Abstract
Nitrogen (N) and phosphorus (P) are vital nutrients in terrestrial ecosystems, essential for vegetation growth, plant survival, development, and the maintenance of plant diversity. While the effects of N and P additions on plant nutrient concentrations and diversity have been extensively studied, the impact of various stages of natural forest ecosystem restoration after disturbance, particularly on plant N, P nutrients, and diversity, is less understood. This study investigates the influence of anthropogenic disturbance on soil N: P ratios, plant community-level N: P, and species diversity in Liaodong oak forests in northern China during early (about 10 years), mid-to-late (about 30 years), and late (about 40 years) restoration stages (it is a space for time substitution method). We observed that soil total nitrogen to total phosphorus ratio (TN: TP) and soil available nitrogen to phosphorus ratio (AN: AP) were significantly lower in the mid-to-late and late stages of restoration compared to the early restoration stages. Concurrently, plant community level leaf nitrogen-phosphorus ratio (CWMN: P) were notably lower in these later stages. Additionally, species richness showed a decline as restoration time increased. These findings highlight that decreasing soil N: P ratio over time exacerbates plant N limitation and reduces plant richness, emphasizing the critical role of soil nutrient dynamics in forest restoration post-disturbance. Our results provide important insights for forest management and restoration practices, contributing to a better understanding of ecosystem restoration processes.
Similar content being viewed by others
Introduction
Environmental degradation is of worldwide concern in part owing to its impact the survival of humankind and on sustainable socio-economic development1. Natural succession is a commonly used site restoration method that can effectively control soil erosion, address ecological degradation, enhance environmental quality and ensure ecosystem sustainability2,3,4. As time post-restoration advances, plant communities not only increase biodiversity and change community structure5but also change plant traits6, increase soil nutrient content7, restore erosion-induced soil function8, enhance soil carbon sequestration9, and mitigate soil erosion10. Therefore, understanding the impact of natural restoration processes on plant and soil properties in critically eroded soils is important for improving ecological functions and providing guidance for the reconstruction and restoration of degraded ecosystems.
Nitrogen (N) and phosphorus (P) are essential nutrients that limit plant growth, reproduction, and productivity in various terrestrial ecosystems11. Coupling and balancing of N and P have a major impact on the structure and function of different biological tissues from the molecular level to the community level12, and they are strongly influenced by human activities13. In recent decades, more studies have focused on the effects of atmospheric N deposition on biodiversity14,15,16. Furthermore, most studies have found that increasing N inputs significantly reduced plant diversity in terrestrial ecosystems16,17,18,19. However, the rate of N deposition is slow, and whether anthropogenic N addition experiments are consistent with the effects on species diversity of changes over restoration time in the natural state is a scientific question of interest. For instance, Li et al.20 found that species richness increased during secondary succession at two different spatial scales in a subalpine meadow. Conversely, it has also been shown to reduce forest ecosystem species diversity as the duration of secondary succession increases. The two ecosystems mentioned above do not vary consistently in terms of species diversity in their natural environments, and it is possible that soil nutrient content is relevant, as how much nutrients are available in the soil limits the size of the ecological niche21. In addition, plant leaf N and P contents were highly influenced by succession time22. However, how altered soil nutrients with increasing successional time affect plant diversity and plant nutrient concentrations of N and P is largely unknown to us.
Liaodong oak (Quercus wutaishanica) is a dominant species in China’s northern temperate forest system, which plays an important role in soil and water conservation, climate regulation and ecosystem services in the region23. However, since the 1980s, human activities have seriously damaged the forest system in our study site until around 2010, when it was effectively protected with the national emphasis on the ecological environment and the improvement of people’s living conditions. In this study, we investigated Liaodong scrub at different restoration periods (about 10, 30 and 40 years) after anthropogenic damage. We predicted that (1) Species diversity decreases with increasing restoration time, as longer restoration times may increase plant competition for nutrients and light, thereby limiting the growth and settlement of some poorly competitive plants. (2) Plant community level leaf N: P decreases with increasing restoration time because as restoration time increases, it improves the productivity and reproductive capacity of the plant community24, increasing plant nutrient availability from the soil nutrient pool, especially the plant’s need for N.
Materials and methods
Study site
The study site is located in Liupanshan National Nature Reserve (N: 35°15′−35°41′, E: 106°09′−106°30′). The region is in the northern agricultural and pastoral zone and the warm temperate zone of semi-humid to semi-arid regions over the edge. In summer, the climates of this region are cold dry airflow, cold temperature and humidity airflow with high temperatures and rain, while the winter is dry and cold. In the region, the coldest month is January, and the mean monthly temperature is −7 ℃. The hottest month is July, and the mean monthly temperature is 17.4 ℃. The mean annual temperature is 5.8 ℃. The region has about 120 days for the frost-free period, the annual sunshine hours are between 2,100 and 2,400 h. The mean annual precipitation in the region is 676 mm, with most of the rainfall concentrated in June to September, and the annual evaporation is 1,426 mm. In the soil division belongs to the gray brown soil area. There are more dissolved ash brown soil, mountain gray brown soil and calcareous gray brown soil in the Yin slope forest; and the dark gray brown soil in the Yang slope vegetation25. The original forests in the Liupan Mountains area have been severely damaged by indiscriminate logging and overgrazing and have been restored to form most of the current natural secondary forests.
In July 2019, through consulting with the staff of Liupanshan Forestry Bureau, Q. wutaishanica scrubs, we selected three plots with different restoration times (it is a space for time substitution method), that is, Xilianggou (10 years), Dawan (30 years) and Dadaogou (40 years) (Fig. 1). Each plot has an area of about 100 ha and each distance is over 1 km. The dominant tree species of the study area are mainly Q. wutaishanica. In the Q. wutaishanica scrub, there are mainly trees such as Q. wutaishanica and Tilia paucicostata, and shrubs such as Crataegus kansuensis, Cotoneaster multiflorus and Syringa microphylla; and herbaceous plants such as Brachypodium sylvaticum, Carex tristachya and Phlomis umbrosa.
Vegetation survey
To investigate the species diversity and community structure with different successional stages, we respectively selected four sample lines in Xilianggou (10 a), Dawan (30 a) and Dadaogou (40 a), each sample line distance is more 20-m, and a 10 m×10 m vegetation sample square was randomly set up in each sample line. Using the sample line survey method, all woody plants with a basal diameter of ≥ 1 cm in each sample plot were counted, and their species name, plant height, number of plants, and cover were recorded. Three 1 m × 1 m herbaceous samples were set up on the diagonal of each sample, and the species name, plant height, number of plants, and cover of herbs were recorded.
The species of trees and shrubs that ranked in the top six of the community were calculated using the importance value method (Importance value (IV) = (relative density + relative cover + relative frequency)/3)26.
Collection of plant leaves and soil samples
In September 2019, we collected 500 intact leaves of five mature trees of different major species randomly from each vegetation sample square. All leaves were placed between two pieces of soaked filter paper, put into self-sealing bags, and brought back to the laboratory for measurement of relevant parameters. Leaves were dried at 80 ℃ for 30 min to kill the chlorophyll, then cooled to 60 ℃ and dried to a constant weight. The samples were crushed after being removed and slightly cooled, and all the crushed samples were passed through a 0.25 mm sieve. N and P concentrations of plant leaves were determined using the Kjeldahl nitrogen determination and the molybdenum-antimony colorimetric method, respectively.
We randomly set up four 5 m × 5 m soil sample squares in each vegetation sample square. Then we set up four 1.5 m × 1.5 m small sample squares in each soil sample square. We randomly selected three sampling points in each small sample square, from which we collected the soil from 0 to 10 cm depth, for a total of 48 soil samples. The collected soil samples were placed in self-sealing bags and brought back to the laboratory. Total soil N (TN) was measured by the Kjeldahl method27.Total soil P (TP) was determined by acid digestion with a H2SO4 + HClO4solution27. After samples were extracted with 0.5 M Na2CO3, available phosphorus (AP) was measured by molybdenum blue colorimetric method28. Soil available N, which includes ammonium nitrogen, nitrate nitrogen and alkali-hydrolyzed nitrogen. Ammonium nitrogen and nitrate nitrogen were determined by flow analyzer after 2 mol KCl29. Alkali-hydrolyzed nitrogen was measured by titration with a dilute solution of H2SO4 after samples had been extracted with a mixture of FeSO4and NaOH30.
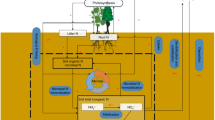
Plant communities N and P were calculated as follows:
Where \(Pi\) is the relative mass of species i in the community, and \(trait\:i\) is the nutrient concentration value of species i31.
Statistics and analyses
The effects of restoration years on soil nutrients, plant nutrients and species richness were analyzed using ANOVA. Then Tukey test was used to analyze the differences between different restoration stages of soil total nitrogen (TN), soil total phosphorus (TP), soil total nitrogen: soil total phosphorus (TN: TP), soil available nitrogen (AN), soil available phosphorus (AP), soil available nitrogen: soil available phosphorus (AN: AP), plant community weighted mean nitrogen (CWMN), phosphorus (CWMP), (CWMN: P), and species richness, respectively. Eventually, all data were taken to Log10 to analyze the regression relationship between leaf N: P ratio and species richness at the plant community level and soil TN: TP, AN: AP, respectively. A significance level of p < 0.05 was used. All data were transformed in R 4.0.2 and plots were done using Sigmplot 12.5.
Result
Soil TN after 30 and 40 years of restoration was significantly lower than soil TN after 10 years of restoration. (Fig. 2, A). There were no significant differences in soil TP across restoration times. (Fig. 2, B). Soil TN: TP was significantly reduced after 30 and 40 years of restoration compared to 10 years of restoration (Fig. 2, C). Soil AN after 10 and 40 years of restoration was considerably higher than that after 30 years of restoration (Fig. 2, D). Soil AP improved with increasing restoration time (Fig. 2, E), while soil AN : AP decreased with increasing restoration time (Fig. 2, F).
Effect of succession time on soil nitrogen and phosphorus content and their ratio. A: Total soil nitrogen. B: Total soil phosphorus. C: The ratio of total soil nitrogen to total soil phosphorus. D: Available soil nitrogen. E: Available soil phosphorus. F: The ratio of available soil nitrogen to available soil phosphorus. Note: Lowercase letters indicate that the difference in the same indicator reached the significance level at different restoration times. Data shows (mean ± standard error, n = 4).
Plant community-level leaf N concentrations were clearly higher after 10 and 40 years of restoration than after 30 years of restoration. (Fig. 3, A). Plant community-level leaf P concentration significantly decreases with increasing time in succession (Fig. 3, B). Plant community level leaf N: P was significantly higher after 10 years of restoration than after 30 and 40 years (Fig. 3, C). Species richness reduced with increasing restoration time (Fig. 3, D).
Effect of succession time on nitrogen and phosphorus concentrations, ratio and plant richness of plant communities. A: Plant community weighted mean nitrogen (CWMN). B: Plant community weighted mean phosphorus (CWMP). C: The ratio of the plant community weighted mean nitrogen to the plant community weighted mean phosphorus (CWMN: P). D: Plant species richness. Note: lower case letters indicate that the difference in the same indicator reached the significance level at different restoration times. Data shows (mean ± standard error, n = 4).
Plant community-level leaf N: P was positively correlated with soil TN: TP and AN: AP, respectively (Fig. 4, A, B). Similarly, plant richness was positively correlated with soil TN: TP and AN: AP, respectively (Fig. 4, B, C).
Relationship between soil nitrogen and phosphorus ratios and plant nitrogen and phosphorus ratios and species richness of plant communities. A: The relationship between the ratio of the plant community weighted mean nitrogen to the plant community weighted mean phosphorus (CWMN: P) and the ratio of the total soil nitrogen to the total soil phosphorus (TN: TP). B: The relationship between the ratio of the plant community weighted mean nitrogen to the plant community weighted mean phosphorus (CWMN: P) and the ratio of the available soil nitrogen to the available soil phosphorus (AN: AP). C: The relationship between the plant species richness and the ratio of the total soil nitrogen to the total soil phosphorus (TN: TP). D: The relationship between the plant species richness and the ratio of the available soil nitrogen to the available soil phosphorus (AN: AP). Note: ^p < 0.1, **p < 0.01.
Discussion
In our extensive field experiment, we investigated soil nutrient contents, plant community nutrient concentration, and species richness during different stages of restoration of Liaodong oak in northern China. We discovered that the ratios of total nitrogen to total phosphorus (TN: TP) and available nitrogen to available phosphorus (AN: AP) significantly decreased with increasing restoration time. Additionally, plant community cover increased (Fig S), species richness decreased, and nitrogen (N) limitation at the plant community level increased over the longer recovery period.
Contrary to previous studies suggesting an increase in soil nutrients over successional time32,33,34, our findings showed that soil total nitrogen content was higher in the first 10 years of restoration than in the subsequent 30 and 40 years. This could be attributed to plants absorbing more nitrogen as succession progresses and increasing aboveground biomass in the forest system35. However, soil available nitrogen content was higher both in the early stage (10 years) and later stage (40 years) of restoration than in the intermediate stage (30 years), possibly due to lower aboveground biomass initially and then increased microbial diversity leading to accelerated decomposition of organic material later36. Soil available phosphorus (P) concentrations increased with restoration time, possibly due to K-strategist plant growth rates decreasing in later restoration stages37. Large trees, which require more P for reproduction, may secrete substances or change the rhizosphere environment, thus stimulating the transformation of soil P into more available forms and increasing soil available P.
A clear decline in plant species richness with increased restoration time supports our hypothesis. Early-stage Q. wutaishanicaforests provide a conducive environment for plant settlement due to low plant community productivity, relatively abundant soil nutrients, and less nutrient limitation38,39. However, as restoration progresses, increased plant community productivity exacerbates nutrient limitation40,41. In later restoration stages, the increased height and cover of the plant community reduce direct light radiation to the surface, heightening competition for light42,43,44, especially impacting canopy and herbaceous plants. This aligns with Doležal et al. (2013)45, who reported plant diversity is linked to resource availability. Prolonged restoration has also been found to reduce plant diversity in North American wet prairies46and semi-arid grasslands, where short-term restoration after grazing enhances diversity, but long-term restoration reduces species richness due to increased plant density and diminished light intensity47. These findings support the concept that resource availability determines plant diversity44,48,49.
Nitrogen and phosphorus are crucial nutrients limiting plant growth, development, and reproduction50. Our study showed that plant community level N concentration was significantly higher after 10 and 40 years of restoration compared to 30 years, suggesting variations in N nutrient limitation. In the early restoration stage, rapid plant growth, typified by r-strategist behavior, requires substantial nitrogen for protein synthesis, resulting in higher leaf N concentration51. After 40 years, higher plant N levels may be due to increased soil microbial diversity, enhanced litter decomposition, and consequent soil N accumulation52. In later stages, woody plants producing seeds and fruits require more elemental P. According to the stoichiometric homeostasis hypothesis53, plants maintain a stable N: P ratio, with leaf P content increasing over time, beneficial for plant reproduction24. Foliar N: P ratios are indicative of N or P limitations54. A review of 40 fertilization studies reveals that an N: P ratio > 16 indicates P limitation on a community level, while an N: P ratio < 14 is indicative of N limitation55. At N: P ratios between 14 and 16, either N or P can be limiting or plant growth is colimited by N and P together55. Our study found a significant decrease in the N: P ratio of plants with restoration time. Foliar N: P ratio > 14 after 10 years of restoration suggests co-limitation by N and P or neither, whereas foliar N: P ratios < 14 after 30 and 40 years of restoration indicate N limitation55. Similarly, a study of temperate forest systems in northern China found that with increasing successional time, exacerbated plant N demand24. Moreover, our study found that as the restoration time extended, both the ratio of total nitrogen to total phosphorus (TN: TP) and the ratio of available nitrogen to available phosphorus (AN: AP) decreased. This implies a reduction in the nitrogen-to-phosphorus ratio of the soil within the nutrient pool, thereby exacerbating the nitrogen limitation for plants.
Our study provides valuable insights into the complex interplay between soil nutrient dynamics, plant nutrient concentrations, and biodiversity in forest ecosystems undergoing restoration. These findings offer important implications for forest management and restoration strategies, highlighting the need to consider nutrient dynamics and plant community responses in ecosystem restoration efforts.
Conclusion
Our study showed that short-term restoration increased plant species richness, but prolonged restoration reduced species richness in a boreal forest system in China. This is indicated that species richness was mainly affected by interspecific competitive relationships. In the early stages, plants have less available nutrient limitation and less competition for light, and later increased nutrient and light limitation with increasing restoration time. In addition, we found that leaf N: P ratio, soil TN: TP ratio, soil AN: AP ratio all reduced with increasing restoration time. This is mainly attributed to the increase in productivity the longer the recovery time, which increased soil N uptake and lowered soil nutrient concentrations. Although our study found that both soil and plant community level N: P ratios were reduced with increasing restoration time, however, there are shortcomings in our study, for example, focusing only on leaf blade nutrient changes and not on other plant organs. In addition, we did not test the effects of nutrient addition on plant communities at different stages of recovery, and there is a lack of systematic understanding of the thresholds for nitrogen and phosphorus limitation in this system. We will focus on these issues in later studies to better understand the nutrient dynamics of different restored boreal temperate forest systems after anthropogenic damage.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Ahmad, N., Youjin, L., Žiković, S. & Belyaeva, Z. The effects of technological innovation on sustainable development and environmental degradation: evidence from China. Technol. Soc. 72, 102184 (2023).
Gann, G. D. et al. International principles and standards for the practice of ecological restoration. Restor. Ecol. 27, S1–S46 (2019).
Bardgett, R. D. et al. Combatting global grassland degradation. Nat. Reviews Earth Environ. 2, 720–735 (2021).
Wang, Z. & Zheng, F. Impact of vegetation succession on leaf-litter-soil C: N: P stoichiometry and their intrinsic relationship in the Ziwuling area of China’s loess plateau. J. Forestry Res. 32, 697–711 (2021).
LetcherSG Phylogenetic structure of angiosperm communities during tropical forest succession. Proc. Royal Soc. B: Biol. Sci. 277, 97–104 (2010).
Chang, C. C. & Turner, B. L. Ecological succession in a changing world. Wiley Online Libr. :503–509. (2019).
Mitchell, R., Marrs, R., Le Duc, M. & Auld, M. A study of succession on lowland heaths in Dorset, Southern England: changes in vegetation and soil chemical properties. J. Appl. Ecol. :1426–1444. (1997).
Zethof, J. H., Cammeraat, E. L. & Nadal-Romero, E. The enhancing effect of afforestation over secondary succession on soil quality under semiarid climate conditions. Sci. Total Environ. 652, 1090–1101 (2019).
Deng, L., Wang, K., Chen, M., Shangguan, Z. & Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the loess plateau. China Catena. 110, 1–7 (2013).
Chen, J., Li, Z., Xiao, H., Ning, K. & Tang, C. Effects of land use and land cover on soil erosion control in Southern China: implications from a systematic quantitative review. J. Environ. Manage. 282, 111924 (2021).
Elser, J. J. et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 (2007).
Elser, J. et al. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 3, 540–550 (2000).
Liang, X. et al. Extreme drought exacerbates plant nitrogen–phosphorus imbalance in nitrogen enriched grassland. Sci. Total Environ. 849, 157916 (2022).
Siddique, I. et al. Nitrogen and phosphorus additions negatively affect tree species diversity in tropical forest regrowth trajectories. Ecology 91, 2121–2131 (2010).
Avolio, M. L. et al. Changes in plant community composition, not diversity, during a decade of nitrogen and phosphorus additions drive above-ground productivity in a tallgrass prairie. J. Ecol. 102, 1649–1660 (2014).
Tian, Q. et al. An integrated belowground trait-based Understanding of nitrogen‐driven plant diversity loss. Glob. Change Biol. 28, 3651–3664 (2022).
Chalcraft, D. R. et al. Scale-dependent responses of plant biodiversity to nitrogen enrichment. Ecology 89, 2165–2171 (2008).
Reich, P. B. Elevated CO2 reduces losses of plant diversity caused by nitrogen deposition. Science 326, 1399–1402 (2009).
Granjel, R. R., Allan, E. & Godoy, O. Nitrogen enrichment and foliar fungal pathogens affect the mechanisms of multispecies plant coexistence. New Phytol. 237, 2332–2346 (2023).
Li, W. et al. Biomass Temporal stability increases at two Spatial scales during secondary succession. J. Ecol. 111, 1575–1586 (2023).
Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 19, 605–611 (2004).
Duffin, K. I., Li, S. P. & Meiners, S. J. Species pools and differential performance generate variation in leaf nutrients between native and exotic species in succession. J. Ecol. 107, 595–605 (2019).
Luo, Y. et al. Response of seedling growth characteristics to seed size and cotyledon damage in Quercus Wutaishanica. Forests 14, 1905 (2023).
Zhang, P., Lü, X., Li, M. H., Wu, T. & Jin, G. N limitation increases along a temperate forest succession: evidences from leaf stoichiometry and nutrient resorption. J. Plant. Ecol. 15, 1021–1035 (2022).
Luo, Y. et al. Density-dependent seed predation of Quercus Wutaishanica by rodents in response to different seed States. Animals 13, 1732 (2023).
Curtis, J. T. The Vegetation of Wisconsin: an Ordination of Plant Communities (University of Wisconsin Pres, 1959).
Bremner, J. M. Total nitrogen, Methods of Soil Analysis, Agronomy American Society of Agronomy, Madison WI, No. 9, Part 2, ;pp. 1149–1176. (1976).
Kowalenko, C. & Babuin, D. Interference problems with phosphoantimonylmolybdenum colorimetric measurement of phosphorus in soil and plant materials. Commun. Soil Sci. Plant Anal. 38, 1299–1316 (2007).
Cawse, P. The determination of nitrate in soil solutions by ultraviolet spectrophotometry. Analyst 92, 311–315 (1967).
Sun, J. et al. Seasonal and Spatial variations in soil biochemical properties in areas with different degrees of mining subsidence in central China. Catena 1, 106984 (2023).
Lavorel, S. et al. Assessing functional diversity in the field–methodology matters. Funct. Ecol. 22, 134–147 (2008).
Di Palo, F. & Fornara, D. A. Plant and soil nutrient stoichiometry along primary ecological successions: is there any link? Plos One. 12, e0182569 (2017).
Lucas-Borja, M. E. & Delgado-Baquerizo, M. Plant diversity and soil stoichiometry regulates the changes in multifunctionality during pine temperate forest secondary succession. Sci. Total Environ. 697, 134204 (2019).
Liu, Y., Fang, Y., An, S. & How, C. N: P stoichiometry in soils and plants responds to succession in Robinia pseudoacacia forests on the loess plateau, China, forest ecology and management. ;475:118394. (2020).
Wu, H. et al. Tree growth rate and soil nutrient status determine the shift in nutrient-use strategy of Chinese Fir plantations along a chronosequence. For. Ecol. Manag. 460, 117896 (2020).
Liu, D. et al. Shaping the succession patterns of different soil nutrients, enzyme stoichiometry, and microbial communities through rotation systems. Catena 236, 107740 (2024).
Zhou, Z., Wang, C., Jiang, L. & Luo, Y. Trends in soil microbial communities during secondary succession. Soil Biol. Biochem. 115, 92–99 (2017).
Jin, X. et al. Changes of plant species diversity and biomass with reclaimed marshes restoration. J. Forestry Res. 32, 133–142 (2021).
Wu, C., Deng, L., Huang, C., Chen, Y. & Peng, C. Effects of vegetation restoration on soil nutrients, plant diversity, and its Spatiotemporal heterogeneity in a desert–oasis ecotone. Land. Degrad. Dev. 32, 670–683 (2021).
Bazzaz, F. Plant species diversity in old-field successional ecosystems in Southern Illinois. Ecology 56, 485–488 (1975).
Denslow, J. S. Patterns of plant species diversity during succession under different disturbance regimes. Oecologia 46, 18–21 (1980).
Rajaniemi, T. K. Why does fertilization reduce plant species diversity? Testing three competition-based hypotheses. J. Ecol. 90, 316–324 (2002).
Hautier, Y., Niklaus, P. A. & Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 324, 636–638 (2009).
Eskelinen, A., Harpole, W. S., Jessen, M. T., Virtanen, R. & Hautier, Y. Light competition drives herbivore and nutrient effects on plant diversity. Nature 611, 301–305 (2022).
Doležal, J., Yakubov, V. & Hara, T. Plant diversity changes and succession along resource availability and disturbance gradients in Kamchatka. Plant Ecol. 214, 477–488 (2013).
Klopf, R. P., Baer, S. G., Bach, E. M. & Six, J. Restoration and management for plant diversity enhances the rate of belowground ecosystem recovery. Ecol. Appl. 27, 355–362 (2017).
Li, J., Zheng, Z., Xie, H., Zhao, N. & Gao, Y. Increased soil nutrition and decreased light intensity drive species loss after eight years grassland enclosures. Sci. Rep. 7, 44525 (2017).
Stevens, M. H. H. & Carson, W. P. Resource quantity, not resource heterogeneity, maintains plant diversity. Ecol. Lett. 5, 420–426 (2002).
Hartmann, M. & Six, J. Soil structure and Microbiome functions in agroecosystems. Nat. Reviews Earth Environ. 4, 4–18 (2023).
Anneberg, T. J. & Segraves, K. A. Neopolyploidy causes increased nutrient requirements and a shift in plant growth strategy in Heuchera cylindrica. Ecology :e4054. (2023).
Siah, K. G., Perakis, S. S., Pett-Ridge, J. C. & van der Heijden, G. Nitrogen-bedrock interactions regulate multi-element nutrient limitation and sustainability in forests. Biogeochemistry :1–25. (2023).
Mahmud, K., Makaju, S., Ibrahim, R. & Missaoui, A. Current progress in nitrogen fixing plants and Microbiome research. Plants 9, 97 (2020).
Jia, G., Cao, J., Wang, C. & Wang, G. Microbial biomass and nutrients in soil at the different stages of secondary forest succession in Ziwulin, Northwest China. For. Ecol. Manag. 217, 117–125 (2005).
Sterner, R. W. & Elser, J. J. Ecological Stoichiometry: Biology of Elements from Molecules To the Biosphere (Princeton University Press, 2002).
Koerselman, W. & Meuleman, A. F. The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. :1441–1450. (1996).
Acknowledgements
This work was funded by research grants from the National Natural Science Foundation of China (32160344). We thank the Forestry Bureau of Liupan Mountain, Jingyuan County, Guyuan City, Ningxia Hui Autonomous Region, for supporting this study. Thanks Caixia Yuan, senior engineer from Wetland Protection and Management Center of Guyuan Forestry Bureau for her contribution to species identification.
Author information
Authors and Affiliations
Contributions
Y.L. J.C. and X.Y. designed the experiment, M.M S.W. L.P. and C.Z made formal analysis, J.Z. and J.C. made investigation, Y.L. and J.C. wrote the main manuscript text, X.Y. revised the article resources: M.Z. H.Y. J.W. and Z.H prepared table and all figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, Y., Cheng, J., Mahmood, M. et al. Plant community restoration time is a key factor affecting plant nutrient and species richness. Sci Rep 15, 15752 (2025). https://doi.org/10.1038/s41598-025-00326-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00326-6