Abstract
With the emphasis on sustainable health, understanding the neural dynamics associated with sustainable practices such as widely practiced yoga has gained significant importance. In this work, we explored the underlying neural mechanisms of yoga training by means of electroencephalogram recordings. The EEG data was recorded before and after the yoga training of 13 participants, for a total of 39 trials, with each trial recorded on consecutive days. The temporal analysis was performed by means of microstates and the changes in the oscillatory rhythms were also evaluated via spectral and statistical analysis. Spectral analysis revealed changes in the oscillatory rhythms of β,γ,α,θ over the electrode regions of O2, P8 and FC6. An analysis of the changes in the temporal microstates revealed > 65% global variance in the topographic clusters, with a significant effect on the occurrence and time coverage parameters of the microstates before and after yoga training. This study highlights that yoga training significantly influences microstate dynamics associated with brain regions, including the visual network, insular cortex, and frontal gyrus, thereby potentially enhancing functions related to attention and cognitive decisions. These findings may suggest a multinetwork neurophysiological basis for the role of yoga in improving mental focus and adaptive decision processes.
Similar content being viewed by others
Introduction
The impact of physical exercise has been documented by studies highlighting improved health benefits related to working memory and attention1 and has an important role in preventing coronary artery disease2 and other cardiovascular diseases, managing elevated triglyceride levels, and hypertension3. Some of these physical exercises embed themselves within traditions and have also been studied as mind-body practices, such as tai chi4,5,6, hatha yoga7,8 and meditation9,10,11. Studies on these practices reported optimal training of motor functions such as postural control12, fall prevention13, agility14, muscle strength15,16 and better cognitive performance17,18 among practitioners. These mind-body practices induce physiological and anatomical changes in the brain. For example, tai chi intervention in older adults induced significant changes in cortical thickness, functional connectivity, homogeneity of the brain, and executive network neural function14 and had a strong effect on brain plasticity, resulting in an increase in gray matter in the left middle occipital gyrus, left superior temporal gyrus and right middle temporal gyrus and an increase in functional connectivity19.
Traditions of yoga have been rooted in ancient Indian philosophy as a comprehensive practice focused on harmony between the body and mind20,21,22,23. Yoga exercises involve dynamic postures and movements that require coordination, balance and flexibility, engaging multiple sensory and motor systems that stimulate various regions of the brain involved in motor control24,25,26,27. Studies have indicated that physical exercise, such as yoga, has positive effects on several pulmonary28, respiratory29,30 and neurological disorders31,32.
A longer duration and consistent practice of yoga have been reported to enhance health markers and cardiovascular health as well as reduce anxiety and stress33,34. Decreased levels of anxiety35, depression36 and improved emotional well-being have also been reported in relation to the practice of complex movements. By influencing synaptic plasticity in the brain, yoga-like techniques37 have improved working memory functions38,39. A study also revealed enhanced coherence and symmetry among the two brain hemispheres, which was attributed to improved neurocognitive functions40. Another study41 examined the neurobiological correlates of yogic practices by imaging techniques and reported variations in gray matter volume structurally and attributing changes to prefrontal cortex regions compared with control subjects who did not practice such methods. A similar influence has been observed with a bilateral increase in hippocampal size following a 6-month history of yoga42.
The long-term use of techniques such as Sahaja yoga has been associated with functional changes in connectivity, enhancing frontal attention and the default mode network (DMN) in the resting state43. The DMN is composed44 of the medial prefrontal cortex, posterior cingulate cortex, dorsal medial prefrontal cortex, salience network (anterior insula and anterior cingulate cortex) and angular gyrus and plays a key role in function when an individual is at rest or is not attentionally focused on the external environment. Several of these regions45 have increased activity for internally focused thought processes46, such as self-reflection, mind wandering and the resting state, and reduced activity in these regions indicates increased effort in cognitive tasks such as attention and decision making47.
An increase in delta activity within the centroparietal regions48, as well as an increase in alpha activity across frontal, central and parietal electrode regions, has been observed after yoga practice. It was also observed that there were faster auditory reaction times with the P300 ERP after 8 weeks of yoga training. Another review-based study49 reported that the practice of yoga influences cerebral activity by reflecting changes in alpha, beta, and theta oscillatory patterns, which subsequently augment memory retention and alleviate anxiety. A 12-week yoga study50 assessing N200 and N450 ERP components before and after yoga training across healthy controls and type 2 diabetes patients reported improved cognitive performance through mechanisms that improved glycemic control.
Neural activity dynamics have been analyzed by segmenting EEG time series into several clusters and defining each cluster as stable states for a period referred to as microstates51. The microstates are thought to reflect the functional integration and synchronization of neural networks, offering a window into the underlying functional architecture of the brain52. By examining changes in microstate parameters, such as duration, frequency, and topographical characteristics, researchers can discern alterations in cognitive processing and mental states induced by external stimuli or internal practices53,54. Microstate analysis entails the segmentation of data into brief epochs of stable topographies, representing transient states of neural functioning51,55,56. In one study57, four microstates were reported (A: orientations of the right frontal to left posterior cortical micro zones; B: orientations of the left frontal to right posterior cortical micro zones; C: orientations of the frontal to occipital cortical micro zones; D: orientations of the fronto-medial to occipital cortical micro zones), which were mapped to different regions of the brain (see Table 1). Two more topographic orientations (E: left to right cortical micro zones and F: posterior medial to frontal cortical micro zones) have been added58. Studies have reported that the characteristics of MS time series vary across behavioral states59, personality types and neuropsychiatric disorders.
It is crucial to understand how sustainable practices such as yoga can contribute to health and wellness for both individuals and communities through a better comprehension of its brain correlates, and to our knowledge, a study of the effects of yoga on brain microstates (MSs) has never been performed yet.
In this work, to study qualitative and quantitative neural dynamics before and after yoga, we analyzed the impact of a fifteen-minute yoga training sequence known traditionally as sun salutation postures8,60,61,62,63 and evaluated its impact via brain microstate analysis and EEG spectral variations. This study explores the distinct functional connectivity changes that are regional to networks during the resting state after short-term yoga training.
Results
Yoga training leverages global brain States in circuit regions related to microstates B and D
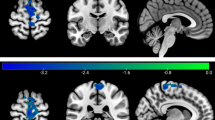
From the data, clustering indicated 4 topographies as the optimal number of clusters with a combined variance of 68%. Before yoga training, GEV reported 68.5%, and 66% GEV reported after yoga. The cluster maps representing the different orientations observed were A, B, C & D (see Fig. 1a (i)) before yoga training and B, C, D, & E after yoga training (see Fig. 1a (ii)). Microstates B has shown a decrease of GEV 16–5% after yoga training (see Fig. 1b).
The microstates B (strong activation in the left and right cortices, including BA 17, 18 & right insula) and D (strong activation in the right inferior parietal lobe & right insula (BA13)) revealed significant alterations after yoga training (see Fig. 1c), and the parameter occurrence of the microstate B map decreased from 1.47 Hz (± 0.346) to 0.628 Hz (± 0.510) per second, p = 0.0048. Similarly, microstate D decreased from 0.679 Hz (± 0.443) to 0.154 Hz (± 0.259) after yoga training, p = 0.013. In this study, the temporal coverage (see Fig. 1d) of microstate B decreased from 38.3% (± 15.8%) to 10.5% (± 9.5%), p value = 0.0008. Microstate C (attributed to the posterior cingulate cortex) was associated with an increase in neural activation from 25.2% (± 14%) to 67.7% (± 16%), p value = < 0.0001.
After yoga training, microstate C increased in duration (see Fig. 1e) from 187 ms (SD ± 71) to 563 ms (SD ± 311), p = 0.0096. Tukey’s multiple comparison analysis revealed a difference between the occurrence of microstates and time coverage (see Table S2 with p values).
Microstate transition probabilities attenuated after yoga training
In this study, we observed the transition probabilities before and after yoga training and found a decrease in the probability of a transition from one MS to another (see supplementary data Table S6 for the percentage of observed transitions). The t test results revealed a significant decrease in the transition probability for microstate B as a node [B →◊D t (13) = 2.98, p value = 0.011] to the microstate D transition after yoga training (see Fig. 2). Similarly, we observed a significant decrease in the number of network transitions with microstate D as a node [D →◊B t (13) = 3.58, p value = 0.003] after yoga training (see supplementary data Table S7 for other t test results).
Diminished variance across temporal activity after yoga training
The temporal variability across microstate B networks after yoga was lower than that across relaxed state networks before yoga (p = 0.0022). However, the temporal variability of microstate C networks after yoga was greater than that before yoga training (p = 0.0010) (see Fig. 3a). Similarly, we observed a decrease in temporal variability across microstate D networks after yoga (p = 0.0022). However, spatial variability (Fig. 3b) was not observed.
Spatial configuration of MSs and analysis of temporal parameters. (a) Topographic plots of the MSs. (i) Data from before yoga training show cluster maps of A, B, C & D, and (ii) data from after yoga training reveal cluster maps of B, C, D & E. Estimated GEV (b), occurrence (c), time coverage (d), and mean duration (e) from four MSs before and after yoga training. Maps B & D decreased in frequency after yoga training from 1.47 Hz (± 0.346) to 0.628 Hz (± 0.510) (p = 0.048). (Gray color indicates pre-yoga, and orange color indicates post-yoga). The decreased occurrence of MSs may suggest enhanced attention and focus after yoga training among subjects.
Transition probabilities before (gray color) and after (orange color) yoga training (p values of 0.0332 (*), 0.0021 (**), 0.0002 (***)). The decreased probability of transition (from B →◊D t (13) = 2.98, p value = 0.011) was not significant (to 3% after yoga training). The transition may implicate changes in network activity from visual to salience network regions.
Functional connectivity networks assessed before and after yoga training. (a) The temporal variability of microstate B decreased from 0.994 to 0.985, and that of microstates C & D decreased from 0.99 to 0.96. (b) The spatial variability for microstate classes B, C, & D did not indicate any major differences. The box plot left of each pair shows variability before yoga training, and the box plot on the right of each pair shows variability after yoga training (** indicates a significant difference with p < 0.05, *** indicates a significant difference with p < 0.001).
Reduced Β and γ activity observed after yoga training
The spectral values over different frequency ranges (for data, see Table 2) before and after yoga training were analyzed (see Fig. 4a and b for comparisons). We observed an increase in alpha rhythms in the occipital area (see Fig. 4b) and a decrease in beta rhythms in the frontal regions after yoga training. The study revealed a comparable reduction in activity within the gamma frequency spectrum after yoga training. Furthermore, the relative percentage was estimated across θ, α, β, and γ oscillations (see Fig. 4c). The relative percentage difference after yoga training indicated a decrease in θ, α, β, and γ oscillations in the F7 and F3 regions (see Fig. 4c). However, we observed increases in the α, β, and γ oscillations in AF3 and AF4. Additionally, we observed an increase in θ oscillations for the FC5, T7, P7, P8, and T8 regions. The FC6 region also indicated an increase in all oscillatory rhythms (see Fig. 4c). β and γ decreased in percentage after yoga in electrode regions F3, FC5, T7, and P7.
There was a statistically significant difference before and after yoga training across electrodes O2, P8 and FC6. The ANOVA findings revealed significant differences in the O2 electrode region (p value 0.044). The nonparametric Wilcoxon test (W) and Mann‒Whitney test (U) revealed that the P8 (p value: 0.047, 0.041) and FC6 (p value: 0.0006, 0.007) regions significantly differed after yoga training (see Table 3 for further values).
Relative power spectral density before and after yoga training (a & b). Frequency domain power topography plots before and after yoga training averaged across all the participants. The power from 5 frequency bins (0–4;4–8; 8–13;13–30;30–45 Hz) before yoga training and after yoga training was averaged over 2 s. The activity distribution before and after yoga clearly differed in the frequency ranges of α, β and γ. (c) The relative percentage changes observed before and after yoga training decreased in the frontal regions at the α,β, and γ frequencies.
Discussion
The present study aims to highlight qualitative and quantitative neural dynamics before and after yoga training. The spectral data revealed that the electrode regions of O2, P8 and FC6 were significant for all the frequency oscillatory rhythms. The P8 electrode region is highly likely localized to regions of the inferior lateral occipital cortex, superior lateral occipital cortex, and angular gyrus (ANG)64; these regions are more involved in object recognition, processing visual stimuli, and interconnect with attentional control and memory. The FC6 electrode region is also highly likely localized to regions of the pre- and postcentral gyri, which are known as the primary somatosensory cortex involved in controlling different bodily movements, and the middle frontal gyrus involved in cognitive functions related to executive control, working memory, attention, and decision-making.
According to previous studies, increased α rhythms in the occipital region indicate the deceleration of cerebral problems and mental stress65. Indeed, EEG-based feedback can be used for training to control alpha band activity, which helps reduce stress levels and mental health66. Practicing yoga may thus help increase α band activity, as we observed significant changes in the mean values. The observed decrease in β oscillations after yoga training may be indicative of alertness, attention and cognitive processing improvements67. Studies have also indicated that increased β activity is correlated with anxiety disorders40,49.
In this study, lower gamma oscillations in the frequency range of 30–60 Hz were reported. Associated with complex tasks and observed when the brain is actively engaged during the states of concentration and heightened focus, the decrease in gamma activity after yoga training could implicate the default mode network: dorsal attention may modify mental attention and the focus mechanism that contributes to how mental fatigue stress may be handled. The increased α oscillations and decreased β and γ oscillations may suggest that yoga training contributes to functional integration in the frontal and parietal networks and may be key in improving mental performance68.
Although the microstate analysis was performed at the level of sensors, the results showed divergent temporal microstates profiles and transition patterns for the sensor regions pertaining to pre and post yoga training data. Studies58,59 have already correlated localization of specific EEG microstates to particular brain regions and in the context of such mapping, this study suggests significant post-yoga training changes in microstates B and D and can be attributed to the regions of right superior and middle frontal gyrus, right superior and inferior parietal lobule, right insula, claustrum, right frontal eye field and cuneus regions.
Microstates B and D had shown decreases across occurrences and time coverages after yoga training. Microstates B and D has been known to involve visual networks including the right and left occipital cortex and right insular cortex and suggesting that fontal and left frontal to right posterior networks exhibited more activity after yoga training. Transition probability and decreased temporal variability related to microstates B and D may suggest that performing Surya namaskar (the yoga practice) correlated to a reduction in neural activity in the regions. The observations from our studies may suggest that there could be an engagement of default mode networks (DMN) attributing changes previously reported as microstates B, C, D and could have links to cognitive processing. The probable transitions from microstates B to D may also imply a change across the visual network to the saliency network56. The increased occurrence of stable microstates orientations through left frontal to right posterior and frontal to occipital orientations is more prevalent after yoga training.
Short-term yoga training has shown relaxing effects and increased focused attention in other studies69,70. Our study on brain activity effects of short-term yoga training reinforce the idea that it could be useful for promoting health and quality of life.
Conclusion
In this study, we analyzed the impact of sustainable short-term yoga practice on global MSs along with the spectral changes in EEG recordings. Our pilot study strongly suggested that performing yoga exercises (Surya namaskar) for daily health may result in short-term variations across the network regions of attention and dominance in visual areas such as the frontal eye field or insular region and the frontal gyrus, which may be interpreted as functions related to attention, cognitive decisions and sensory processing. A longitudinal study with a larger sample size and a longer duration might be critical to evaluate how these networks change across time and across individuals with different age groups.
Methods
Thirty participants aged 20–54 years with a mean age of 42.3 years were recruited for this study, and only data from 13 participants (mean = 34.76 years, 3 trials/subject, 4 male, 9 female; for data, see Table S1) were considered due to impedance errors, sample loss rate and lack of synchrony across electrodes caused by drift noise in the remaining subjects. The study was reviewed and approved by the Institutional Ethics Committee (IEC), Amrita Vishwa Vidyapeetham and were carried out in accordance with their guidelines and regulations. All the subjects involved in the study were informed prior to the objective of data acquisition, and signed informed consent was obtained from all the participants before the protocol was administered. EEG signals were acquired from the subjects via a 14-electrode device (EMOTIV EPOC+) with a sampling rate of 128 Hz.
In this study, the advantage of using consumer grade 14 electrode device was the portability which facilitated data acquisition across subjects. Additionally, its ease of use and minimal setup requirements made it appropriate for non-clinical studies or EEG-based behavioral assessments with large participant samples. However, there were certain limitations compared to high density EEG systems. A 14-electrode device provided significantly lower spatial resolution and had lower sensitivity in data acquisition. Despite these limitations, we determined that for the current microstate analysis on some protocols, these consumer grade devices provided a reliable and faster method to collect valid data71,72,73.
In this study, the computational analysis of all 39 recording trials from 13 subjects was performed via Python (3.11.4), the MNE library (1.5.0)74, NumPy (1.25.2)75, Matplotlib (3.7.1)76 and SciPy (1.11.2)77 on an Apple computer.
Study design
The participants performed yoga training, which involved a set of dynamic postures, including breath control, beginning in the late 1920s, as described earlier8,78. The data were acquired by asking the subjects to close their eyes and relax before yoga training and after yoga training; each subject was asked to perform dynamic postures, which included four rounds of Surya namaskar asanas for a duration of fifteen minutes. After yoga training, the subject was asked to rest for 2 min, and later, the EEG data were acquired by asking the subject to close his or her eyes and relax. In this study, we recorded the data for 2 min and 40 s before and after yoga (see Fig. 5 for the schematic study design). For each subject, the trials were conducted on consecutive days. We limited this work to an experimental group alone, although the studies79,80,81 reported that, compared with the control group (for example, those that do not practice yoga), in this work, we included only the subjects who practice short-term yoga and compared them before and after practice.
Yoga training study design. The study design comprised a total duration of 22 min and 20 s and was divided into distinct phases. The initial phase consisted of prerecording (blue color) with the eyes closed, lasting 2 min and 40 s, which captured baseline measurements prior to yoga training. Following this, participants engaged in a 15-minute session of yoga training (green color), a series of 12 postures performed in a flowing sequence as in pictorial representation. After the training, there was a 2-minute break (gray color) to allow the participants to rest. Finally, a post-yoga recording (pink color) of 2 min and 40 s was conducted with the participants’ eyes closed. This process was repeated for all 39 trials (3 trials each on 13 subjects).
Artifact correction & signal processing
The raw data were extracted for 2 min 40 s, and within that data, 2 min of EEG data were considered for the analysis by filtering out the initial 20 s and the last 20 s. Offline data analysis was performed via custom-made python scripts imported into the ‘mne’ package74 (see Fig. 6a for noise removed preprocessed data). The data were high-pass filtered to minimize drift, and the reference mean was subtracted. Then, the data were detrended, and a band stop filter was used to remove line noise at 50 Hz.
A bandpass filter was used with overlap- add FIR based non-casual band pass filter in the frequency range of 1–50 Hz to obtain the spectral information, for microstates another band pass filtering was applied in range of 1 –20 Hz82 To remove the EMG or EOG artifacts from the data, we used independent component analysis (ICA). Spatial filtering was performed via the python package ‘Pycrostates’83. In this work, the signal processing methods were based on a previous study73.
Temporal dynamics of microstates
The measure of global field power (GFP) computes spatial variance, quantifying the amount of activity across all electrodes at a given point in time and quantifying the strength of the global electric field on the scalp51 (see Eq. (1)). GFP was estimated (Fig. 6b) for each individual trial, and microstate clusters were modeled for different n numbers of clusters (n = 4, n = 5, n = 6, n = 8). From the maxima peaks of the GFP, the clustering algorithm k-means84 was applied (using the ‘Pycrostates’ python package). For each subject and for each condition before training, k-means was repeated, varying from 4–8 clusters (see Fig. 6c) for workflow). The optimal number of clusters (see Fig. 6d) was chosen separately for each condition on the basis of the global explained variance56,59 (GEV) in this study; the data indicated 4 clusters, with the maximum GEV measuring the percentage of data variance in a given state of microstate85. The topographies of the microstate maps were obtained for each participant and averaged over n trials.
where global field power (GFP) is a measure of the root of the mean squared potential differences at all electrode regions K (i.e., Vi (t)) from the mean of potentials across electrodes (i.e., Vmean (t)), and t is the time point.
To obtain the MS parameters, the global maps were fitted back to the original data, and the original data were segmented on the basis of the MS labels. The microstate parameters57,59,86,87 were estimated from the topographies. The “mean duration” is defined as the duration of each microstate, i.e., the mean length of time during which a given microstate remains stable51; the time coverage of a microstate is the fraction of total recording time in which the microstate is dominant; and the ‘occurrence’ or ‘frequency of occurrence’ of each microstate is the average number of times per second the microstate becomes dominant during the recording period51,88. We have also evaluated microstate syntax51,57 which refers to the characteristic sequence and temporal dynamics of short stable (microstate) patterns of global brain activity that lasts for few hundred milliseconds before transition to another state.
Spatiotemporal variability of functional networks based on EEG MSs
The study employed segmented microstate windows in the microstate sequences to estimate the functional connectivity computed via the phase lag index as in a previous study (see Eq. (2))89. Phase‒lag synchronization estimations were computed between the range, with 0 indicating no connectivity and 1 indicating pro connectivity. The variabilities of the brain regions both temporally and spatially from the functional networks were estimated on the basis of an earlier recent study90.
where PLIm(i, j) indicates the functional connectivity for electrode channels i and j within the mth MS window (see Eq. 1), Δφ(t) is the instantaneous phase difference between two signals at time t, sign is the signum function, and ⟨⋅⟩ denotes the average over time at the mth MS window.
Spectral estimation
The spectral frequencies were labeled θ (4–7 Hz) , α (7–13 Hz), β (13–30 Hz), and γ(30–50 Hz) estimated using a multi-taper power spectral density(PSD) methods91,92. The PSDs were compared between the pre- and post-yoga training states as percentage changes.
Statistical analysis
A two-way repeated measure of ANOVA93 analysis along with Tukey’s94 post hoc pairwise comparisons were used to compare the significant differences in the microstate topography maps and the spectral oscillations across the electrode regions before and after sun salutation yoga training. Independent t-test95 was used for the temporal and spatial variability across the microstates and among the transition probabilities of microstates before and after sun salutation yoga training. Multiple non parametric tests of Mann-Whitney(U)96, Kruskal-Wallis test(H)97and Wilcoxon test98 were performed and compared along with repeated measures of ANOVA (F). The statistical tests were performed using the tool GraphPad prism99.
Illustration of global field power and microstate analysis for yoga training data. (a) Noise-removed preprocessed data. (b) Estimated global field power (GFP) across brain topologies. (c) The peaks of the GFP are used for clustering via algorithms such as k-means, typically from n = 4 to n = 8. (d) Fitting the cluster maps to the original data. (e) Microstates segmented from a trial in an epoch for a period of 1 s (x-axis is time in seconds).
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Please write to the contact author shyam@amrita.edu.
References
Boere, K., Lloyd, K., Binsted, G. & Krigolson, O. E. Exercising is good for the brain but exercising outside is potentially better. Sci. Rep. 13, 1140 (2023).
Thompson, P. D. et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Circulation 107, 3109–3116 (2003).
Pinckard, K., Baskin, K. K. & Stanford, K. I. Effects of exercise to improve cardiovascular health. Front. Cardiovasc. Med. 6, (2019).
Guyon, A. Benefits of Tai Chi for physical and mental health. Future Integr. Med. https://doi.org/10.14218/FIM.2023.00088 (2024).
Wu, Y., Wang, Y., Burgess, E. O. & Wu, J. The effects of Tai Chi exercise on cognitive function in older adults: A meta-analysis. J. Sport Health Sci. 2, 193–203 (2013).
Yu, Y., Zuo, E. & Doig, S. The differential effects of Tai Chi vs. Brisk walking on cognitive function among individuals aged 60 and greater. Front. Hum. Neurosci. 16, 821261 (2022).
Gothe, N. P., Kramer, A. F. & McAuley, E. The effects of an 8-week Hatha yoga intervention on executive function in older adults. J. Gerontol. Biol. Sci. Med. Sci. 69, 1109–1116 (2014).
Prasanna Venkatesh, L. & Vandhana, S. Insights on Surya Namaskar from its origin to application towards health. J. Ayurveda Integr. Med. 13, 100530 (2022).
Ahani, A. et al. Change in physiological signals during mindfulness meditation. Int. IEEEEMBS Conf. Neural Eng. Proc. Int. IEEE EMBS Conf. Neural Eng. 1738–1381 https://doi.org/10.1109/NER.2013.6696199 (2013).
Whitfield, T. et al. The effect of Mindfulness-based programs on cognitive function in adults: A systematic review and Meta-analysis. Neuropsychol. Rev. 32, 677–702 (2022).
Zeidan, F., Johnson, S. K., Diamond, B. J., David, Z. & Goolkasian, P. Mindfulness meditation improves cognition: Evidence of brief mental training. Conscious. Cogn. 19, 597–605 (2010).
Ni, M. et al. Comparative impacts of Tai Chi, balance training, and a Specially-Designed yoga program on balance in older fallers. Arch. Phys. Med. Rehabil. 95, 1620–1628e30 (2014).
Tousignant, M. et al. Efficacy of supervised Tai Chi exercises versus conventional physical therapy exercises in fall prevention for frail older adults: A randomized controlled trial. Disabil. Rehabil. 35, 1429–1435 (2013).
Pan, Z. et al. The effects of Tai Chi intervention on healthy elderly by means of neuroimaging and EEG: A systematic review. Front. Aging Neurosci. 10, 110 (2018).
Bhowmik Bhunia, G. & Ray, U. S. Improvement in muscular strength, body flexibility and balance by Yoɡasana and with reduced detraining effects by Yoɡa breathing maneuvers: A non-randomized controlled study. J. Ayurveda Integr. Med. 15, 100815 (2024).
Saud, A. et al. Harnessing the benefits of yoga for myositis, muscle dystrophies, and other musculoskeletal disorders. Clin. Rheumatol. 41, 3285–3297 (2022).
Lam, L. C. W. et al. Interim follow-up of a randomized controlled trial comparing Chinese style Mind body (Tai Chi) and stretching exercises on cognitive function in subjects at risk of progressive cognitive decline. Int. J. Geriatr. Psychiatry. 26, 733–740 (2011).
Wayne, P. M. et al. Effect of Tai Chi on cognitive performance in older adults: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 62, 25–39 (2014).
Cui, L. et al. Tai Chi Chuan vs general aerobic exercise in brain plasticity: A multimodal MRI study. Sci. Rep. 9, 17264 (2019).
Lanman, C. R. The Hindu Yoga-System. Harv. Theol. Rev. 11, 355–375 (1918).
Malhotra, J. C. Yoga and mental hygiene. Am. J. Psychother. 17, 436–442 (1963).
Pandurangi, A. K., Keshavan, M. S., Ganapathy, V. & Gangadhar Bengaluru. N. Yoga: Past and Present. Am. J. Psychiatry 174, 16–17 (2017).
Vaidya, A. D. & Vaidya, R. A century of ‘the science of yoga’ (1921–2021): Revival, renewal and renaissance. J. Ayurveda Integr. Med. 14, 100613 (2022).
Diamond, A. Effects of physical exercise on executive functions: going beyond simply moving to moving with thought. Ann. Sports Med. Res. 2, 1011 (2015).
Fj, K., Ne, C. G., Vb, F. & Kl, H. P. Dance and the brain: a review. Ann. N Y Acad. Sci. 1337, (2015).
Gothe, N. P. & McAuley, E. Yoga and cognition: A Meta-Analysis of chronic and acute effects. Psychosom. Med. 77, 784–797 (2015).
Voelcker-Rehage, C., Godde, B. & Staudinger, U. M. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front. Hum. Neurosci. 5, 26 (2011).
Bellissimo, G., Leslie, E., Maestas, V. & Zuhl, M. The effects of fast and slow yoga breathing on cerebral and central hemodynamics. Int. J. Yoga. 13, 207–212 (2020).
Bhutkar, P. M., Bhutkar, M. V., Taware, G. B., Doijad, V. & Doddamani, B. R. Effect of Suryanamaskar practice on cardio-respiratory fitness parameters: A pilot study. Al Ameen J. Med. Sci. 1, 126–129 (2008).
Bijlani, R. L. et al. A brief but comprehensive lifestyle education program based on yoga reduces risk factors for cardiovascular disease and diabetes mellitus. J. Altern. Complement. Med. N Y N. 11, 267–274 (2005).
Hassan, A., Robinson, M. & Willerth, S. M. Modeling the effects of yoga on the progression of Alzheimer’s disease in a dish. Cells Tissues Organs. 206, 263–271 (2018).
Mehta, U. M. & Gangadhar, B. N. Yoga: balancing the excitation-inhibition equilibrium in psychiatric disorders. Prog Brain Res. 244, 387–413 (2019).
Grabara, M. Could Hatha yoga be a health-related physical activity? Biomed. Hum. Kinet 8, (2016).
Kwok, J. Y. Y. et al. Effects of mindfulness yoga versus conventional physical exercises on symptom experiences and Health-related quality of life in people with Parkinson’s disease: The potential mediating roles of anxiety and depression. Ann. Behav. Med. Publ Soc. Behav. Med. 56, 1068–1081 (2022).
Streeter, C. C. et al. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: A randomized controlled MRS study. J. Altern. Complement. Med. 16, 1145–1152 (2010).
Banerjee, B. et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr. Cancer Ther. 6, 242–250 (2007).
Gothe, N. P., Hayes, J. M., Temali, C. & Damoiseaux, J. S. Differences in brain structure and function among yoga practitioners and controls. Front. Integr. Neurosci. 12, 26 (2018).
Brunner, D., Abramovitch, A. & Etherton, J. A yoga program for cognitive enhancement. PloS One. 12, e0182366 (2017).
Nourollahimoghadam, E., Gorji, S. & Gorji, A. Therapeutic role of yoga in neuropsychological disorders. World J. Psychiatry. 11, 754–773 (2021).
Desai, R., Tailor, A. & Bhatt, T. Effects of yoga on brain waves and structural activation: A review. Complement. Ther. Clin. Pract. 21, 112–118 (2015).
Froeliger, B. E., Garland, E. L., Modlin, L. A. & McClernon, F. J. Neurocognitive correlates of the effects of yoga meditation practice on emotion and cognition: a pilot study. Front. Integr. Neurosci. 6, (2012).
Hariprasad, V. R. et al. Yoga increases the volume of the hippocampus in elderly subjects. Indian J. Psychiatry. 55, S394–S396 (2013).
Barrós-Loscertales, A., Hernández, S. E., Xiao, Y., González-Mora, J. L. & Rubia, K. Resting state functional connectivity associated with Sahaja yoga meditation. Front. Hum. Neurosci. 15, (2021).
Tomasino, B., Fregona, S., Skrap, M. & Fabbro, F. Meditation-related activations are modulated by the practices needed to obtain it and by the expertise: An ALE meta-analysis study. Front. Hum. Neurosci. 6, 346 (2013).
Brewer, J. A. et al. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. U S A. 108, 20254 (2011).
Menon, V. 20 Years of the default mode network: A review and synthesis. Neuron 111, 2469–2487 (2023).
Garrison, K. A., Zeffiro, T. A., Scheinost, D., Constable, R. T. & Brewer, J. A. Meditation leads to reduced default mode network activity beyond an active task. Cogn. Affect. Behav. Neurosci. 15, 712–720 (2015).
Ajjimaporn, A., Rachiwong, S. & Siripornpanich, V. Effects of 8 weeks of modified Hatha yoga training on resting-state brain activity and the p300 ERP in patients with physical disability-related stress. J. Phys. Ther. Sci. 30, 1187–1192 (2018).
Kora, P., Meenakshi, K., Swaraja, K., Rajani, A. & Raju, M. S. EEG based interpretation of human brain activity during yoga and meditation using machine learning: A systematic review. Complement. Ther. Clin. Pract. 43, (2021).
Kanthi, A., Deepeshwar, S., Chidananda, K., Vidyashree, M. & Krishna, D. Event-Related potential changes following 12-week yoga practice in T2DM patients: A randomized controlled trial. Clin. EEG Neurosci. https://doi.org/10.1177/15500594241249511 (2024).
Lehmann, D., Ozaki, H. & Pal, I. EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalogr. Clin. Neurophysiol. 67, 271–288 (1987).
Xu, J. et al. EEG microstates are correlated with brain functional networks during slow-wave sleep. NeuroImage 215, (2020).
Brodbeck, V. et al. EEG microstates of wakefulness and NREM sleep. NeuroImage 62, 2129–2139 (2012).
Schlegel, F., Lehmann, D., Faber, P. L., Milz, P. & Gianotti, L. R. R. EEG microstates during resting represent personality differences. Brain Topogr. 25, 20–26 (2012).
Hu, W. et al. Microstate detection in naturalistic electroencephalography data: A systematic comparison of topographical clustering strategies on an emotional database. Front. Neurosci. 16, 812624 (2022).
Michel, C. M. & Koenig, T. EEG microstates as a tool for studying the Temporal dynamics of whole-brain neuronal networks: A review. NeuroImage 180, 577–593 (2018).
Khanna, A., Pascual-Leone, A., Michel, C. M. & Farzan, F. Microstates in resting-state EEG: current status and future directions. Neurosci. Biobehav Rev. 49, 105–113 (2015).
Custo, A. et al. Electroencephalographic Resting-State networks: source localization of microstates. Brain Connect. 7, 671–682 (2017).
Croce, P., Quercia, A., Costa, S. & Zappasodi, F. EEG microstates associated with intra- and inter-subject alpha variability. Sci. Rep. 10, 1–11 (2020). (2020).
Godse, A. S., Shejwal, B. R. & Godse, A. A. Effects of Suryanamaskar on relaxation among college students with high stress in Pune, India. Int. J. Yoga. 8, 15–21 (2015).
Pal, R. et al. Impacts of the Surya Namaskar on body composition and physiological parameters among yoga and Non yoga professionals: A Quasi-experimental study. J. Clin. Diagn. Res. https://doi.org/10.7860/JCDR/2024/66733.19043 (2024).
Parajuli, N., Shindhe, M. & Pradhan, B. Effect of Surya Namaskara (Sun Salutation) on mental health, self-control and mindfulness of adolescent school children. Complement. Ther. Clin. Pract. 50, 101691 (2023).
Stec, K., Kruszewski, M. & Ciechanowski, L. Effects of Suryanamaskar, an intensive yoga exercise routine, on the stress levels and emotional intelligence of Indian students. Int. J. Environ. Res. Public. Health. 20, 2845 (2023).
Scrivener, C. L. & Reader, A. T. Variability of EEG electrode positions and their underlying brain regions: visualising gel artifacts from a simultaneous EEG-fMRI dataset. bioRxiv 2021.03.08.434424 (2021). https://doi.org/10.1101/2021.03.08.434424
Hughes, S. W. & Crunelli, V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 11, 357–372 (2005).
Tyson, P. D. Task-related stress and EEG alpha biofeedback. Biofeedback Self-Regul. 12, 105–119 (1987).
Khajuria, A., Kumar, A., Joshi, D. & Kumaran, S. S. Reducing stress with yoga: A systematic review based on multimodal biosignals. Int. J. Yoga. 16, 156–170 (2023).
Ganpat, T. S., Nagendra, H. R. & Selvi, V. Efficacy of yoga for mental performance in university students. Indian J. Psychiatry. 55, 349–352 (2013).
Gothe, N. P., Khan, I., Hayes, J., Erlenbach, E. & Damoiseaux, J. S. Yoga effects on brain health: A systematic review of the current literature. Brain Plast. 5, 105 (2019).
Woodyard, C. Exploring the therapeutic effects of yoga and its ability to increase quality of life. Int. J. Yoga. 4, 49–54 (2011).
Sabio, J., Williams, N. S., McArthur, G. M. & Badcock, N. A. A scoping review on the use of consumer-grade EEG devices for research. PLOS ONE. 19, e0291186 (2024).
Dadebayev, D., Goh, W. W. & Tan, E. X. EEG-based emotion recognition: review of commercial EEG devices and machine learning techniques. J. King Saud Univ. - Comput. Inf. Sci. 34, 4385–4401 (2022).
Bodda, S. & Diwakar, S. Exploring EEG spectral and Temporal dynamics underlying a hand Grasp movement. PLOS ONE. 17, e0270366 (2022).
Gramfort, A. et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 7, 1–13 (2013).
Harris, C. R. et al. Array programming with numpy. Nature 585, 357–362 (2020).
Hunter, J. D. & Matplotlib A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Virtanen, P. et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods. 17, 261–272 (2020).
Szaszkó, B. et al. The influence of Hatha yoga on stress, anxiety, and suppression: A randomized controlled trial. Acta Psychol. (Amst). 241, 104075 (2023).
Leach, H. J. et al. Matching exercise volume in active control groups for yoga interventions. Altern. Ther. Health Med. 29, 237–241 (2023).
Sharma, H., Raj, R. & Juneja, M. EEG signal based classification before and after combined yoga and Sudarshan Kriya. Neurosci. Lett. 707, 134300 (2019).
Zetzl, T., Schuler, M., Renner, A., Jentschke, E. & van Oorschot, B. Yoga intervention and reminder e-mails for reducing cancer-related fatigue - a study protocol of a randomized controlled trial. BMC Psychol. 7, 64 (2019).
Antonova, E. et al. EEG microstates: functional significance and short-term test-retest reliability. Neuroimage Rep. 2, 100089 (2022).
Férat, V., Scheltienne, M., Brunet, D., Ros, T. & Michel, C. Pycrostates: a Python library to study EEG microstates. J. Open. Source Softw. 7, 4564 (2022).
Pascual-Marqui, R. D., Michel, C. M. & Lehmann, D. Segmentation of brain electrical activity into microstates: model Estimation and validation. IEEE Trans. Biomed. Eng. 42, 658–665 (1995).
Murray, M. M., Brunet, D., Michel, C. M. & Topographic ERP analyses: A Step-by-Step tutorial review. Brain Topogr. 20, 249–264 (2008).
Das, S. et al. Resting state electroencephalography microstates in autism spectrum disorder: A mini-review. Front. Psychiatry. 13, 988939 (2022).
Koenig, T. et al. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. NeuroImage 16, 41–48 (2002).
Lehmann, D. et al. EEG microstate duration and syntax in acute, medication-naive, first-episode schizophrenia: a multi-center study. Psychiatry Res. 138, 141–156 (2005).
Stam, C. J., Nolte, G. & Daffertshofer, A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 28, 1178–1193 (2007).
Chu, C. et al. Temporal and Spatial variability of dynamic microstate brain network in early Parkinson’s disease. Npj Park Dis. 9, 1–12 (2023).
Babadi, B. & Brown, E. N. A review of multitaper spectral analysis. IEEE Trans. Biomed. Eng. 61, 1555–1564 (2014).
J Thomson, D. Spectrum Estimation and harmonic analysis. Proc. IEEE. 70, 1055–1096 (1982).
Fujikoshi, Y. Two-way ANOVA models with unbalanced data. Discrete Math. 116, 315–334 (1993).
Tukey, J. W. Comparing individual means in the analysis of variance. Biometrics 5, 99–114 (1949).
Student The probable error of a mean. Biometrika 6, 1–25 (1908).
Mann, H. B. & Whitney, D. R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 18, 50–60 (1947).
Kruskal, W. H. & Wallis, W. A. Use of ranks in One-Criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621 (1952).
Wilcoxon, F. Individual comparisons by ranking methods. Biom Bull. 1, 80 (1945).
Motulsky GraphPAD Software Inc. (Firm). Harvey. GraphPAD Prism 7.04: Scientific Graphing, Curve Fitting & Statistics (GraphPAD Software Inc., 2002).
Acknowledgements
This work derives direction and ideas from the Chancellor of Amrita Vishwa Vidyapeetham, Sri Mata Amritanandamayi Devi. This work is funded by Amrita University Seed Grant (ASG2022111), Amrita Viswa Vidyapeetham. The authors would like to thank Amrita Yoga team at Mata Amritanandamayi Math, India for their assistance in yoga training and Prof. Alice Guyon, Aix Marseille University, France for comments and for proofreading the manuscript. Authors would also like to thank Nijin Nizar, Rakhi Radhamani and Priya Chellaiah for their assistance with data collection for the study.
Author information
Authors and Affiliations
Contributions
S.B. and S.D.,designed the experiments, analyzed data, wrote the manuscript and. prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bodda, S., Diwakar, S. Interconnections and global transitions among functional states encode activity-related dynamics as brain topology changes after yoga training. Sci Rep 15, 16845 (2025). https://doi.org/10.1038/s41598-025-00134-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00134-y