Abstract
Ultraviolet (UV) light is never used for gene transfer because it damages DNA and harms cellular and plasmid DNA. A light source selectively radiating Far UV-C (F-UV) light causes less DNA damage. We investigated potential introduction of plasmids into cells by irradiating them with F-UV light. COS-7 and CHO-K1 cells were irradiated with 222 nm F-UV light. Then, DNA solution containing green fluorescent protein (EGFP) plasmid was added to the culture and incubated (37 °C, 5% CO2, 24 h) for fluorescence microscopy. Cytotoxicity of cells irradiated with the same energy as for gene transfer was evaluated. Characteristics of EGFP-positive cells were compared with non-transfected cells by propidium iodide (PI) and Hoechst staining. Cells with distinct green fluorescence were observed after F-UV light irradiation and addition of 200 ng of EGFP plasmid. COS-7 cells showed the highest number of EGFP-positive cells at 0.5 mJ/cm2 irradiation, whereas that for CHO-K1 cells was at 1 mJ/cm2 irradiation. Cytotoxicity was low in COS-7 at ≤ 1 mJ/cm2 irradiation and CHO-K1 at ≤ 0.5 mJ/cm2. Transfected cells did not incorporate PI, and their nuclei did not differ from those of non-transfected cells. We successfully transfected two cell lines with EGFP plasmids by F-UV irradiation.
Similar content being viewed by others
Introduction
The functional analysis of targeted genes by introducing plasmids into cells is one of the fundamental techniques used in molecular biology. Chemical, biological, and physical methods of gene transfer have been developed, and improvements and developments of new methods have been reported1,2.
Gene transfer by light is a physical method that uses various wavelengths of light, including visible or infrared light3,4,5. Deep-ultraviolet (D-UV) light is well absorbed by nuclear DNA because the absorption maximums of the bases (A, G, T, and C) that form DNA are all in the approximate vicinity of 260 nm6,7. Ultraviolet (UV) light has never been used for gene transfer because it causes DNA damage and is harmful to cellular and plasmid DNA.
Recently, a light source was developed that can selectively radiate Far UV-C (F-UV) light, which has an even shorter wavelength than the D-UV light that causes DNA damage. This device uses excimer lamps as the light source and selectively radiates 222 nm F-UV light by using a filter to cut off wavelengths of UV light of 230 nm or longer that cause DNA damage8. Thus, F-UV light of 222 nm is less readily absorbed by DNA than D-UV light of around 260 nm and is less likely to cause DNA damage. As we found F-UV light penetrate cell membrane without DNA damage in a colon cancer cell line9, we speculated that F-UV can be used for gene transfection.
We investigated the possibility of introducing plasmids into cells by irradiating them with F-UV light, which causes less DNA damage.
Results
Cell transfection
Fluorescence images of COS-7 cells 24 h after irradiation with F-UV light and the addition of 200 ng of enhanced green fluorescent protein (EGFP) plasmid showed cells with a distinct green fluorescence. Phase-contrast microscopy showed that cells were almost confluent (Fig. 1A, B).
Plasmid transfection was attempted by varying the irradiation dose from 0.01 to 5 mJ/cm2. Figure 2 shows the results of the number of EGFP-positive cells per value of F-UV irradiation energy. COS-7 cells showed the highest number of EGFP-positive cells at 0.5 mJ/cm2 irradiation, and the efficiency was 0.234%. CHO-K1 cells showed the highest number of EGFP-positive cells at 1 mJ/cm2 irradiation, and the efficiency was 0.200%. In cells with no F-UV irradiation and only the addition of plasmid solution, only 1 or 2 cells showed green fluorescence (Figs. 2 and 3A).
The cytotoxicity of F-UV irradiation is shown in Fig. 3B. Irradiation at 5 mJ/cm2 induced cytotoxicity in both COS-7 and CHO-K1 cells, but cytotoxicity was low in COS-7 (at 1 mJ/cm2 or less) and CHO-K1 (at 0.5 mJ/cm2 or less).
Evaluation of transfected cells
The COS7 cells after plasmid transfection were continuously observed under a fluorescence microscope. The number of EGFP-positive cells increased up to 48 h after transfection, and then fluorescence was maintained for up to 144 h (Fig. 4A).
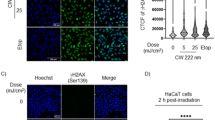
PI staining after transfection showed that among the COS-7 and CHO-K1 cells, PI was not incorporated into the transfected green fluorescence cells, but PI was incorporated into a few dead cells in culture (Fig. 4B). Morphological evaluation of nuclei by Hoechst staining showed no significant differences in nuclear morphology. The area and fluorescence intensity between transfected and non-transfected cells did not differ in the COS-7 and CHO-K1 cells (Fig. 5).
Discussion
UV light has not been used for gene transfer because it damages the DNA of cells and plasmids, but we believe that we were the first in the world to introduce plasmid DNA into cells without causing cell damage, using a light source that selectively emits F-UV. UV radiation can dimerize two adjacent pyrimidines in DNA to covalently bond together, forming dimers. The most common dimer is the cyclobutane pyrimidine dimer (CPD), which forms when two adjacent thymines bond together. This pyrimidine dimer interferes with DNA replication and transcription, leading to cell death, mutation, and chromosome instability10. Thus, UV light, which can damage the DNA of cells and plasmids, was not used for gene transfection.
F-UV light has a shorter wavelength than D-UV and a different absorption spectrum for DNA and proteins. F-UV radiation is less likely to cause DNA damage due to its lower absorption by DNA11. Naito et al., examined UV damage of plasmid DNA at 222 nm F-UV and 254 nm D-UV, which were transformed into E. coli HB101 competent cells after UV irradiation. They reported that the transformation efficiency is not reduced at 222 nm compared to 254 nm. This means that F-UV is harmless to plasmid DNA12. With F-UV irradiation, the amount of pyrimidine dimers in bacteria and cells appears to be lower. We have also reported that irradiation of monolayer-cultured colon cancer cell lines with 30 mJ/cm2 of F-UV induced cell death, but the amount of CPD in that case was extremely small9. We considered that F-UV radiation did not damage DNA based on these results. To detect minor DNA damage, it will be a future challenge to detect pyrimidine dimers such as CPD and 6-4PP and to perform modified comet assays13,14.
F-UV light is absorbed by proteins at about 20 times higher absorbance than is D-UV light15. It has been reported that when enzymes are irradiated with F-UV light in vitro, the enzyme activity is reduced12. In addition, F-UV irradiation significantly reduced the infectivity of adenovirus and coronavirus even though the amount of viral DNA quantified by PCR remained unchanged. F-UV irradiation may affect viral capsids more than nucleic acids16,17. When we irradiated a colon cancer cell line with 30 mJ/cm2 of F-UV, the cells swelled markedly, and the DNA-binding dye PI, which is taken up by cells and bacteria damaged plasma membranes18, was significantly incorporated, suggesting that the target is most likely the proteins of the plasma membrane9. We used F-UV light of extremely low energy and there was no PI uptake, suggesting that the genes were transfected without irreversible plasma membrane damage.
In this study, we could successfully transfect two cell lines with EGFP plasmids and clearly observed the transient expression EGFP. Although various methods of gene transfer have been developed, the problem with all of them is that they cause some degree of cytotoxicity. The advantage of our method is that it causes almost no cell damage during gene transfer. The efficiency of transfection was low, and it may be necessary to optimize the transfection based on the cell or plasmid used. Elucidation of the mechanism of transfer will be a future challenge.
Methods
Cells and cell culture
COS-7 cells (JCRB9127) is an African green monkey kidney fibroblast-like cell line. The cells (JCRB9127) were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS) at 37 °C in a 5% CO₂ atmosphere. CHO-K1 cells (JCRB9018) was derived from the ovary of an adult Chinese hamster. They were cultured in Ham’s F12 medium supplemented with 10% FBS at 37 °C in a 5% CO₂ atmosphere. COS-7 cells and CHO-K1 cells were purchased from JCRB Cell Bank (Japanese Collection of Research Bioresources Cell Bank).
F-UV irradiation equipment
The device that radiates F-UV light with a peak wavelength of 222 nm is the Care222, produced by USHIO INC (Tokyo, Japan). The 222 nm KrCl excimer lamp had a proprietary filter to remove longer and more penetrative wavelength. The irradiance of Care222 is 1 mW/cm2 at a distance of 10 cm from the object. The irradiation dose was adjusted to the values of 0.01, 0.05, 0.1, 0.5 ,1, and 5 mJ/cm² using a polytetrafluoroethylene film, a predetermined irradiation distance, and a fixed irradiation time.
F-UV irradiation
The cells were seeded at a density of 1.0 × 104 cells per well, with a total medium volume of 100 µL, in 96-well plates and incubated at 37 °C with 5% CO₂ for 24 h. Following the removal of the culture medium, the cells were washed twice with PBS and were irradiated with 222 nm light at the following doses: 5, 1, 0.5, 0.1, 0.05, and 0.01 mJ/cm². Exposure time was set between 3 and 6 s. Immediately after F-UV irradiation, 10 µL of DNA solution containing enhanced green fluorescent protein (EGFP) plasmid adjusted to 20 ng/µL with Opti-MEM (GIBCO) was added to the culture. Following 1 h of incubation, 90 µL of culture medium was added, and cells were incubated for 24 h at 37 °C in a 5% CO₂ atmosphere for fluorescence microscopy. Fluorescence images and 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 H-tetrazolium (MTS) assay were evaluated 24 h after irradiation with F-UV light. The plasmid used in this study contains the EGFP gene downstream of the cytomegalovirus promoter and is 4733 bp in size. To investigate the efficiency of the transfection, the MTS values were measured for each cell number and a calibration curve was constructed so that the cell number could be determined from the MTS values. The transfection efficiency was calculated by dividing the number of EGFP-positive cells counted under a fluorescence microscope by the total number of cells, which was determined from the MTS value. The cytotoxicity of cells irradiated with the same energy as for gene transfer was evaluated by MTS assay. The viability rate was calculated as the percentage of the MTS value of cells after F-UV irradiation compared to the MTS value of non-irradiated cells.
PI (Propidium Iodide) and Hoechst stain
After 24 h of plasmid transfection by F-UV light, the medium was removed and washed twice with PBS. Then, 100 µL of a mixture of 3 µg/mL Hoechst and 2 µg/mL propidium iodide (PI) was added to each well and allowed to react for 15 min at 37 °C. The PI was incorporated into the nuclei of dead cells whose cell membranes had been damaged. Hoechst binds to the adenine-thymidine-rich portion of DNA and stains the nuclei. From Hoechst staining images, the nuclear area and nuclear fluorescence intensity of the EGFP-positive cells were compared with those of the non-transfected cells (10 cells each) using ImageJ software. The nuclear area was determined by tracing it in ImageJ software, which facilitates the measurement of the average fluorescence intensity of each pixel within a defined area.
Statistical analysis
All data are presented as means ± SE. Data were compared with Student’s t tests, since the variance was shown to be equal with F test between the groups which were to be compared in this study. Differences were considered to be statistically significant at p < 0.05.
Data availability
Data availability from the corresponding author upon request.
References
Kim, T. K. & Eberwine, J. H. Mammalian cell transfection: the present and the future. Anal. Bioanal. Chem. 397 (8), 3173–3178. https://doi.org/10.1007/s00216-010-3821-6 (2010).
Mehier-Humbert, S. & Guy, R. H. Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Adv. Drug Deliv Rev. 57 (5), 733–753. https://doi.org/10.1016/j.addr.2004.12.007 (2005).
Yao, C. P., Zhang, Z. X., Rahmanzadeh, R. & Huettmann, G. Laser-based gene transfection and gene therapy. IEEE Trans. Nanobiosci. 7 (2), 111–119. https://doi.org/10.1109/TNB.2008.2000742 (2008).
Torres-Mapa, M. L. et al. Transient transfection of mammalian cells using a Violet diode laser. J. Biomed. Opt. 15 (4), 041506. https://doi.org/10.1117/1.3430730 (2010).
Stevenson, D. et al. Femtosecond optical transfection of cells: viability and efficiency. Opt. Express. 14 (16), 7125–7133. https://doi.org/10.1364/oe.14.007125 (2006).
Voet, D., Gratzer, W. B. & Cox, R. A. Doty Paul. Absorption spectra of nucleotides, polynucleotides, and nucleic acids in the Far ultraviolet. Biopolymers 1, 193–208. https://doi.org/10.1002/bip.360010302 (1963).
Sinha, R. P. & Häder, D. P. UV-induced DNA damage and repair: a review. Photochem. Photobiol Sci. 1 (4), 225–236. https://doi.org/10.1039/b201230h (2002).
Buonanno, M. et al. Germicidal efficacy and mammalian skin safety of 222-nm UV light. Radiat. Res. 187 (4), 483–491. https://doi.org/10.1667/RR0010CC.1 (2017).
Nishikawa, J. et al. Far-Ultraviolet light at 222 Nm affects membrane integrity in monolayered DLD1 Colon cancer cells. Int. J. Mol. Sci. 25 (13), 7051. https://doi.org/10.3390/ijms25137051 (2024).
Brash, D. E. & Haseltine, W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature 298 (5870), 189–192. https://doi.org/10.1038/298189a0 (1982).
Kreusch, S. et al. UV measurements in microplates suitable for high-throughput protein determination. Anal. Biochem. 313 (2), 208–215. https://doi.org/10.1016/s0003-2697(02)00460-8 (2003).
Naito, K., Sawadaishi, K. & Kawasaki, M. Photobiochemical mechanisms of biomolecules relevant to germicidal ultraviolet irradiation at 222 and 254 Nm. Sci. Rep. 12 (1), 18217. https://doi.org/10.1038/s41598-022-22969-5 (2022).
Azqueta, A. et al. Technical recommendations to perform the alkaline standard and enzyme-modified comet assay in human biomonitoring studies. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 843, 24–32. https://doi.org/10.1016/j.mrgentox.2019.04.007 (2019).
Collins, A. et al. Measuring DNA modifications with the comet assay: a compendium of protocols. Nat. Protoc. 18 (3), 929–989. https://doi.org/10.1038/s41596-022-00754-y (2023).
Goldfarb, A. R., Saidel, L. J. & Mosovich, E. The ultraviolet absorption spectra of proteins. J. Biol. Chem. 193 (1), 397–404 (1951).
Oh, C., Sun, P. P., Araud, E. & Nguyen, T. H. Mechanism and efficacy of virus inactivation by a microplasma UV lamp generating monochromatic UV irradiation at 222 Nm. Water Res. 186, 116386. https://doi.org/10.1016/j.watres.2020.116386 (2020).
Kitagawa, H. et al. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control 49 (3), 299–301. https://doi.org/10.1016/j.ajic.2020.08.022 (2021).
Rosenberg, M., Azevedo, N. F. & Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 9 (1), 6483. https://doi.org/10.1038/s41598-019-42906-3 (2019).
Acknowledgements
This work was financially supported by Ushio Inc.
Author information
Authors and Affiliations
Contributions
J. N. and Y. Shimizu contributed to the preparation of the manuscript. M. N., T. F., Y. Okada, T. Y., Y. Ogawa and T.K. performed the experiments and analyzed the data; Y. Suehiro and T. Yamasaki supervised the studies and contributed conceptual advice.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nishimura, M., Shimizu, Y., Fujii, T. et al. Transient transfection using 222 nm far UV-C irradiation. Sci Rep 15, 16787 (2025). https://doi.org/10.1038/s41598-025-00477-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00477-6