Abstract
Biomass ash is produced as a by-product of boiler combustion and most of this ash is disposed of in landfills. Biomass ash has a relatively low reactivity and alkali activated ash systems have poor mechanical properties. The inclusion of Fe-rich material in the precursor composition would increase the mechanical properties of alkali-activated binders. This study focuses on an alkali-activated binder made from blends of biomass fly ash from a power plant and iron sludge from a ship repair company. In the precursor blends, some of biomass fly ash were substituted by iron sludge, which consisted of high-volume Fe-rich compounds. After curing, the compressive strength was determined, and the strength values were explained by mineral composition and microstructure. Samples cured at higher temperature (at 60 °C for 24 h), iron sludge acted as a reactive component and reacted with NaOH to reduce its (NaOH) content in the system. For the samples cured at ambient temperature iron sludge acted as a less reactive component or like filler. Calcium aluminosilicate hydrate was not detected, but a higher amount of N-A/F-S-H and C-(A/F)-S-H gel formed compared to samples cured at higher temperature. Iron sludge incorporated into the matrix, making it more compact. These factors led to an increase in the strength values of the alkali-activated biomass fly ash incorporating the IS. The highest compressive strength of 9.8 MPa was achieved by samples cured at ambient temperature with 30% iron sludge. Thus, creating alkali activated binders is a promising way to use Fe-rich residues such as iron sludge from a ship repair company.
Similar content being viewed by others
Introduction
To reduce the consumption of natural building materials, it will be appropriate to recycle production waste and to produce environmentally friendly binders, such as alkali-activated materials. Yu et al.1 stated that due to suitable chemical composition, biomass bottom ash (BBA) can be used as precursor for alkaline binders. In another study2 agricultural waste ash is a type of biomass ash and can be used as a precursor for the preparation of alkali activated binders or as a supplementary cementitious material in Ordinary Portland cement systems. Blesson et al.3 concluded that ash from the agricultural industry exhibits both pozzolanic and hydraulic bonding properties and can be a suitable material to produce alkali-activated binders. The use of calcined aluminosilicate initial materials in this case resulted in better mechanical properties of the binders than non-calcined materials. The study4 is unique in that silica-rich biomass ash such as rice husk ash, sugar cane bagasse ash, and corn cob was used as a precursor and alkali activator (sodium silicate solution) for the preparation of alkali activated materials.
Some silicon- and aluminum-rich precursors (biomass ash) have low reactivity, and it is therefore possible to develop certain blends to increase the reactivity of precursors. Ling et al.5 found that BBA has a relatively low reactivity and should therefore be blended with another type of aluminum- and silicon-rich materials, such as granulated ground blast furnace slag and fly ash, which increases the reactivity of the precursor. Substitution BBA with granulated ground blast furnace slag led the improved the mechanical properties, with an optimum replacement rate of 10% by weight. In this case, the maximum compressive strength of the samples was 54.6 MPa. Alonso et al.6 used a blend precursor made from blast furnace slag, oil biomass ash and bottom ash to produce an alkali-activated binder. The KOH solution was used as an alkaline activator. After 28 days of hydration, the compressive strength of hardened cement pastes made from 30 wt% of blast furnace slag and 30 wt% of olive oil biomass ash was 18–33 MPa. In another study7 the precursor of alkali activated binders were made from three ingredients: sugarcane bagasse ash, granulated blast furnace slag and metakaolin. The mixture of sodium hydroxide and sodium silicate (water glass) solution was used as the activator. After 91 days, the compressive strength of the blends made from 15% by weight of sugarcane bagasse ash was 117.7 MPa. The higher amount of this type of ash reduced the compressive strength of the hardened cement paste. Blesson et al.8 produced a precursor from blends of calcined and non-calcined agricultural waste ash with granulated blast furnace slag. The use of calcined biomass ash increased the compressive strength (23.46 MPa) of the mortar by 11.02% compared to the mortar with non-calcined biomass ash (21.13 MPa) after 28 days of hydration. Thus, the calcination of agricultural waste ash slightly increased the compressive strength of the mortar. In the study9, a precursor for alkali-activated materials was made from the blends of biomass fly ash (BFA) and glass powder. The compressive strength of samples made only from BFA was 2.85–3.69 MPa. The incorporation of glass powder in the preparation of the precursor improved the compressive strength, which was 7.20-19.62 MPa after 14 days.
Wood bottom ash is another type of biomass ash. In some studies, wood ash is used as a precursor for the production of alkali-activated binders. Ayobami et al.10 concluded that the optimum of wood bio-ash in the aluminosilicate precursor blends improved the mechanical properties of the alkali-activated systems. Thi et al.11 included a significant amount of wood bottom ash in an alkali-activated blends of ground granulated blast furnace slag and class F fly ash. The use of biomass ash improved the mechanical properties of the samples. After 56 days of hydration, the compressive strengths ranged from 14.6 to 50.0 MPa. The highest compressive strength was obtained with 30% by weight of biomass ash, however the compressive strength was reduced with higher amounts of biomass ash between 50 and 70%. In other study12, ground wood ash has been used as an aluminosilicate precursor for to produce alkali-activated binders. The precursors were made of ground granulated blast furnace slag and blends of ground or unground wood ash. The highest amount of wood ash was 20 wt%. It was found that ground wood ash resulted in higher mechanical properties of the samples than unground wood ash. The aluminosilicate blends of precursors in the study13 were made from metakaolin and wood ash. Substituting wood ash with metakaolin improved the microstructure of alkali-activated blends. This improvement can be attributed to the compensatory effect of negative charges in the tetrahedra of SiO4 units, which form in alkali-activated compounds similar to zeolite structures. Silva et al.14 used eucalyptus ash as precursor of alkali activated systems. In this case the blends of precursors were made from silica fume and eucalyptus ash. After 28 days of hydration, the mortars activated with 5 mol/l NaOH solution had a compressive strength higher than 55 MPa.
The precursors in convenient alkali-activated materials are aluminum- and silicon-rich materials. Several studies have reported that iron-rich precursors can be successfully used for the production of alkali-activated materials. Koçyiğit et al.15 studied a mortar with an alkali-activated binder made from the blends made from ferrochrome slag waste and fly ash. The highest mechanical properties (compressive strength of 23.92 MPa) and durability (minimal water absorption of 7.26%) were achieved in the samples with a precursor made of 40% fly ash and 60% ferrochrome slag. An experimental study16 was carried out on alkali-activated bricks with a precursor made of iron ore tailings and ground granulated blast furnace slag. The best ratio of iron ore tailings to ground and granulated blast furnace slag was 85:15 with a compressive strength of 31.72 MPa. Moreover, the finer iron ore tailings particles led to higher compressive strength. Iron ore tailings reacted more slowly in alkaline environments than ground granulated blast furnace slag and a small amount of iron compound stabilizes the C-S-H gel. Larger amount of iron ore tailings in the alkali-activated slag matrix acted as an aggregate. The study of Levandoski et al.17 focused on the incorporation of iron ore tailings in the alkali activated sugar cane bagasse ash and hydrated eggshell lime blends. The maximum strength was 6.59 MPa and N-A-S-H-like gels were formed as hydration products in the samples. In this study18, the effect of iron-rich precursors on the high mechanical properties of alkali-activated materials was assessed. The optimum alkaline activator was soluble glass with a silicate modulus of 1.2–1.6. The samples reached compressive strength over 100 MPa. These high mechanical properties are explained by iron’s ability to participate in the formation of new hydration products and the transformation of Fe2+ to Fe3+ is now taking place.
Previous studies have shown that biomass ash has a relatively low reactivity and should therefore be blended with another type of Al- Fe- and/or Si-rich materials. Several studies have reported that iron-rich precursors can be successfully used for the production of alkali-activated materials. So, the scientific literature contains data on the use of various iron compounds such as ferrochrome slag waste, iron ore tailings, red mud alkaline-activated systems. In the present study iron sludge (IS) was used as high-volume Fe-rich component. The novelty of this study is that IS from Lithuanian ship repair company as local available by-product was included in the precursor blends. Due to BFA relatively low reactivity and low amount of aluminum, it is commonly landfilled and IS is expected to enrich the system with iron and improve the reactivity of the precursor. The aim of this study is to determine the effect of IS by-product on the main properties of alkali-activated BFA. Two types of curing conditions were used: higher temperature (at 60 °C for 24 h) and at ambient temperature. This has led to the study of ecological and technological problems.
Materials and methods
Initial materials
In this study, the precursor was made from the blends of biomass fly ash (BFA) and iron sludge (IS).
BFA was collected from local Lithuanian biofuel boiler plant. The biofuel boiler is equipped with a bubbling fluidized bed system using silica sand in the fraction of 0.5–0.8 mm. The boiler has a combustion temperature of approximately 835–860 °C. After the biofuel has burned, the hot mixture of sand and ash is cooled with water and transported to the ash bin. In this ash SiO2 and CaO dominated: the sum of these oxides consisted of 65.62 wt% (Table 1).
The second material of precursor is IS, and this waste is also of indigenous origin (Lithuanian). This waste comes from a ship repair company and is dominated by metal production waste dust. Its recovery is quite problematic, and ways are being sought to recover this waste more cheaply and efficiently. One possible solution is the use of this waste in the production of an alkali-activated binder. In IS iron oxide dominated, and it consists of more than 98%wt (Table 1).
Mineral composition of BFA and IS according to X-ray diffraction (XRD). Q is quartz SiO2 (pdf No 5-490), CC is calcite CaCO3 (pdf No 1-837), K is calcium silicate hydrate Ca1.5SiO3.5·H2O (pdf No 33–306), AN is anhydrite CaSO4 (pdf No 72–503), CO is calcium oxide CaO (pdf No 77-2376), A is ferrite, Fe (pdf No 3-1050); B is iron oxide Fe0.974O (pdf No 73-2143); C is hematite Fe2O3 (pdf No 73–603); D is magnetite Fe3O4 (pdf No 3-863).
The mineral composition of BFA and IS was determined by XRD analysis (Fig. 1). In BFA quartz and calcite is dominated and small amount of calcium silicate hydrate, anhydrite, and calcium oxide detected as crystalline chemical compounds. Similar chemical compounds were determined by Mladenović et al.13. Calcite and larnite dominated in investigated wood ash according to XRD analysis. According to Lei et al.19 the main crystalline phases of biomass ash from agricultural residues are quartz and albite. Crystalline compounds such as ferrite, iron oxide, hematite and magnetite form the mineral composition of IS.
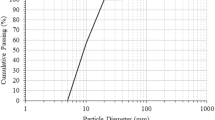
Scanning electron microscope analysis was used for the evaluation of granulometric composition for precursor materials (Fig. 2). Most of the BFA particles are irregularly shaped and angular, while the shape of the IS consisted of round particles. A similar irregular shape of BFA particles was found in this study by Munawar et al.20. The particle size distribution of BFA ranged from 0.1 μm to 500 μm with a mean diameter of 58.7 μm (Fig. 2). IS particles are almost twice as large as BFA particles, ranging in size from 1.8 μm to 450 μm (mean diameter – 108.92 μm).
Preparation and test of the samples
The reference samples were produced from BFA only and the other samples were formed by replacing part of the BFA with IS in different proportions. The proportions of the sample mixtures are given in Table 2.
The samples used in the study were formed by mixing BFA and IS according to the scheme from Fig. 3. This mixture was poured with sodium hydroxide (NaOH) solution. The resulting mass was then stirred for 3–10 min until a flowable mortar was formed. This mortar was used to fill molds of size 20 × 20 × 20 mm. The mortar in the molds was vibrated on a vibratory plate to remove additional air and compact the mortar. Finally, the molds are prepared for curing or heating by placing the mortar with the mold in a sealed plastic bag to prevent the evaporation of the air needed for hydration.
One part of the prepared samples was placed in the heating oven. The samples were cured at 60 °C for 24 h, then taken out and left to harden up to 7 and 28 days under ambient conditions. On the first day after forming, the samples are cured at ambient temperature for 24 h to allow the formation of crystallization seeds, which accelerates the formation of hydration products such as zeolitic structures21,22. Previous research demonstrated that oven-hardened samples had higher compressive strength values than those hardened at ambient temperature23. It is known that a curing temperature of 60 ◦C and a curing time of 24 h are optimal for best mechanical properties of alkali activated systems24.
The other half of the samples weren’t heated, but simply left to harden up to 28 and 90 days under ambient temperature.
Experimental techniques
The elemental composition of the initial materials was determined by X-ray fluorescence spectrometry (XRF). The XRF analysis of the initial materials was carried out on a Bruker X-ray S8 Tiger WD using a rhodium (Rh) tube, an anode voltage Ua up to 60 kV, and an electric current I up to 130 mA.
The mineral composition of the initial materials for the precursor and alkali activated samples was determined by X-ray diffraction analysis (XRD). A Bruker D8 Advance X-ray diffractometer with Bragg-Brentano geometry, using Ni-filtered CuKα radiation, operating up to a voltage of 60 kV, scanning at 0.02 degree increments was used. Oxford Cryosystems Crystallographica Search-Match software was used to determine the reflections and individual phases.
Microstructures of initial materials and alkali activated binders were evaluated according to scanning electron microscopy (SEM). The high-resolution SEM microscope Hitachi S–3400 N Type II was used with the employed acceleration voltages were 5 and 15 kV.
Particle size distribution analysis was carried out using a laser. Therefore, the analyses were performed with the Cilas 1090 laser particle size analyzer, with a measuring range of 0.1–500 μm. The analyses were carried out in a dry dispersion mode.
Particle size distribution analysis was carried out using a laser. Therefore, the analyses were carried out with a laser particle size analyzer Cilas 1090 with a measuring range of 0.1–500 μm. The analyses were performed in dry dispersion mode.
Compressive strength tests of the hardened alkali activated binders were carried out by using ToniTechnik 2020.0600/132/02 computerized press. The compression test was performed according to the European standard EN 13,279–2. The compression test rate was 0.6 MPa/s for a reference area of 4 cm2.
Results and discussion
Mechanical properties of alkali activated binders made from a blend of biomass ash and iron sludge
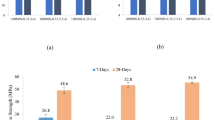
The main properties of construction materials are mechanical properties, and the most popular of the mechanical properties is compressive strength. After 7 days of hydration, the addition of IS had a positive effect on the compressive strength, only for the first group of samples, where the content of sodium hydroxide solution was lowest. In general, when comparing the mean values of the maximum compressive strengths of the reference group of samples with those of all the other samples with the IS, the change in the maximum strength is not significant. These results are presented in Fig. 4. The maximum strength value of the reference samples was 6.7 MPa, while after the addition of iron sludge the strength decreased slightly to 6.3 MPa. When the curing period was extended to 28 days, only one sample exceeded the compressive strength of the reference sample. After 28 days the maximum compressive strength was 6.9 MPa and it was slightly higher than reference sample without the IS (6.7 MPa). Thus, the longer curing period and the different IS content of the heated specimens did not appreciably affect the compressive strength of the alkaline-activated BFA-IS mixture.
Another similar study by M.A. Gomez-Casero et al.25 investigated alkali-activated systems made from ground black steel slag and biomass bottom ash. The compressive strength of the heated (60 ⁰C for 24 h) samples increased from 22 MPa to 41 MPa after 28 days, when the ratio between BBA and BBS changed from 100:0 to 50:50. However, the paper notes that this increase in compressive strength is due to the higher CaO content and the reduced Si/Al molar ratio.
The samples cured under ambient conditions showed more significant changes in compressive strength compared to the reference samples as shown in Fig. 5. The samples cured for 28 days had a compressive strength of 6.2 MPa, whereas the reference samples had a compressive strength of 8 MPa. In contrast, the samples cured for 90 days reached a compressive strength of 9.8 MPa, whereas the reference samples reached 8.9 MPa. It was also observed that the compressive strength increased continuously with increasing IS content.
The compressive strength of the samples cured for 28 days under ambient conditions decreased or did not change at all with increasing iron content in the precursor mixtures. A similar result was also obtained by another researcher26 who used high-calcium ashes and iron ore tailings. However, iron does not have a positive effect on the compressive strength of the samples cured in ambient conditions for 28 days, but the results change when the samples are cured for 90 days. In the current study, all the samples to which IS was added showed an increase in compressive strength after 90 days compared to the values obtained after 28 days. This trend was also observed in a study by the authors25. Iron has a significant effect on the BFA when the samples are cured for at least 90 days under ambient conditions.
Further analysis of the data was carried out by evaluating the compressive strength through the molar ratios of the chemical elements (Na + Ca)/(Al + Fe) and Si/(Al + Fe). The diagrams for this analysis are shown in Fig. 6. The results for the heated samples cured for 28 days are given in part (a) and the enlarged zone of maximum compressive strength is given in part (c). For samples cured under ambient conditions for 90 days, the graph is given in part (b) and the enlarged area of the maximum compressive strength is given in part (d).
Meanwhile, for the samples cured at higher temperature, the zone of maximum compressive strength was found to be at (Na + Ca)/(Al + Fe) ratios of 5–7 and Si/(Al + Fe) ratios of 2.5–4.5. In contrast, the zone of maximum compressive strength of the samples cured under ambient conditions is at 1.5–2.5 molar ratio of Si/(Al + Fe) and at 3–4 molar ratio of (Na + Ca)/(Al + Fe). The obtained molar ratios are similar to the ratios for the maximum compressive strength of metakaolin-based alkali-activated concretes, with Si/Al and Na/Al ratios of 1.9–2.5 and 1.0-1.3, respectively27. Although the test program did not come close enough to the range of optimal ratios, it is sufficient to make some conclusions. The molar ratios obtained for samples cured for 90 days under ambient conditions are decreasing (compressive strength increasing) to the range of the optimum ratios for alkali-activated concretes based on metakaolin. This means that the compressive strength of samples cured for 90 days under ambient conditions can still increase when the iron content is increased, with a decrease in the molar ratio.
The ratios of the heated samples are different from the theoretical ratios of alkaline-activated concretes, which indicate that only a limited amount of iron is reacted, while the remainder acts as an inert aggregate. It cannot be excluded that the heating treatment may have accelerated the hydration process in early age and may have resulted in a poorer microstructure, which may have adversely affected the mechanical properties at a later age of curing28.
Mineral composition of alkali activated biomass ash and iron sludge
After 28 days of curing, the three types of samples (cured at 60 °C for 24 h) with the highest compressive strength values were selected to determine the mineral composition (Fig. 7). The X-ray diffraction analysis of hardened pastes revealed all three samples have similar mineral composition and the incorporation of IS significantly does not change the mineral composition of samples.
X-ray diffraction patterns of alkali activated BFA with IS after 28 days of hydration cured at 60 ⁰C for 24 h. Notes: Q – quartz, SiO2 (77–1060); P – portlandite, Ca(OH)2 (44–1481); K – calcium silicate hydrate, Ca1.5SiO3.5x H2O (33–306); S – sodium carbonate hydrate Na2CO3(H2O) (70–845); Z – calcium aluminum silicate hydrate Ca5.57 Al12.3 Si12 O49.2 H2.34 (76–1507); X – calcium aluminum oxide carbonate sulfide hydrate Ca4Al2O6(CO3)0.67(SO3)0.33·11H2O (41–476).
In all samples, quartz and portlandite dominated as crystalline compounds. The main hydration products such as calcium silicate hydrate, calcium aluminum silicate hydrate and calcium aluminum oxide carbonate sulfide hydrate are possible detect. In addition, the carbonation process produces sodium carbonate hydrate. No crystalline iron phases were detected in the mineral composition of the alkali activated BFA and IS blends. Similar conclusions have been reported in previous studies29,30, which reported that no new iron phases were observed during the studies. Comparable crystalline compounds were detected by Silva et al. and Du et al.9,31. After alkali activation quartz and calcite were the major crystalline phases presented in the alkali activated biomass ash and these minerals are incompletely reacted by alkaline reactions. In a previous study32, quartz was thus found to be the main crystalline compound in an alkali-activated BFA system. In addition to these two minerals from precursor (biomass ash) new chemical compounds such as calcium silicate hydrate and calcium aluminum silicate hydrate was detected33. In this study slightly higher peaks of calcium aluminum silicate hydrate (Z) was determined for the H28-Fe-10-1 sample compared to the samples with higher amount of IS (H28-Fe-30-1). In this case, the compressive strength of this sample was the highest and the newly formed calcium aluminum silicate hydrate may have had a positive effect on the strength values. Newly formed similar hydration products such as calcium (aluminum) silicate hydrate after alkali activation of BFA have been identified by Ates et al.34.
A similar mineral composition of the crystalline compounds was found for the ambient hardened samples (Fig. 8). Quartz was the predominant crystalline compound, while portlandite, calcium silicate hydrate, sodium carbonate hydrate, calcium alumina carbonate sulfide hydrate was detected as traces. The formation of phases with iron was also not detected by XRD. The addition of IS (A90-Fe-30-1) also did not detect calcium aluminum silicate hydrate. Davidovits et al.35 stated that the low solubility of iron under alkaline conditions slowed the formation of crystalline hydration products. The low solubility (reactivity) of iron compounds is due to the formation of low amounts of hydration products in alkali-activated matrix systems36. So, according to XRD for the samples (with or without IS) cured at higher temperature the main hydration products were calcium silicate hydrate, calcium aluminum silicate hydrate and calcium aluminum oxide carbonate sulfide hydrate. For the ambient hardened samples, the incorporation of IS (A90-Fe-30-1) slowed down the formation of crystalline hydration products due to low solubility of iron compounds. In this case calcium aluminosilicate hydrate was not detected.
X-ray diffraction patterns of alkali activated BFA with IS after 90 days of hydration. Q – quartz, SiO2 (77–1060); P – portlandite, Ca(OH)2 (44–1481); K – calcium silicate hydrate, Ca1.5SiO3.5x H2O (33–306); S – sodium carbonate hydrate Na2CO3(H2O) (70–845); Z – calcium aluminum silicate hydrate Ca5.57 Al12.3 Si12 O49.2 H2.34 (76–1507); X – calcium aluminum oxide carbonate sulfide hydrate Ca4Al2O6(CO3)0.67(SO3)0.33·11H2O (41–476).
FT-IR analysis was used to identify amorphous and crystalline compounds alkali activated BFA after curing period (Fig. 9).
The first weak band at 3635 cm− 1 corresponds to portlandite. According to Hoyos-Montilla et al.37, this band is attributed to the O–H stretching vibration of calcium hydroxide. The broad bands at 3247–3384 cm− 1 and about 1649 cm− 1 are attributed to stretching and bending vibrations of the hydroxyl groups of water respectively38. Zeolite structure which formed during alkali activation process related to the weak band at 2360 cm− 139. The bands around 865, 1400 and 1446 cm− 1 are associated with C-O bonds. The first carbonate group at 1446 cm− 1 presents asymmetric stretching vibration carbonate group of the calcium carbonate. The second group at 1400 cm− 1 is assigned to sodium carbonate hydrate. Band at 865 cm− 1 corresponds to C-O vibrations in CaCO38,37. Peaks 1099 cm− 1 are observed for all samples correspond to Si–O asymmetrical stretching vibration and this band is attributed to the quartz from BFA9,40.
Two main bands at about 937 cm− 1 and 1031 cm− 1 assigned to alkali activation reaction products which are closely related to the alkali activation level. The – Si–O–T (T = Al, Fe or Si), group’s vibration of the band (1031 cm− 1) evidences the presence of N-A/F-S-H gel. The bands, which appear at approximately 937 cm− 1 vibration are related to the presence of C-(A/F)-S-H gel37,41. The higher alkali activation has samples hardened at ambient condition (A90-Fe-0-1 and A90-Fe-30-1) and the bands at about 937 cm− 1 and 1031 cm− 1 have a higher intensity compared to the samples cured at higher temperature in this case. By comparing samples cured at higher temperature the highest alkali activation has samples H28-Fe-10-1. Higher alkali activation led to higher strength (Figs. 4 and 5).
In summary, the following can be said according to FT-IR analysis the higher alkali activation reaction is presented by samples hardened at ambient condition (A90-Fe-0-1 and A90-Fe-30-1). Two main bands at about 937 cm− 1 and 1031 cm− 1 assigned to alkali activation reaction products: N-A/F-S-H and C-(A/F)-S-H gel. Higher alkali activation led to higher strength values.
Microstructure of alkali activated biomass Ash and iron sludge
The effect of IS on the microstructure of alkali activated BFA is shown in Fig. 10. In all cases, the matrix of alkali activated BFA was heterogeneous. At the higher curing temperature porous and voids materials can be detected in all samples, but the addition of IS changes the compactness of this matrix. The densest matrix was found in the H28-Fe-10-1 sample containing 10 wt % IS. The higher content 30 wt % of IS increased the porosity of the matrix (H28-Fe-30-1). After 28 days of hydration, the IS of the heated sample reacted with sodium hydroxide and the lower NaOH content of the system leads to a reduction in alkali activation reaction. This reduction is related to the mechanical strength decrease. Thejas et al.1,42 determined a similar influence of the amount of waste iron ore tailings on the alkali activated matrix. The more compact matrix is due to the inclusion of a 40% iron compound compared to samples using 90% waste iron ore tailings.
Different impact of IS is in the samples cured in ambient conditions. In this case 30 wt % IS (A90-Fe-30-1) led to the formation of the dense and compact matrix which led to the increase in compressive strength. A similar trend was found in their studies by Marathe et al.43. Under ambient conditions, the IS acted as a low-reactive compound or filler. According to XRD analysis, no calcium aluminum silicate hydrate is formed in sample A90-Fe-30-1 (Fig. 8). It is possible that the IS particles were incorporated into the matrix, making it more compact and with better mechanical properties. However, part of the IS takes part in the alkali activation reaction, forming two types of gels: N-A/F-S-H and C-(A/F)-S-H (Fig. 9).
This study is relevant from the perspectives of engineering, economics, and environmental protection. Closely linked to environmental protection are the saving of natural resources, the recovery of waste and the development of new type binder. This prevents environmental pollution and reduces landfill space. In the economical case, the production of this binder is based on the circular economy. The use of local waste or by-products saves transport costs, energy and makes the production technology less expensive than conventional binders. The relevance of technical factors of the proposed binders relate to their durability, as they contain hydration products of a zeolitic nature, which give the specimens their durability properties. Kryvenko et al.44 published a similar review study and argued that alkali-activated cements as sustainable materials have engineering, economics, and environmental protection benefits.
Conclusions
When samples cured at higher temperature, IS acted as a reactive component and reacted with NaOH to reduce its content in the system reducing alkali activation reaction. In samples cured at ambient temperature, IS acted as a less reactive component or as a filler: no zeolitic crystalline phase (calcium aluminum silicate hydrate) were detected, but a higher gel content was formed and IS was incorporated into the matrix making it more compact. The incorporation of IS in alkali activated BFA systems changed the mechanical properties of samples which is close related to the mineral composition and microstructure.
-
After 28 days of curing, the maximum compressive strength of heated sample was 6.9 MPa (90% BFA and 10% IS) and it was slightly higher than reference sample without the IS (6.7 MPa). The additional IS content may have slightly increased the calcium aluminum silicate hydrate content and increased the intensity of the alkaline reaction, but an include of more than 10% in IS content reduces these effects, resulting in a less compatible matrix.
-
Meanwhile the samples cured under ambient conditions showed more significant changes in compressive strength compared to the reference samples (8.9 MPa). The highest compressive strength of 9.8 MPa was achieved by samples cured for 90 days on a mixture of 30% IS and 70% BFA. The incorporation of IS (A90-Fe-30-1) slowed down the formation of crystalline hydration products due to low solubility of iron compounds. In this case calcium aluminosilicate hydrate was not detected. The higher alkali activation reaction was presented by to alkali activation reaction products in FT-IR: N-(A/F)-S-H and C-(A/F)-S-H gel. Higher alkali activation led to higher strength values.
-
In summary, it is difficult to compensate for the aluminum deficiency in alkali-activated BFA systems, but the results show that the deficiency can be partially compensated for by iron. However, the use of iron has its disadvantages: in a heated system (60 oC), the use of iron is rather limited to 10%. In an alkali-activated system, under ambient conditions, iron prolongs the setting time, acts as a retarder and prevents the formation of crystalline calcium aluminosilicate hydrate.
-
Although the practical use of the alkaline-activated concrete made from BFA and IS in this work is quite limited, the possibility cannot be ruled out that such concrete could be used as a leveling layer, which is necessary for the installation of reinforcement and concrete casting.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Yu, X. et al. Review of the materials composition and performance evolution of green alkali-activated cementitious materials. Clean Technol. Environ. Policy. 25 (5), 1439–1459. https://doi.org/10.1007/s10098-023-02478-3 (2023).
Thomas, B. S. et al. Biomass ashes from agricultural wastes as supplementary cementitious materials or aggregate replacement in cement/geopolymer concrete: A comprehensive review. J. Building Eng. 40, 102332. https://doi.org/10.1016/j.jobe.2021.102332 (2021).
Blesson, S. & Rao, A. U. Agro-industrial-based wastes as supplementary cementitious or alkali-activated binder material: a comprehensive review. Innovative Infrastructure Solutions. 8 (4), 125. https://doi.org/10.1007/s41062-023-01096-8 (2023).
Liu, Z., Deng, P. & Zhang, Z. Application of silica-rich biomass Ash solid waste in geopolymer preparation: A review. Constr. Build. Mater. 356, 1291425. https://doi.org/10.1016/j.conbuildmat.2022.129142 (2022).
Ling, X., Chen, W., Schollbach, K. & Brouwers, H. J. H. Valorization of biomass bottom Ash in alkali-activated GGBFS-fly Ash: impact of biomass bottom Ash characteristic, silicate modulus and aluminum-anodizing waste. Constr. Build. Mater. 428, 136408. https://doi.org/10.1016/j.conbuildmat.2024.136408 (2024).
Alonso, M. M. et al. Olive biomass Ash as an alternative activator in geopolymer formation: A study of strength, radiology and leaching behaviour. Cem. Concr. Compos. 104, 103384. https://doi.org/10.1016/j.cemconcomp.2019.103384 (2019).
Sousa, L. N. et al. Effect of non-calcined sugarcane Bagasse Ash as an alternative precursor on the properties of alkali-activated pastes. Molecules 27 (4), 1185. https://doi.org/10.3390/molecules27041185 (2022).
Blesson, S., Rao, A. U., Bhandary, R. P., Shetty, P. P. & Thomas, B. S. Comparative characteristics assessment of calcined and uncalcined agro-based waste Ash with GGBS and its application in an alkali-activated binder system. Cogent Eng. 10 (1), 2220483. https://doi.org/10.1080/23311916.2023.2220483 (2023).
Silva, G. J. B., Santana, V. P. & Wójcik, M. Investigation on mechanical and microstructural properties of alkali-activated materials made of wood biomass Ash and glass powder. Powder Technol. 377, 900–912. https://doi.org/10.1016/j.powtec.2020.09.048 (2021).
Ayobami, A. B. Performance of wood bottom Ash in cement-based applications and comparison with other selected Ashes: overview. Resour. Conserv. Recycl. 166, 105351. https://doi.org/10.1016/j.resconrec.2020.105351 (2021).
Thi, K. D. T., Liao, M. C. & Vo, D. H. The characteristics of alkali-activated slag-fly Ash incorporating the high volume wood bottom Ash: mechanical properties and microstructures. Constr. Build. Mater. 394, 132240. https://doi.org/10.1016/j.dibe.2023.100125 (2023).
Teker Ercan, E. E., Cwirzen, A. & Habermehl-Cwirzen, K. The effects of partial replacement of ground granulated blast furnace slag by ground wood Ash on Alkali-Activated binder systems. Materials 16 (15), 5347. https://doi.org/10.3390/ma16155347 (2023).
Mladenović Nikolić, N. N. et al. Radiological and structural characterization of Raw and Alkali-Activated wood Ash and Metakaolin blends. Sustainability 14 (20). https://doi.org/10.3390/su142012960 (2022).
Silva, T. H., Lara, L. F., Silva, G. J., Provis, J. L. & Bezerra, A. C. Alkali-activated materials produced using high-calcium, high-carbon biomass Ash. Cem. Concr. Compos. 132, 104646. https://doi.org/10.1016/j.cemconcomp.2022.104646 (2022).
Koçyiğit, Ş. Performance evaluation of geopolymer mortars containing waste ferrochrome slag and fly Ash for sustainable green Building. Sci. Rep. 14 (1), 14606. https://doi.org/10.1038/s41598-024-65552-w (2024).
Kang, X. et al. Synthesis and characterization of sustainable Eco-Friendly Alkali-Activated High-Content Iron ore tailing bricks. Buildings 13 (11), 2743. https://doi.org/10.3390/buildings13112743 (2023).
Levandoski, W. M. K., Ferrazzo, S. T., Bruschi, G. J., Consoli, N. C. & Korf, E. P. Mechanical and microstructural properties of iron mining tailings stabilized with alkali-activated binder produced from agro-industrial wastes. Sci. Rep. 13 (1), 15754. https://doi.org/10.1038/s41598-023-42999-x (2023).
Ponomar, V., Yliniemi, J., Adesanya, E., Ohenoja, K. & Illikainen, M. An overview of the utilisation of Fe-rich residues in alkali-activated binders: mechanical properties and state of iron. J. Clean. Prod. 330, 129900. https://doi.org/10.1016/j.jclepro.2021.129900 (2022).
Lei, Z. & Pavia, S. Geopolymer based on biomass Ash from agricultural residues. Constr. Build. Mater. 441, 137471. https://doi.org/10.1016/j.conbuildmat.2024.137471 (2024).
Munawar, M. A. et al. Biomass Ash characterization, fusion analysis and its application in catalytic decomposition of methane. Fuel 285, 119107. https://doi.org/10.1016/j.fuel.2020.119107 (2021).
Nguyen, D. K. et al. Effects of aging and hydrothermal treatment on the crystallization of ZSM-5 zeolite synthesis from bentonite. RSC Adv. 13 (30), 20565–20574. https://doi.org/10.1039/D3RA02552G (2023).
Rees, C. A., Provis, J. L., Lukey, G. C. & Van Deventer, J. S. The mechanism of geopolymer gel formation investigated through seeded nucleation. Colloids Surf., A. 318 (1–3), 97–105. https://doi.org/10.1016/j.colsurfa.2007.12.019 (2008).
Kumaravel, S. Development of various curing effect of nominal strength geopolymer concrete. J. Eng. Sci. Technol. Rev. 7, 116–119 (2014).
Nasvi, M. M., Gamage, R. P. & Jay, S. Geopolymer as well cement and the variation of its mechanical behavior with curing temperature. Greenh. Gases Sci. Technol. 2, 46–58. https://doi.org/10.1002/ghg.39 (2012).
Gómez-Casero, M. A., Pérez-Villarejo, L., Castro, E. & Eliche-Quesada, D. Effect of steel slag and curing temperature on the improvement in technological properties of biomass bottom Ash based alkali-activated materials. Constr. Build. Mater. 302, 124205. https://doi.org/10.1016/j.conbuildmat.2021.124205 (2021).
Bezerra, A. C. D. S., França, S., Magalhães, L. F. D. & Carvalho, M. C. R. D. Alkaline activation of high-calcium Ash and iron ore tailings and their recycling potential in Building materials. Ambiente Construído. 19 (3), 99–112. https://doi.org/10.1590/s1678-86212019000300327 (2019).
Rowles, M. & O’connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 13 (5), 1161–1165. https://doi.org/10.1039/B212629J (2003).
Emmanuel, A. C., Krishnan, S. & Bishnoi, S. Influence of curing temperature on hydration and microstructural development of ordinary Portland cement. Constr. Build. Mater. 329, 127070. https://doi.org/10.1016/j.conbuildmat.2022.127070 (2022).
Ozturk, M., Bankir, M. B., Bolukbasi, O. S. & Sevim, U. K. Alkali activation of electric Arc furnace slag: mechanical properties and micro analyzes. J. Building Eng. 21, 97–105. https://doi.org/10.1016/j.jobe.2018.10.005 (2019).
Lemougna, P. N., Mackenzie, K. J. D., Jameson, G. N. L. & Chinje, H. R. U. F. The role of iron in the formation of inorganic polymers (geopolymers) from volcanic Ash: a 57Fe Mossbauer spectroscopy study. J. Mater. Sci. 48, 5280–5286. https://doi.org/10.1007/s10853-013-7319-4 (2013).
Du, Y. et al. A review of biomass wood Ash in alkali-activated materials: treatment, application, and outlook. J. Compos. Sci. 8 (5), 161. https://doi.org/10.3390/jcs8050161 (2024).
Cabrera, M., Galvin, A. P., Agrela, F., Carvajal, M. D. & Ayuso, J. Characterisation and technical feasibility of using biomass bottom Ash for civil infrastructures. Constr. Build. Mater. https://doi.org/10.1016/j.conbuildmat.2014.01.087 (2014).
Rajamma, R., Labrincha, J. A. & Ferreira, V. M. Alkali activation of biomass fly ash–metakaolin blends. Fuel 98, 265–271. https://doi.org/10.1016/j.fuel.2012.04.006 (2012).
Ates, F., Park, K. T., Kim, K. W., Woo, B. H. & Kim, H. G. Effects of treated biomass wood fly Ash as a partial substitute for fly Ash in a geopolymer mortar system. Constr. Build. Mater. 376, 131063. https://doi.org/10.1016/j.conbuildmat.2023.131063 (2023).
Davidovits, J. & Davidovits, R. Ferro-Sialate geopolymers (-Fe-O-Si-O-Al-O-). Geopolymer Institute library. Www Geopolymer Org. https://doi.org/10.13140/RG.2.2.25792.89608/2 (2020).
Djobo, J. N. Y. & Tome, S. Insights into alkali and acid-activated volcanic ash-based materials: A review. Cem. Concr. Compos. 105660. https://doi.org/10.1016/j.cemconcomp.2024.105660 (2024).
Hoyos-Montilla, A. A., Puertas, F., Mosquera, J. M. & Tobón, J. I. Infrared spectra experimental analyses on alkali-activated fly ash-based binders. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 269, 120698. https://doi.org/10.1016/j.saa.2021.120698 (2022).
Liang, X., Dong, H., Li, Z., van Zijl, M. B. & Ye, G. Utilization of biomass fly ash in alkali-activated materials. In 4th International Rilem Conference on Microstructure Related Durability of Cementitious Composites, 805–812 (Delft University of Technology, 2021).
Kaplan, G. et al. Effect of quartz powder on mid-strength fly Ash geopolymers at short curing time and low curing temperature. Constr. Build. Mater. 329, 127153. https://doi.org/10.1016/j.conbuildmat.2022.127153 (2022).
Lee, W. K. W. & Van Deventer, J. S. J. Use of infrared spectroscopy to study geopolymerization of heterogeneous amorphous aluminosilicates. Langmuir 19 (21), 8726–8734. https://doi.org/10.1021/la026127e (2003).
Saikia, B. J., Parthasarathy, G. & Sarmah, N. C. Fourier transform infrared spectroscopic Estimation of crystallinity in SiO2 based rocks. Bull. Mater. Sci. 31, 775–779. https://doi.org/10.1007/s12034-008-0123-0 (2008).
Thejas, H. K. & Hossiney, N. Alkali-activated bricks made with mining waste iron ore tailings. Case Stud. Constr. Mater. 16, e00973. https://doi.org/10.1016/j.cscm.2022.e00973 (2022).
Marathe, S., Sheshadri, A. & Sadowski, Ł. Agro-industrial waste utilization in air-cured alkali-activated pavement composites: properties, micro-structural insights and life cycle impacts. Clean. Mater. 14, 100281. https://doi.org/10.1016/j.clema.2024.100281 (2024).
Kryvenko, P. et al. Alkali-activated cements as sustainable materials for repairing building construction: a review. J. Build. Eng. 109399. https://doi.org/10.1016/j.jobe.2024.109399 (2024).
Acknowledgements
This project has received funding from the Research Council of Lithuania (LMTLT), agreement No [S-PD-24-125].
Author information
Authors and Affiliations
Contributions
Author Contributions: D.Ž.: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Investigation. D. V.: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. R. P.B.: Writing – review & editing, Visualization, Validation, Formal analysis, Data curation, Conceptualization.L. V.: Writing – review & editing, Methodology, Formal analysis, Data curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Žūrinskas, D., Vaičiukynienė, D., Borg, R.P. et al. Sustainable alkali activated binders from a blend of biomass ash and iron sludge precursor. Sci Rep 15, 16428 (2025). https://doi.org/10.1038/s41598-025-00455-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00455-y