However, the distinct immunogenic properties on these platforms remain less understood. We traced neutralizing antibodies, memory B cells, and T cells longitudinally in cohorts that received either mRNA (BNT162b2 or mRNA-1273) or adenoviral vector (ChAdOx1) vaccines with homologous or heterologous regimens (total 9 groups, n = 26–28 for each group) at 4 weeks interval. The priming and boosting effects on various immune parameters were comparably assessed between mRNA and adenoviral vector platforms. We found that initial priming by adenoviral vector vaccine elicited robust T cell responses, but B cell responses, including antibody titers, were relatively lower than those elicited by mRNA priming. The dissociation between T cell and antibody responses were exaggerated at greater extents after the homologous booster with the adenoviral vector vaccine, resulting in 5-19-fold lower antibody titers despite comparable spike-specific T cell numbers at day 28 after the boost. Robust IFN-γ and few IL-2 and IL-5 production characterized T cell functionality primed by adenoviral vector. Boosting with mRNA vaccines restored their IL-2 and IL-5 production at some extents, but the IL-5 T cell responses elicited by adenoviral vector/mRNA heterologous regimen waned faster than those by mRNA homologous regimen. Thus, our data revealed that the cytokine production of helper T cells was skewed by adenoviral vector priming, leading to the attenuated IL-2 and IL-5 responses which were prolonged even after mRNA boosting, suggesting an imprinting of T-cell functionality depending on the vaccine platform used for initial priming. These results highlight the importance of selecting vaccine platforms based on the immunogenic properties.
Similar content being viewed by others
Introduction
The swift development of multiple COVID-19 vaccines within one year after viral genome isolation greatly contributed to the pandemic response and the end of Public Health Emergency of International Concern on May 2023. While two types of COVID-19 mRNA vaccines (BNT162b2 and mRNA-1273) have been administered for primary series of vaccination in several countries including Japan, the other platforms of vaccines, such as adenoviral vector (ChAdOx1 nCoV-19), recombinant protein (NVX-CoV2373), and inactivated (CoronaVac) vaccine were also utilized for the prompt acquisition of herd immunity in other regions. Numerous studies have been performed to profile immune responses elicited by COVID-19 vaccines and extended our understanding of vaccine-induced immunity as well as their potential relevance to the vaccine effectiveness. Many immune profiling studies have been performed in mRNA vaccinees1,2,3,4,5,6,7,8,9,10, as the populations in many regions were vaccinated with mRNA platform at highest percentage. However, it is important to explore the immunogenic properties of other vaccine platforms or prime-boost vaccination regimen by heterologous vaccine platforms, as the multiple vaccine platforms and regimens are required for the preparedness of the next pandemic caused by unknown pathogen X for which we cannot predict the appropriate platforms to elicit protective immunity.
The main aim of the current COVID-19 vaccination is to durably elicit multiple layers of memory responses that are coordinated by neutralizing antibodies, memory B, and T cells. Neutralizing antibodies are relatively easy to quantitate by standardized methods in high-throughput manner while the memory B and T cells in the second layer requires immunological techniques with in vitro culture and flow cytometric analysis hampering standardization and high-throughputness. The neutralizing antibodies serve as the first line of defense and are shown to correlate with the vaccine effectiveness (VE) within 3 months after the booster when high antibody titers are maintained, providing the basis for using the neutralizing antibody titers as the immune correlate of protection11,12,13,14. However, the gap between neutralizing antibody titers and VE expands along with an antigenic mismatch between the vaccine and infected strains occurs as we experienced by the emergence of Omicron variants. Moreover, the waning of neutralizing antibody titers with time enhances the gap, because the contribution of memory B and T cells on the VE increases in such situation, especially against more severe diseases15. Indeed, the longer persistence of VE against severe diseases relative to preventing infection is frequently observed16. Therefore, a detailed evaluation of the vaccine-specific immune responses with relevance of VE requires the profiling of not only neutralizing antibodies but also memory B and T cells.
In the early COVID-19 pandemic period, the ChAdOx1 nCoV-19 (AZD1222) vaccine accounted for over one third of all global COVID-19 vaccine doses administered in 2021. Although ChAdOx1 received regulatory approval as a two-dose regimen with 4 to 12 weeks interval longer than homologous mRNA vaccination, an association with immune thrombocytopenia with the adenoviral vector vaccination hampered the booster vaccination by this platform17,18. As a result, the substantial numbers of individuals primed by a ChAdOx1 vaccine were boosted with mRNA vaccine. The heterologous prime-boost regimen (ChAdOx1/mRNA vaccine) elicited higher neutralizing antibodies and comparable numbers of IFN-γ-producing T cells, along with the greater VE19,20. However, the factors underlying the enhanced immunogenic properties by the ChAdOx1 priming remains unknown, even though it was used in more countries than any other COVID-19 vaccine21. This point can be addressed using the cohorts which were given by the prime-boost regimens with the same time interval with homologous vaccination. Here, we compared vaccine-elicited neutralizing antibody and memory B and T cell responses in various cohorts which received mRNA (BNT162b2 or mRNA-1273) or adenoviral vector (ChAdOx1) vaccines with homologous or heterologous regimen with the same 4 weeks interval, elucidating the priming and boosting properties of ChAdOx1 vaccines on T cells which are previously unappreciated.
Results
Study design
We enrolled a total of 270 healthy volunteers as subjects (Fig. 1). The subjects were separated into 9 groups (n = 30) who then received any possible combination of BNT162b2, mRNA-1273, and ChAdOx1 with a uniform 28-days-interval. We enrolled subjects with > 40 ages for ChAdOx1 doses, because the vaccine was approved for > 40 years adults in Japan (Fig. S1A and B). Blood samples were collected for analyses on serum antibody titers and peripheral blood mononuclear cells (PBMCs) at 0, 28, 56, 84, and 168 days after the primary vaccination. We first measured anti-nucleocapsid antibody titers to assess SARS-CoV-2 infection histories throughout the study period (Fig. S1C and D). We excluded 26 subjects (2 to 4 subjects per group) who seroconverted by day 56 from further longitudinal analyses. The subjects seroconverted at day 84 or 168 (1 to 6 subjects per group, 34 subjects in total) were excluded from the analyses at all time points later than seroconversion. We analyzed anti-spike IgG titers, neutralization titers, memory B cells, and T cells in peripheral blood (Fig. S1A). For memory B cell analyses, we focused on receptor-binding domain (RBD)-reactive B cells, since RBD is a main target of potently neutralizing antibodies22,23,24,25,26,27. It is important to mention that the numbers of spike-reactive B cells and RBD-reactive B cells highly correlate each other in the vaccinees7. Memory B cells were detected as CD19+CD20+CD21+CD27+IgM−IgA−IgG+ B cells that bind SARS-CoV-2 spike and RBD probes (Fig. S2A). Spike-specific T cells were analyzed as CD4+ or CD8+ in CD3+ T cells with activation-induced markers (AIM), CD69 and CD137, expression after overnight stimulation of PBMCs with spike overlapping peptides (Fig. S2B). The study design with a uniform prime/boost interval and any possible combination provided us with a rare and relatively straightforward approach to compare the priming/boosting effects between adenoviral vector and mRNA platforms.
Robust T-cell responses but attenuated antibody and B-cell responses following adenoviral vector priming
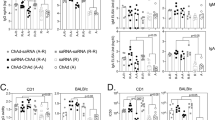
First, we compared the immune responses induced by the primary vaccination at 28 days. Among three vaccines, mRNA-1273 was most potent for eliciting anti-spike titers, neutralization titers, and RBD-reactive memory B cells (Fig. 2A-C). BNT162b2 and ChAdOx1 were comparable for these responses except for anti-spike titers, where BNT162b2 induced slightly higher titers than ChAdOx1. Regarding T cell responses, mRNA-1273 and ChAdOx1 induced higher frequencies of CD4 and CD8 T cells than BNT162b2 (Fig. 2D), indicating that the three vaccines have distinct immunogenic properties for priming antibody, T cell, and B cell responses. Overall, adenoviral vector ChAdOx1 induced robust T-cell responses, although its antibody and B-cell responses were equal or lower than those by two types of mRNA vaccines. To visualize the T-cell biased responses, the ratios of the antibody responses over the CD4+ T-cell responses were plotted (Fig. 2E). Indeed, the ratios were lower in ChAdOx1 compared to BNT162b2 and mRNA-1273, while the numbers were equivalent among the mRNA vaccines. The similar analysis from CD8+ T cells revealed more profound reduction in ChAdOx1 compared to BNT162b2 and mRNA-1273 (Fig. 2F), supporting the adenoviral vector-directed biases for priming T-cell responses over antibody/B-cell responses.
Robust T-cell responses but attenuated antibody and B-cell responses following adenoviral vector priming. Immune responses were analyzed at 28 days after primary vaccination with BNT162b2, mRNA-1273, and ChAdOx1. Subjects with ≥ 40 years of age were analyzed. (A) Serum anti-spike titers were measured with ECLIA. (B) Neutralization titers against authentic SARS-CoV-2 virus were measured. (C) Frequencies of CD19 + CD20 + IgG + B cells which bind SARS-CoV-2 spike RBD were measured with flow cytometry. (D) Frequencies of spike-specific CD4 and CD8 T cells were measured using AIM assay. (E) Indicated humoral immune parameters were normalized to spike+ CD4 T cell frequencies. (F) Indicated humoral immune parameters were normalized to spike+ CD8 T cell frequencies. Data were analyzed by Kruskal-Wallis test and subsequent Dunn’s multiple comparison test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
ChAdOx1 vaccinees were composed of > 40 years adults only, whereas the mRNA vaccinees included < 40 years adults as well owing to the distinct age eligibility. To assess the possible biases posed by differential age distribution between the groups, we stratified the mRNA vaccinees into > 40 and < 40 ages (Fig. 3). Most immune parameters, except RBD B cells from BNT162b2, were comparable between two age groups in the mRNA vaccinees, showing the minor contribution of ages in these parameters. Indeed, the comparison between adenoviral vector and mRNA vaccinees > 40 ages reproduced the similar trend without the age adjustment (Fig. 2); robust T-cell responses but equal or lower antibody/B-cell responses were observed after adenoviral vector priming compared to mRNA priming. In sum, the T cell-biased responses over antibody/B-cell responses are adenoviral vector-dependent event rather than age-dependent events.
Immune responses induced by primary vaccination in young and aged subjects. Immune responses were analyzed at 28 days after primary vaccination with BNT162b2, mRNA-1273, and ChAdOx1. For mRNA vaccinees, subjects were classified into < 40 and ≥ 40 years of age were analyzed. (A) Serum anti-spike titers were measured with ECLIA. (B) Neutralization titers against authentic SARS-CoV-2 virus were measured. (C) Frequencies of CD19+CD20+IgG+ B cells which bind SARS-CoV-2 spike RBD were measured with flow cytometry. (D) Frequencies of spike-specific CD4 and CD8 T cells were measured using AIM assay. (E) Indicated humoral immune parameters were normalized to spike+ CD4 T cell frequencies. (F) Indicated humoral immune parameters were normalized to spike+ CD8 T cell frequencies. Data were analyzed by Kruskal-Wallis test and subsequent Dunn’s multiple comparison test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Comparable T-cell responses but attenuated antibody responses following homologous adenoviral vector booster
We next analyzed the immune parameters induced by the homologous and heterologous booster vaccination with any possible combination. The booster vaccinations recalled the comparable numbers of T-cells by any combination of adenoviral vector and mRNA vaccines from day 56 (day 28 after the boost) to day 168 (day 140 after the boost) (Fig. 4A). B cell numbers were also within the similar ranges among the different platform combination (Fig. 4B). In contrast, both spike-binding and neutralizing antibody titers from the homologous ChAdOx1 booster regimen were 5–19 folds lower than those from any other regimens that include mRNA platform for either priming or boosting at day 56 (Fig. 4C and D). These results indicate that the usage of mRNA vaccines for either priming or boosting as the heterologous regimens compensated the poor immunogenicity of homologous ChAdOx1 regimen on eliciting antibodies. Antibody titers elicited by heterologous booster regimens also persisted from day 56 to day 168 as well as those by homologous mRNA booster regimen (Fig. 4E and F). Besides the vaccine platform, the differential antibody titers following homologous booster regimen were notified between the mRNA vaccines (BNT162b2 vs. mRNA-1273), with higher antibody responses observed at days 56 and 168 from mRNA-1273 group (Fig. 4G and H). The superior immunogenicity in mRNA-1273 is observed from several studies28,29,30, possibly accounting for the higher antibody responses in this study.
Comparable T-cell responses but attenuated antibody responses following adenoviral vector priming and boosting. (A–D) Overall kinetics of spike-specific CD4 and CD8 T cells (A), RBD-reactive IgG B cells (B), serum anti-spike titers (C), and neutralization titers (D) were analyzed. Medians and IQRs were depicted. (E–H) Serum anti-spike titers (E, G) and neutralization titers (F, H) at 56 and 168 days after the primary vaccination (28 and 140 days after the booster vaccination, respectively) were analyzed. Data were analyzed by Mixed-effects analysis of time points (Time) and vaccine combination (Vac.) (A–D) and Kruskal-Wallis test followed by Dunn’s multiple comparison test (E–H) (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Differences in ages were included in as fixed effects the mixed-effects model.
Cytokine profiles of spike-reactive T cells
To investigate mechanisms underlying the differences in antibody responses induced by the ChAdOx1 vaccination, we analyzed the frequencies of circulating follicular helper T (cTfh) cells, which has been reported to correlate with antibody responses (Fig. S2C). After the primary vaccination, mRNA-1273 and ChAdOx1 induced higher frequencies of cTfh cells in total CD4 T cells (or in total PBMCs) than BNT162b2 did (Figs. 5A and S3A), which does not explain the difference in antibody titers at the primary vaccination. We confirmed the frequency of spike-specific cTfh cells were comparable among the three groups before the vaccination (Fig. S4A). Likewise, cTfh cells were almost comparable between the nine groups after the homologous and heterologous booster vaccination (Figs. 5B and S3B). Hence, we concluded that cTfh cell induction is not determinant for the differential antibody responses in our study.
Cytokine profiling of T cells. (A and B) Frequencies of spike-specific cTfh cells at 28 days after the primary (A) and booster (B) vaccination were analyzed. (C and E) Cytokines secreted into supernatants of AIM culture at 28 days after the primary (C) and booster (E) vaccination were analyzed. (D) Ratio of indicated cytokines were calculated for data from 28 days after the primary vaccination. In (A, C, and D), subjects with ≥ 40 years of age were analyzed. Data were analyzed by Kruskal-Wallis test and subsequent Dunn’s multiple comparison test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). In (E), the analyses were performed between the homologous ChAdOx1 group and the others.
To identify the T-cell derived correlates for differential antibody responses, we next analyzed cytokines secreted from spike-reactive T cells. We measured major cytokines produced by CD4 T cell subsets, including Th0 (IL-2), Th1 (IFN-γ), Th2 (IL-4 and IL-5), Th17 (IL-17A), and regulatory T (IL-10) cells. We confirmed the cytokine producing ability of spike-specific T cells were comparable among the three groups before the vaccination (Fig. S4B), indicating the differences below were induced by the vaccination. After the primary vaccination, the spike-reactive T cells showed different patterns of cytokine production, indicating that these vaccines induce qualitatively different helper T cell responses (Fig. 5C). Notably, ChAdOx1 priming induced robust IFN-γ-producing T cells at the levels higher than those by BNT162b2 and comparable to mRNA-1273. mRNA-1273 also elicited T cells that produce other cytokine, such as IL-2 and IL-5, which were not produced by spike-specific T cells induced by ChAdOx1. Indeed, the ratios of IFN-γ versus IL-2 or IL-5 revealed 2.4–5.5 or 2.3–4.3 folds skewing, respectively, of ChAdOx1 priming for IFN-γ production (Fig. 5D). Since the provision of IL-2 and IL-5 enhances the antibody responses from in vitro stimulated B cells, the results suggest that induction of IL-2 and/or IL-5 T cells may have contributed to higher antibody responses in the mRNA-1273 vaccinees. After the booster vaccination, the IL-2 and IL-5 production remained low in the homologous ChAdOx-1 group (Fig. 5E), while other groups receiving mRNA vaccines for either priming or boosting showed more elevated IL-2 and IL-5 production. Especially, IL-5 production was significantly lower in the homologous ChAdOx1 group compared with any other groups. Collectively, these results suggest that IL-5 levels in the stimulated culture may be one of correlates for attenuated antibody responses observed following homologous ChAdOx1 boosters compared to other booster regimens including mRNA vaccines for either priming or boosting.
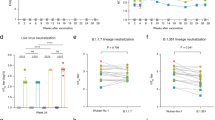
Durability of the IL-5-producing T cells is imprinted by the primary vaccine
Finally, we examined kinetics of the IL-5-producing T cells in each group (Fig. 6A). IL-5-producing T cells remained below the detection limit throughout the period following homologous ChAdOx1 booster; however, IL-5-producing T cells were elicited above the detectable levels in heterologous booster groups at day 28 (Fig. 6A), indicating the usage of mRNA vaccines for either priming or boosting compensates the immunogenic properties for eliciting IL-5-producing T cells above detectable levels, similar to the antibody titers. Of note, IL-5-producing T cells detected at day 28 in the heterologous booster (adenoviral vector/mRNA) groups waned to undetectable levels over time (Fig. 6B). In contrast, the heterologous booster groups with reversed vaccination order sustained higher numbers of IL-5-producing T cells, showing the adenovirus vector priming rather than boosting affected more profoundly on the waning of IL-5 T cells, the phenomenon similar to immunological imprinting. Such imprinting was specific to the IL-5-producing T cells since cTfh cells and IL-2-producing T cells were sustained throughout the period (Fig. 6C and D).
Durability of the IL-5-producing T cells is imprinted by the primary vaccine. (A) Overall kinetics of IL-5 secreted into supernatants of AIM culture were analyzed. Medians and IQRs were depicted. (B) IL-5 secreted into supernatants of AIM culture at 168 days after the primary vaccination (140 days after the booster vaccination) were analyzed. (C and D) Overall kinetics of cTfh cell frequencies (C) and IL-2 secreted into supernatants of AIM culture (D) were analyzed. Medians and IQRs were depicted. Data were analyzed by Mixed-effects analysis of time points (Time) and vaccine combination (Vac.) (A, C, and D, *P < 0.05, **P < 0.01, ****P < 0.0001) and Kruskal-Wallis test followed by Dunn’s multiple comparison test (B, groups not sharing a common letter are significantly different (P < 0.05)). Differences in ages were included in as fixed effects the mixed-effects model.
Discussion
We have performed the immune profiling analysis following heterologous booster regimens with a combination of adenoviral vector and mRNA vaccines, and demonstrated the distinct immunogenic properties by adenoviral vector and mRNA vaccines, particularly in the elicited T-cell functionality and durability. Adenoviral vector priming induced T-cell responses characterized by robust IFN-γ production with an attenuated IL-2 and IL-5 production and anti-spike antibody titers. This cytokine skewing appears to have long-lasting effects, as the IL-5 production detected in this study were significantly less durable in individuals primed with the ChAdOx1 vaccine, even after a subsequent mRNA vaccine boost.
IL-2 and IL-5 have been reported to be involved in humoral immune responses. IL-5 is a well-established type 2 helper cytokine for humoral immune responses, inducing B cell differentiation into antibody-secreting cells31. Although IL-2 is not so established for B cell help functions as IL-5, several studies revealed that IL-2 promotes B cell proliferation and differentiation into antibody-secreting cells32,33,34. Hence, the attenuated IL-2 and IL-5 T cell responses in adenoviral vector vaccination may contribute to the modest antibody responses.
One of the key implications in this study is to shed new lights on the concept of “immunological imprinting” depending on the initial vaccine platform. The ChAdOx1 adenoviral vector vaccine not only influenced the magnitude of the T-cell response but also appeared to establish a prolonged bias in T-cell functionality that persisted at least after the boosting with mRNA vaccine platform. This suggests that the initial exposure to a particular vaccine platform could influence the quality of the immune response to future vaccinations, underscoring the need to understand the immunogenic properties of individual vaccine platforms for applying heterologous vaccine regimens. The balance of T cell responses is regulated by innate antigen-presenting cells and microenvironmental milieu, such as cytokines35,36. Our results showed that the primary dose of ChAdOx-1 induced higher production of IFN-γ by spike-stimulated T cells, which could skew Th balance towards Th1. Further, one dose of ChAdOx-1 has been reported to induce trained immunity, where monocytes isolated from ChAdOx-1 vaccinees highly produced IFN-γ in response to stimulation for months after the vaccination37. Hence, one possible scenario is that the IFN-γ-producing T cells and monocytes induced by primary ChAdOx-1 dose dampen Th2 cell response and impair durability of IL-5-producing T cells after heterologous mRNA booster dose.
Our results also stress the importance of immune profiling studies not only for quantitating neutralizing antibody titers but also for detecting the multiple parameters associated with vaccines-elicited protective immunity. Although neutralizing antibody titers serve as one of immune correlates of protection in individuals who have completed vaccination regimens (COVID-19, influenza, rabies, mumps, and more)38, the other immune parameters, such as non-neutralizing antibody titers, B-cell and T-cell responses are also suggested to contribute to VE15. Notably, the VE for severe COVID-19 is maintained for long periods even after the waning of neutralizing antibody titers16, supporting the contribution of immune parameters other than neutralizing antibody titers for durable protection. The prolonged skewing of T-cell cytokine production observed in our study suggests that vaccine strategies need to be tailored not just to elicit neutralizing antibody responses in the early time points, but also to ensure that T-cell and B-cell responses are well-balanced and capable of supporting long-term immune protection which is contributed by multiple layers of immune responses as represented by antibody, B-cell, and T-cell responses.
In conclusion, this study highlights the important roles of the initial vaccine platform in shaping the long-term immune responses, with significant implications for the design of future vaccination regimens. The concept of vaccine-induced T-cell imprinting should be taken into consideration when planning booster doses at least one time, as it could influence the durability of the protective immune response and VE as well. Further research into the mechanisms underlying this imprinting and its impact on other aspects of the immune response, such as antibody and B-cell helper function, will be essential for optimizing vaccine strategies in the emerging and re-emerging infectious diseases.
Limitations of the study
We analyzed combinations of three COVID-19 vaccines to compare immunogenic differences of vaccine platforms with well-coordinated cohorts for comparable study. For more generalizable information about immunogenic properties of vaccine platforms, further analyses on cohorts with other vaccine platforms are needed. We also did not trace the cohorts after third vaccine dose. Further studies are needed to examine how much extents the imprinting effects of ChAdOx1 priming on IL-5-producing T cells can be alleviated by an additional mRNA vaccine booster or not. Our study compared the effects of vaccines at clinically used dose (30 µg of BNT162b2, 100 µg of mRNA-1273, and 5 × 1010 viral particles of ChAdOx1) to evaluate immune responses in real world. The differences in antigen dose may have affected the immune responses, e.g., higher antibody responses in mRNA-1273 than BNT162b2. The responses may also be affected by differences in conformation of spike antigen, as two-proline mutations are introduced in BNT162b2 and mRNA-1273 to stabilize prefusion conformation of the spike39, which increase antibody titer40. Further detailed study will be needed for elucidating differences in immune responses induced by each vaccine platform.
Methods
Human participants
Healthy volunteers were recruited and grouped into the nine regimens in order of their first visit. Only the participants with age of > 40 years were included in groups where participants received ChAdOx1 as a primary or booster vaccine. We obtained written informed consent from all the participants before enrollment. The vaccination was conducted in double-blind. This study was approved by Certified Review Board of National Center for Global Health and Medicine (NCGM-C-004337-00). All methods were performed in accordance with the principles of the Declarations of Helsinki.
Sample preparation
Blood samples were longitudinally collected from the participants at PS Clinic (Fukuoka, Japan). For serum samples, blood was collected in Venoject II (Terumo) and left at room temperature for 30 min. After centrifuge at 4 °C and 3000 rpm for 30 min., serum samples were collected and stored at -80 °C. PBMCs were prepared as previously described41. Briefly, blood was collected in Vacutainer CPT tubes (BD biosciences), followed by centrifugation at 1800 g for 20 min. PBMCs suspended in plasma were transferred into conical tubes followed by further centrifugation at 300 g for 15 min. PBMC pellets were washed with PBS three times followed by cryopreservation in CELLBANKER 1 plus (Zenogen pharma).
ECLIA
Serum anti-spike and nucleocapsid protein antibody titers were measured as cut-off index (COI) values using Cobas e411 (Roche) with Elecsys Anti-SARS-CoV-2 S (Roche) and Elecsys Anti-SARS-CoV-2 (Roche), respectively, according to the manufacturer’s instruction.
Viral neutralization assay
Serum neutralization titers against authentic SARS-CoV-2 viruses were measured as described previously9,42. Briefly, serum samples were serially diluted (2-fold dilutions starting from 1:5) in Dulbecco’s modified Eagle’s medium (D-MEM) supplemented with 2% fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin, and were mixed with 100 TCID50 SARS-CoV-2 WK-521 (hCoV-19/Japan/TY-WK-521/2020, ancestral strain), followed by incubation at 37 °C for 1 h. The virus-plasma mixtures were placed on VeroE6/TMPRSS2 cells (JCRB1819) seeded in 96-well plates and cultured at 37 °C with 5% CO2 for 5 days. After the culture, the cells were fixed with 20% formalin (Fujifilm Wako Pure Chemicals) and were stained with crystal violet solution (Sigma-Aldrich). The mean cut-off dilution index with > 50% cytopathic effect from 2 to 4 multiplicate series was presented as the neutralizing titers.
Flow cytometry
RBD-reactive B cells were analyzed by flow cytometry as described previously43. Briefly, cryopreserved PBMCs were thawed at 37 °C and immediately washed with D-MEM supplemented with 2% FBS. The PBMCs were suspended in D-MEM supplemented with 2% FBS containing the spike- and RBD-probes and 10 µM biotin, followed by incubation for 30 min at room temperature. The cells were then incubated with D-MEM supplemented with 2% FBS containing fluorochrome-conjugated antibodies against surface antigens, 10 µM biotin, and Brilliant stain buffer plus (BD Biosciences), and incubated for 30 min at room temperature followed by washing. The cells were suspended in D-MEM supplemented with 2% FBS. Flow cytometry was performed with FACSymphony A3 (BD Biosciences). Data were analyzed with FlowJo software (BD Biosciences).
AIM assay
Spike-specific T cells were analyzed using AIM assay as previously described44. Briefly, cryopreserved PBMCs were suspended in R10 medium [RPMI1640 supplemented with 10% heat-inactivated human AB serum (Sigma-Aldrich), 10 mM Hepes (Thermo Fisher Scientific), 1% minimum essential medium non-essential amino acid (Thermo Fisher Scientific), 1 mM L-glutamine (Thermo Fisher Scientific), and 100 U/mL penicillin/streptomycin (Thermo Fisher Scientific) ] and incubated at 0.5–1.5 × 106 cells/well in 96-well U-bottom plates with or without overlapping (15 oligomers with 11 amino acids overlap) peptide pools spanning the Wuhan spike glycoprotein (spike-OLPs) at 37 °C for 16 h in a 5% CO2 incubator. The spike-OLPs were purchased from JPT (PM-WCPV-S-1) and Myltenyi Biotec (PepTivator SARS-CoV-2 Prot_S Complete), reconstituted with dimethyl sulfoxide (DMSO) and distilled water, respectively, and used at a final concentration of 1 µg/ml. Supernatants were harvested for cytokine quantification. Cells were washed with staining buffer (PBS supplemented with 2% fetal bovine serum and 0.01% NaN3), incubated with Fc Receptor Blocking Solution (Human TruStain FcX, Biolegend) on ice for 20 min, and then stained for 1 h on ice with the following antibodies: CD3-BUV805 (SK7, BD Biosciences), CD4-BV480 (SK3, BD Biosciences), CD8-BUV496 (RPA-T8, BD Biosciences), CD69-FITC (FN50, BioLegend), CD137-APC (4B4-1, BioLegend), and CD185-PE-Cy7 (J252D4, BioLegend). After staining, cells were washed twice with staining buffer and then subjected to flow cytometry using a FACSymphony A3 (BD Biosciences). Data were saved as FCS files and analyzed using FlowJo software (v. 10.8.0, BD Biosciences), and the frequencies of spike-specific CD4 T cells and cTfh cells in total CD4 T cells, and spike-specific CD8 T cells in total CD8 T cells were calculated. We also calculated the frequency of cTfh cells in total PBMCs.
The concentrations of cytokines secreted into the culture supernatant were quantified using the cytometric bead array kit (BD Biosciences) according to the manufacturer’s instructions. Culture supernatant was diluted two-fold for analysis. Data were acquired using a FACSCanto II cytometer (BD Biosciences) and analyzed using FCAP Array Software Version 3.0 (BD Biosciences). The cytokine quantities in the supernatant were normalized by the number of PBMCs. The data represent the amount of cytokines produced by one million PBMCs.
Statistical analysis
Statistical analyses were performed using Prism software (GraphPad). Detailed methods used for the statistical analyses are indicated in figure legends.
Materials availability
All unique and stable materials generated in this study are available from the lead contact under a Material Transfer Agreement.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Goel, R. R. et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 374, abm0829 (2021).
Painter, M. M. et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity https://doi.org/10.1016/j.immuni.2021.08.001 (2021).
Cho, A. et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 600, 517–522 (2021).
Sokal, A. et al. mRNA vaccination of Naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity 54, 2893–2907 (2021).
Mazzoni, A. et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J. Clin. Invest. 131, e149150 (2021).
Tong, P. et al. Memory B cell repertoire for recognition of evolving SARS-CoV-2 Spike. Cell 184, 4969–4980 (2021).
Goel, R. R. et al. Distinct antibody and memory B cell responses in SARS-CoV-2 Naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 6, eabi6950 (2021).
Mateus, J. et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science eabj9853 (2021).
Kotaki, R. et al. SARS-CoV-2 Omicron-neutralizing memory B-cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci. Immunol. eabn8590 (2022).
Takano, T. et al. Distinct immune cell dynamics correlate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. Cell. Rep. Med. https://doi.org/10.1016/j.xcrm.2022.100631 (2022).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 375, 43–50 (2022).
Earle, K. A. et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 39, 4423–4428 (2021).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Arashiro, T. et al. COVID-19 vaccine effectiveness against severe COVID-19 requiring oxygen therapy, invasive mechanical ventilation, and death in Japan: A multicenter case-control study (MOTIVATE study). Vaccine 42, 677–688 (2024).
Schultz, N. H. et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 384, 2124–2130 (2021).
Greinacher, A. et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 384, 2092–2101 (2021).
Nordström, P., Ballin, M. & Nordström, A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: A nationwide cohort study. Lancet Reg. Health Eur. 11, 100249 (2021).
Pozzetto, B. et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature 600, 701–706 (2021).
Shoemaker, K. et al. Long-term safety and immunogenicity of AZD1222 (ChAdOx1 nCoV-19): 2-year follow-up from a phase 3 study. Vaccines 12, 883 (2024).
Barnes, C. O. et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588, 682–687 (2020).
Zost, S. J. et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 584, 443–449 (2020).
Piccoli, L. et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 183, 1024–1042e21 (2020).
Rogers, T. F. et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 369, 956–963 (2020).
Andreano, E. et al. Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. Cell 184, 1821–1835 (2021).
Cao, Y. et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 182, 73–84 (2020).
Dickerman, B. A. et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in US Veterans. N. Engl. J. Med. 386, 105–115 (2022).
Puranik, A. et al. Comparative effectiveness of mRNA-1273 and BNT162b2 against symptomatic SARS-CoV-2 infection. Med 3, 28–41e8 (2022).
Abu-Raddad, L. J., Chemaitelly, H., Bertollini, R. & National Study Group for COVID-19 Vaccination. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N. Engl. J. Med. 386, 799–800 (2022).
Takatsu, K. Interleukin 5 and B cell differentiation. Cytokine Growth Factor Rev. 9, 25–35 (1998).
Mingari, M. C. et al. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature 312, 641–643 (1984).
Le Gallou, S. et al. IL-2 requirement for human plasma cell generation: Coupling differentiation and proliferation by enhancing MAPK-ERK signaling. J. Immunol. 189, 161–173 (2012).
Hipp, N. et al. IL-2 imprints human Naive B cell fate towards plasma cell through ERK/ELK1-mediated BACH2 repression. Nat. Commun. 8, 1443 (2017).
Zhou, L., Chong, M. M. W. & Littman, D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Geginat, J. et al. Plasticity of human CD4 T cell subsets. Front. Immunol. 5, 630 (2014).
Murphy, D. M. et al. Trained immunity is induced in humans after immunization with an adenoviral vector COVID-19 vaccine. J. Clin. Invest.. https://doi.org/10.1172/JCI162581 (2023).
Plotkin, S. A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17, 1055–1065 (2010).
Wrapp, D. et al. Cryo-EM structure of the 2019-nCoV Spike in the prefusion conformation. Science 367, 1260–1263 (2020).
Mercado, N. B. et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586, 583–588 (2020).
Kotaki, R. et al. Repeated Omicron exposures redirect SARS-CoV-2-specific memory B cell evolution toward the latest variants. Sci. Transl. Med. 16, eadp9927 (2024).
Moriyama, S. et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity 54, 1841–1852 (2021).
Onodera, T. et al. CD62L expression marks SARS-CoV-2 memory B cell subset with preference for neutralizing epitopes. Sci. Adv. 9, eadf0661 (2023).
Terahara, K. et al. SARS-CoV-2-specific CD4+ T cell longevity correlates with Th17-like phenotype. iScience 25, 104959 (2022).
Acknowledgements
We thank Akira Dosaka, Eriko Izumiyama, Rieko Iwaki, Megumi Koda, Saori Tachibana, and Ryoko Itami at Research Center for Drug and Vaccine Development, NIID for their technical support. We thank Akiko Sataka and Rena Sakamoto at Department of Pathology, NIID for their support for the antibody measurement. We also thank Hiroko Kumashiro at Clinical Research Division, SOUSEIKAI medical group for coordinating clinical research. This study was supported by the Japan Agency for Medical Research and Development (JP21nf0101637 to TS, YT, and TT, JP24gm1810004 to YT, JP243fa627005 to YT, JP243fa627009 to YT, and JP243fa727002 to YT).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.H., T.S., Y.T., and T.T. Funding acquisition: Y.H., T.S., Y.T., and T.T. Investigation: M.I., T.O., A.A., R.K., T.K., S.S., M.T., K.T., M.H. Methodology: M.I., T.O., and A.A. Project administration: T.S., Y.T., and T.T. Resources: M.H., Y.H., T.S., Y.T., and T.T. Supervision: T.S., Y.T., and T.T. Visualization: M.I., T.O., A.A., and R.K. Writing – original draft: R.K. and Y.T. Writing – review & editing: M.I., T.O., A.A., T.K., S.S., M.T., K.T., M.H., Y.H., T.S., and T.T.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Isogawa, M., Onodera, T., Ainai, A. et al. Prolonged effects of adenoviral vector priming on T-cell cytokine production in heterologous adenoviral vector/mRNA COVID-19 vaccination regimens. Sci Rep 15, 18684 (2025). https://doi.org/10.1038/s41598-025-00054-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00054-x