Abstract
The value of intravascular ultrasound (IVUS) in the diagnosis and treatment of the venous system is not well established. Introducing a novel approach to utilizing IVUS to evaluate cerebral venous sinus (CVS) stenosis and select stent. Idiopathic intracranial hypertension (IIH) patients with CVS stenosis who underwent IVUS-guided stenting were included in the data analysis from January 2014 to February 2022. The degree of maximum stenosis was determined based on the cross-sectional area (CSA) measured by IVUS, and a stent selection method was applied in the study. Follow-up evaluations were conducted at 6 months to 1 year after endovascular treatment to assess symptom improvement. Additionally, repeated digital subtraction angiography (DSA) or Magnetic resonance venography (MRV) / CT venography(CTV) was performed to evaluate the stent patency at 6 months to 1 year post-procedure. The study included 61 patients. IVUS indicated a lower degree of stenosis compared to conventional DSA measurements when evaluating the degree of stenotic segments preprocedure (74.84 ± 10.12% vs. 78.48 ± 8.72%, p = 0.035). Post-procedural CSA of the most severe stenotic segments showed significant improvement (36.44 ± 8.07 mm2 vs. 7.42 ± 3.28 mm2, p < 0.001). The stent achieved complete expansion (mean stent expansion index, 0.93 ± 0.20) with no significant change in the structure of the reference segment. The trans-stenotic mean pressure gradients (MPGs) across 61 patients significantly decreased from 11.00 ± 6.23 mmHg to 2.09 ± 2.34 mmHg. 47 out of 61 patients received imaging follow-up; among them, 44 (93.6%) demonstrated stent patency in the follow-up imaging. IVUS has great potential to evaluate the degree and extent of CVS stenosis, assist stent selection, and optimize stent position during the interventional procedure in conjunction with DSA.
Similar content being viewed by others
Introduction
It was well recognized that cerebral venous sinus (CVS) stenosis is common among patients with Idiopathic Intracranial Hypertension (IIH),1 and CVS stenting has increasingly become an effective treatment for these cases, particularly in refractory IIH cases2,3.
The cross-section view of the CVS more closely corresponds to a triangular shape4,5,6, which poses a difficulty in lesion assessment and appropriate stent selection using DSA. In addition to angiography, intravascular ultrasound (IVUS) can provide cross-sectional images of vessels and nearby structures, optimizing stent implantation. Recently, IVUS has been recommended for assessment of indeterminate coronary artery disease, carotid artery stenosis, or intracranial artery stenosis 7,8,9 However, the value of IVUS in the diagnosis and treatment of the venous system is not well established6.
The study aimed to assess the severity of CVS stenosis using real-time IVUS, and to propose a method for assisting stent selection based on IVUS.
Methods
Patients selection
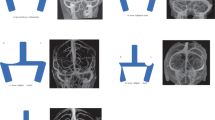
Patients presenting with IIH symptoms and undergoing endovascular treatment (EVT) for CVS stenosis between January 1, 2014, and February 1, 2022, in our center were prospectively enrolled. IIH diagnosis was based on: (1) papilledema; (2) normal neurological examination, except for cranial nerve abnormalities; (3) neuroimaging revealing normal brain parenchyma without hydrocephalus, mass, or any structural lesion; (4) normal CSF (cerebrospinal fluid) composition; (5) elevated CSF opening pressure (greater than 250 mmH2O); and (6) additional neuroimaging features supporting an IIH diagnosis. In addition, a diagnosis of IIH can be made even in the absence of papilledema if criteria 2 and 3 are satisfied for unilateral or bilateral abducens nerve palsy10. Candidates for VSS were selected based on intractable headaches, progressive papilledema, refractory to standard conservative treatment, and a diagnosis of CVS stenosis ≥ 50% with trans-stenotic MPGs of at least 8 mmHg6. MRV, CTV, or DSA was used for screening and following up on CVS stenosis. Exclusion criteria included concurrent venous sinus thrombosis and poor-quality of images. A total of 272 patients were intial enrolled in the study. Of these cases, 85 cases underwent IVUS assessment, and 61 of them underwent IVUS-guided stent implantation (Fig. 1).
The study was approved by the institutional review boards of our hospitals, and informed consent was obtained pre-procedure.
DSA and IVUS assessment
DSA examination was necessary to confirm the diagnosis and assess the CVS stenosis before and after stent placement. IVUS was conducted with a 3.5 F, 20 MHz Eagle Eye IVUS probe transducer. The probe range was set to 20 mm for comprehensive visualization of the venous wall. DSA and IVUS images were assessed by two experienced readers who were blinded to the patient’s clinical information. Any discrepancies were resolved by a third experienced reader.
In IVUS (Volcano Corporation, Rancho Cordova, CA, USA) assessment, the luminal lesion border was traced using a slow manual pullback technique at rates of approximately 1.0 mm/s, to calculate the luminal CSA. The reference segments in this study were defined as a segment 5 mm proximal and distal to the stenosis border and were confirmed as healthy segments without stenosis by IVUS. The method for calculating the degree of maximum stenosis of the lesion on IVUS is depicted in Fig. 2.
A diagram presented CVS stenosis induced by arachnoid granulations, accompanied by IVUS images of the resulting stenosis. (A) The diagram desplayed cross-sectional and longitudinal views of CVS stenosis sites caused by arachnoid granulations, along with reference sections from the proximal and distal segments. The blue section represented the CVS, while the black circles in the cross-sections marked the tips of the IVUS transducer. Sections b and d showed the cross-sectional and longitudinal views of the proximal and distal segments, respectively. Section c displayed the cross-sectional and longitudinal views of the stenosis sites caused by arachnoid granulations. Sections a and a1 exhibited significant arachnoid granulations within the CVS, leading to CVS stenosis. (B) IVUS images displayed cross-sectional and longitudinal views of CVS stenosis sites caused by arachnoid granulations. CSA 1 indicated the luminal CSA at the site of the most severe stenosis caused by arachnoid granulation, while CSA2 and CSA3 indicated the luminal CSA at the distal and proximal reference segments, respectively (a’ depicted significant arachnoid granulation within the CVS, resulting in CVS stenosis). The Fig. 2A was generated by Adobe Illustrator CC 2022 (http://www.adobe.com/cn/creativecloud/roc/business.html) .
Three measurements were recorded: the luminal CSA of the site with the most severe stenosis (CSA 1), the luminal CSA of the distal reference segment (CSA 2), and the luminal CSA of proximal reference segment (CSA 3). The formula for the degree of stenosis is (1-[CSA 1/(CSA 2 + CSA 3)/2])100%. The length of CVS stenosis was defined as the distance between the distal and the proximal ends of the stenosis border.
Stenosis severity on DSA was determined using the method outlined by James et al.11, the narrowest luminal diameter affected CVS, as well as the normal diameter of the CVS in the worst view on angiographic projection. Percent stenosis was calculated using the formula: (1 - [smallest diameter/normal diameter]) × 100%. The length of stenosis on DSA is calculated as the sum of the narrowest segment 1 (length between the distal stenosis border and maximum stenosis) and narrowest segment 2 (length between the proximal stenosis border and maximum stenosis). The formula of the length of stenosis on DSA is narrowest segment 1 + narrowest segment 2.
Stent selection
Consedering that the CVS corresonds more closely to a triangular shape in cross-section view, the study introduced a novel stent selection method. The stent size and length were calculated using formulas derived from IVUS measurements of the proximal and distal reference segments of the lesion.
As illustrated in Fig. 3, R2 represents the radius of circle A2, calculated using the formula: \(\:\sqrt{{A}_{2}/2{\uppi\:}}\). Here, A2 refers to the average triangular area of the proximal (CSA1) and distal (CSA2) reference segments of the lesion.
Schematic illustration of the stent diameter selection based on IVUS measurement. (A) Cross-sectional illustration of venous sinuses and adjacent anatomical structures, the blue section indicated the CVS. (B) Cross-sectional illustration of proximal reference segment of the lesion. C1 denoted the perimeter of triangle CVS in this segment, while CSA1 represented its area. The yellow region indicated the area of the inscribed circles within the CVS triangle of the proximal reference segment. (C) Cross-sectional illustration of the distal reference segment of the lesion. C1 denoted the perimeter of the CVS triangle in this segment and CSA2 represented its area. The yellow region indicated the area of the inscribed circles within the CVS triangle of the diatal reference segement. (D) Schematic illustration of the hypothesized lesion structure based on IVUS measurements of the proximal and distal reference segments. C3 denoted the perimeter of the lesion’s CVS triangle, derived from the IVUS measurements of C1and C2. A2 represented the area of the lesion’s CVS triangle, derived from CSA1 and CSA2, while A3 indicated the inscribed circles within this triangular structure, inferred from both reference segments. (E) Illustration of stent size selection for lesion, based on cross-sectional area and inscribed circles within the CVS triangular structure, derived from proximal and distal reference segments. The blue area represented the cross-sectional area of the CVS. The yellow area represented the area of inscribed circles within the CVS. The small black squares depicted the cross-sectional area of the stent. R2 represented the radius calcaluted from the average cross-sectional area of proximal and distal reference segments of the CVS. R3 was derived from the average area of inscribed circles within proximal and distal reference segments of the CVS. R1 was the stent radius, which was between R2 and R3. (C) Cross-sectional illustration of stent deployment in the CVS. The figure was generated by Adobe Illustrator CC 2022 (http://www.adobe.com/cn/creativecloud/roc/business.html) .
R3 denotes the radius of circle A3, derived from the formula: \(\:\sqrt{{A}_{2}/{\text{C}}_{3}}\). A3 represents the inscribed circles of the lesion within the triangular structure of the CVS, as inferred from the proximal and distal reference segments. Additionally, C3 is defined as the average perimeter of the triangular perimeter C1 and C2, representing the proximal and distal reference segments, respectively.
The stent diameter (2R1) was determined to be within the range defined by 2R2 and 2R3, expressed mathematically as 2\(\:\sqrt{{A}_{2}/2{\uppi\:}}\)≤ 2D1 ≤\(\:2\sqrt{{A}_{2}/{\text{C}}_{3}}\) and 2(CSA1+CSA2)/(C1 + C2) ≤2D1 ≤2\(\:\sqrt{\left(CS{A}_{1}+CS{A}_{2}\right)/2\pi\:}\). The stent length was determined by adding 10 mm to both sides of the distal and proximal stenosis borders, in addition to the length of the stenosis segment.
Self-expanding stents (Precise Carotid stent, Cordis, U.S.A; Wallstent, Boston Scientific, USA) with diameters ranging from 7.0 mm to 9.0 mm and lengths between 30 mm and 50 mm were utilized for stent implantation.
Stenting procedure
Before stenting, all patients received a daily dose of 75 mg clopidogrel and 100 mg aspirin for 3–5 days. Procedures were conducted under general anesthesia with intraoperative anticoagulation therapy (dosage 4000–6000 units) and intravenous heparin to maintain an activated clotting time above 250s12. Prior to stenting, DSA was performed through arterial femoral access to assess stenosis features and select the worst view. IVUS evaluation and stenting followed a femoral transvenous approach using a short 8-Fr sheath. An 8-Fr guiding catheter was placed at the end of the internal jugular vein (IJV). A 6F intermediate catheter was advanced into the distal transverse sinus (TS) through the 8-Fr guiding catheter. The microcatheter (Rebar-27, Medtronic, Minneapolis, MN) was used to access the superior sagittal sinus (SSS) under microwire guidance. Contrast agent was injected through the microcatheter into the SSS, TS, and sigmoid sinus (SigS). The transtenotic MPGs were measured by a microcatheter-attached pressure transducer, using a venographic roadmap with the worst projection angle of the stenosis and the mid-axillary line as the zero reference point. If the transtenotic MPGs of the CVS stenosis exceeded 8 mmHg, IVUS evaluation and stenting would be carried out. An IVUS probe transducer was advanced across the stenotic sinus segment over a microwire. The radiopaque IVUS transducer probe was slowly retracted from the distal reference segment point to the proximal reference segment point of the lesion using a slow manual pullback technique at rates of approximately 1 mm/Sect. 6. All IVUS images were displayed and recorded on a workstation. Following IVUS assessment the stenosis, balloon angioplasty predilatation was performed as the initial treatment. Predilatation was conducted at maximum of 6 atm for 60 s using a 5.0–6.0 mm balloon catheter (Sterling balloon catheter, Boston Scientific, U.S.A). Subsequently, stenting procedures were performed. The IVUS assessment was then repeated from the distal to proximal stenting site, yielding another recording. Post-stenting balloon diatation was performed if the residual stenosis of the CSA exceeded 50% at the target lesion site, or if the transtenotic MPGs exceeded 8 mmHg post-stenting. The size of the post-stenting balloon was determined based on 0.8 times the target CVS reference diameter, ensuring adequate the stent stenosis coverage. Subsequently, IVUS and transtenotic MPGs were re-evaluated. The procedure ended when the residual stenosis of the CSA was less than 30% at the target lesion or when the transtenotic MPGs was less than 8 mmHg post dilatation.
Anticoagulation and antiplatelet
The patients were administered with low-molecular-weight heparin for 3 days post-procedure. They continued to take 100 mg of aspirin and 75 mg of clopidogrel daily for 3 months, followed by life-long use of aspirin or clopidogrel.
Follow-up protocol
Clinical assessments and radiographic examinations were performed 6 months to 1 year after stent placement. Restenosis was defined as stenosis exceeding 50% within the stent or at the edge of the stent, as verified by angiography.
Statistical analysis
Statistical analyses used SPSS Statistics version 21.0 (IBM SPSS, Armonk, NY, USA). Baseline and outcome data were presented as means (standard deviations) or medians (25th and 75th percentiles) for continuous variables and as frequencies or proportions for categorical variables. The paired-sample T test or nonparametric test (Wilcoxon test) was employed to compare mean differences. The categorical and binary data were compared between the groups using the χ2 test or Fisher exact test, respectively. A p-value < 0.05 was considered statistically significant.
All methods were in accordance with the Declaration of Helsinki and approved by the Ethical Committee of Beijing Tiantan Hospital. All participants were fully informed of the nature of the study protocol and all gave their written consents regarding participation.
Results
Clinical characteristics of the patients before the procedure
Patients’ baseline characteristics are reported in Table 1, with 52 out of the 61 patients (85.2%) being female. The mean body mass index (BMI) of the group was 27.8 ± 4.4 kg/m2. The most common symptom was visual dysfunction, affecting 50 patients (82.0%). All patients exhibited increased CSF opening pressure, averaging 300.9 ± 46.1 cm H2O. The findings also indicated that the primary lesion sites were frequently situated at the junction of the TS and SigS, impacting 45 patients (73.8%). Stenosis was most commonly found on the right side of the venous sinus, affecting 41 patients (67.2%).
IVUS and DSA for CVS stenosis assessment
Table 2 displays that the degree of maximum stenosis evaluated by DSA was more severe than that evaluated by IVUS before the procedure (p = 0.035), and milder than the IVUS assessment after the procedure (p < 0.001).
IVUS-guided stent implantation
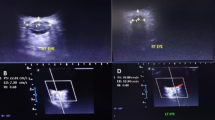
A total of 74 stents were used in the 61 patients, with a single stent implanted in 50 cases and 24 stents in 11 cases due to long or tandem lesions. The stent sizes employed in this study were detailed in Table 3. The mean diameter of the stent was 7.94 ± 0.56 mm, with a mean length of 48.36 ± 11.76 mm across the 61 patients. IVUS-guided stent implantation was successful in all cases. A representative case of IVUS-guided CVS stenting is shown in Fig. 4.
The patient presented with a headache and persistent deterioration of visual function, despite medical treatment for 8 months. A and B : The angiogram with venous phase images shows severe stenosis of the right transverse sinus before stenting from both the anterior-posterior and oblique views, the transtenotic mean pressure gradient was 14mmHg. Note that there is stenosis of both transverse sinuses. A1 and B1: Angiogram with venous phase images demonstrating right transverse sinus post stenting from anterior-posterior view and oblique view. Note obvious change in flow dynamics with 2mmHg of transtenotic mean pressure gradient. D: Intravascular ultrasound (IVUS) demonstrating echogenic material with a significantly decreased diameter of the right transverse sinus. (a) distal reference segment of tight stenosis on IVUS. (b) tight stenosis on IVUS. (c) proximal reference segment of tight stenosis on IVUS. E. a1. distal reference segment on IVUS post-stenting. b1. IVUS post-stent deployment demonstrated increased cross-sectional area (CSA) at the narrowest site of the right transverse sinus prior to stenting. c1. proximal reference segment on IVUS post-stenting.
Accroding to the IVUS measurement results presented in Table 4, an appropriately sizeed and lengthened stent was selected for EVT. Two patients underwent post-stenting balloon dilatation due to the residual stenosis with a CSA exceeding 50% at the target lesion site. After stenting or post-stenting balloon dilatation, there was a significant improvement in the diameter and CSA of the most severely stenosed segments. The stents fully covered the stenotic segment (p < 0.001), and the CSA of the stenosis significantly improved post-procedure (p < 0.001), while the reference segment of the target lesion did not show significant change post-stenting (p = 0.287). The trans-stenotic MPGs across the 61 patients significantly decreased from 16.27 ± 8.71 mmHg pre-procedure to 1.30 ± 1.60 mmHg post-procedure.
Follow-up outcomes
Clinical information follow-ups were completed for all patients (Table 5). Repeat radiological studies were performed in 47 out of 61 (77.0%) patients. Among the patients who had headaches as a symptom, 91.7% showed improvement or disappearance of their symptoms. The visual function did not significantly change for 5 patients, besides 2 patients experienced worsened symptoms. All 5 patients had a prolonged history of symptoms, and ophthalmoscopic examination revealed severe optic atrophy before the procedure.
Discussion
The cross-section view of the CVS more closely corresponds to a triangular shape4,5,6, which poses difficulty in lesion assessment and appropriate stent selection. Boddu et al. measured the diameter of CVS stenosis by using IVUS and DSA. The results suggested that the degree of stenosis observed on DSA was significantly higher than that on IVUS13. Boddu SR et al. used MRV and IVUS to evaluate the baseline CVS. Compared with IVUS measurements, three-dimensional contrast enhancement MRV overestimated the measurements of dimensions, including maximum diameter, area, and perimeter5. Although IVUS was used to assess the lesion and guide stent implantation, none of the studies addressed how to select an appropriate stent. Therefore, in our patient cohort, we introduced a method for stenosis evaluation and stent selection using IVUS. In our study, we found that calculating the degree of stenosis for the triangular structure of CVS using CSA and perimeter measurements obtained by IVUS may be more reasonable. As shown in Table 2, the degree of the maximal stenosis measured by DSA was higher preprocedure and lower postprocedure than that measured by IVUS, indicating potential overestimate or underestimate by DSA compared with IVUS measurements. While there were no significant differences in stenosis length between DSA and IVUS measurements, DSA may overestimate the lesion length compared with IVUS measurements. These findings support previous studies13.
For stent implantation in patients with CVS stenosis, using an oversized stent may not only significantly alter the original triangular contour of the CVS, but also impede the outflow of cortical veins as they enter the CVS, resulting in life-threatening complications such as hemorrhage, venous infarction, and seizures14,15. Conversely, an undersized stent may increase the risk of restenosis. Therefore, selecting the appropriate stent size and length can prevent in-stent stenosis and reduce the duration of medical treatment16,17. In our study, we employed a new method to select the stent using IVUS. With this method, the mean stent size was 7.94 ± 0.56 mm, which fell between the diameter of the circle with an area equal to the average area of the reference segments (7.95 ± 0.65 mm) and the average diameter of an inscribed circle within the CVS, plus the average triangle perimeter of the reference segments (5.99 ± 0.81 mm). The mean stent expansion index was 0.93 ± 0.20. Our data demonstrated that the stent achieved complete expansion (expansion index > 0.8) per IVUS assessment criteria for coronary artery stent implantation18. Additionally, the transtenotic MPGs of all patients significantly decreased after the procedure, showing promising results.
Despite most of the patients reporting significant improvement in their symptoms during the follow-up, three patients complained of aggravated headaches, two of whom had poor visual function and exacerbated tinnitus. A follow-up DSA revealed that one patient developed a dural arteriovenous fistula (DAVF), the symptom was largely improved after DAVF embolization. The other two patients experienced stent restenosis. One patient presented tandem lesions, and a stent was implanted for each lesion, resulting in a total of two stents. Repeat imaging suggested that the restenosis was located at the edge of the stent between the two. For another patient with in-stent stenosis, the lesion was too extensive to be adequately covered by one stent, so two stents were implanted. Radiological follow-up detected that the stent had separated from its former overlapped site, and restenosis occurred at this site. However, the symptoms of two patients were resolved after a third stent was implanted at the stenotic site. The in-stent restenosis at the stent edge of was probably caused by negative remodeling or intimal hyperplasia19. Conversely, using a tandem stent may lead to subacute thrombosis development20. Regarding the patients whose visual function did not significantly change after the stenting, all of them had a long history of symptoms, and ophthalmoscopic examination revealed severe optic atrophy before the procedure, which was difficult to reverse21.
Limitations
The small sample size and our single center of IIH patients with CVS restricted our study. Further large-scale studies are needed to validate our evaluation method for CVS stenosis and stent selection using IVUS. Additionally, the stents utilized in the procedure are not tailored for CVS stenosis. The future development of dedicated devices for CVS stenosis will hopefully overcome this limitation.
Conclusions
IVUS possesses potential to assess the severity and extent of CVS stenosis, aid in stent selection, and optimize stent position during the interventional procedures when used alongside DSA.
Data availability
The data used in this study can be available upon reasonable request from the corresponding Author.
References
Satti, S. R., Leishangthem, L. & Chaudry, M. I. Meta-analysis of Csf diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am. J. Neuroradiol. 36, 1899–1904 (2015).
Kanagalingam, S. & Subramanian, P. S. Cerebral venous sinus stenting for pseudotumor cerebri: A review. Saudi J. Ophthalmology: Official J. Saudi Ophthalmological Soc. 29, 3–8 (2015).
Fargen, K. M. et al. Recommendations for the selection and treatment of patients with idiopathic intracranial hypertension for venous sinus stenting. J. Neurointerv Surg. 10, 1203–1208 (2018).
Lublinsky, S. et al. Automated cross-sectional measurement method of intracranial dural venous sinuses. AJNR Am. J. Neuroradiol. 37, 468–474 (2016).
Boddu, S. R., Gobin, P., Oliveira, C., Dinkin, M. & Patsalides, A. Anatomic measurements of cerebral venous sinuses in idiopathic intracranial hypertension patients. PloS One. 13, e0196275 (2018).
Raynald, Chen, N. et al. Intravascular ultrasound characteristics of different types of stenosis in idiopathic intracranial hypertension with venous sinus stenosis. J. Neurointerventional Surg. 16, 506–511 (2024).
Levine, G. N. et al. 2011 Accf/aha/scai guideline for percutaneous coronary intervention. A report of the American college of cardiology foundation/american heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. J. Am. Coll. Cardiol. 58, e44–122 (2011).
Clark, D. J., Lessio, S., O’Donoghue, M., Schainfeld, R. & Rosenfield, K. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheterization Cardiovasc. Interventions: Official J. Soc. Cardiac Angiography Interventions. 63, 355–362 (2004).
Joan, M. M. et al. Utility of intravascular ultrasound examination during carotid stenting. Ann. Vasc. Surg. 23, 606–611 (2009).
Raynald, Huo, X. et al. Characteristics and outcomes of the idiopathic intracranial hypertension treatment in intrinsic and extrinsic stenosis: A single-center experience in China. Neurol. Therapy. 10, 1029–1044 (2021).
West, J. L. et al. Correlation between angiographic stenosis and physiologic venous sinus outflow obstruction in idiopathic intracranial hypertension. J. Neurointerv Surg. 11, 90–94 (2019).
Asif, H. et al. Idiopathic intracranial hypertension: 120-day clinical, radiological, and manometric outcomes after stent insertion into the dural venous sinus. J. Neurosurg. 129, 723–731 (2018).
Boddu, S. D. M. & Patsalides, A. Role of intravascular ultrasound in the intracranial cerebral venous sinus angioplasty and stenting. J. Neurointerv Surg. 7(Suppl (1), A92–A93 (2015).
Raper, D. M. S. et al. Patency of the vein of Labbe after venous stenting of the transverse and sigmoid sinuses. J. Neurointerv Surg. 9, 587–590 (2017).
Dinkin, M. J. & Patsalides, A. Venous sinus stenting for idiopathic intracranial hypertension: where are we now? Neurol. Clin. 35, 59–81 (2017).
Lenck, S. et al. Stenting of the lateral sinus in idiopathic intracranial hypertension according to the type of stenosis. Neurosurgery 80, 393–400 (2017).
Shazly, T. A. et al. Venous sinus stenting shortens the duration of medical therapy for increased intracranial pressure secondary to venous sinus stenosis. J. Neurointerv Surg. 10, 310–314 (2018).
Sonoda, S. et al. Current clinical use of intravascular ultrasound imaging to guide percutaneous coronary interventions. Cardiovasc. Intervention Ther. 35, 30–36 (2020).
Ichimoto, E. et al. Mechanism of edge restenosis after sirolimus-eluting stent implantation. J. Invasive Cardiol. 24, 55–57 (2012).
Sun, X. et al. Risk factors of subacute thrombosis after intracranial stenting for symptomatic intracranial arterial stenosis. Stroke 48 784–786 (2017).
Attia, R. et al. Risk factors associated with progression from papilloedema to optic atrophy: results from a cohort of 113 patients. BMJ Open. Ophthalmol. 8 (2023).
Acknowledgements
The Study Funded by Beijing Municipal Science & Technology Commission (Z221100007422053) and the Beijing Municipal Administration of Hospitals Incubating Program (PX2017009) to Dapeng Mo and Youth Training Program of Beijing Hospitals Authority (No. QML20190505) to Gang Luo.
Author information
Authors and Affiliations
Contributions
Gang Luo: drafted the manuscript; Raynald -, Xu Tong, Dapeng Sun: critical revision of manuscript for intellectual content; Yinuo Huang, Zifei Han, Bo Wang, Baixue Jia, Zhenbo Shi,: performed the data collection and data analysis; Feng Gao, Ning Ma: study supervision; Zhongrong Miao and Dapeng Mo: study concept and design, analysis and interpretation of data, critical revision of manuscript for intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, G., Raynald, Tong, X. et al. Real time intravascular ultrasound evaluation and stent selection for cerebral venous sinus stenosis associated with idiopathic intracranial hypertension. Sci Rep 15, 16381 (2025). https://doi.org/10.1038/s41598-025-00505-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00505-5