Abstract
Xanthoceras sorbifolia is a unique woody oilseed tree in China, and its leaves are rich in flavonoids, which are involved in plant growth, development and defense. However, the mining of flavonoid synthesis-related genes in Xanthoceras sorbifolia leaves is lacking. In this study, 226 leaves of Xanthoceras sorbifolia from eight provinces in the key distribution areas were measured for flavonoid content, and the differences in flavonoid content of Xanthoceras sorbifolia were analysed to screen out excellent seed sources and six excellent single plants with higher flavonoid content. Genome-wide association analysis (GWAS) was used to identify genes controlling the synthesis of flavonoids, and 62 significant Single nucleotide polymorphism (SNP) sites were identified, which were closely associated with 8 traits, and a total of 11 genes coding for proteins. We found that these genes mainly encode proteins such as WPP domain-associated protein (WAP) (Fragment), Protein pleiotropic regulatory locus 1 (PRL1) and Phosphomevalonate kinase, peroxisomal (PMK), etc. We found that these proteins may directly or indirectly affect the synthesis of flavonoids, which will provide a data base for molecular breeding and genetic improvement of Xanthoceras sorbifolia.
Similar content being viewed by others
Introduction
Xanthoceras sorbifolia, also known as papaya and wenge, is a deciduous shrub or small tree of the genus Xanthocera in the family Sapindaceae. Xanthoceras sorbifolia is highly resilient and grows well in cold, dry and saline soils1, and is widely distributed in the northern and northeastern regions of China, such as Inner Mongolia, Liaoning and Shaanxi.
The leaves of Xanthoceras sorbifolia are rich in flavonoids, mainly including Myricitrin, Quercetin, Rutin, Kaempferol and L-Epicatechin. Studies have shown that flavonoids, as a widely distributed secondary metabolite in plants, are an important component of the odor and color of fruits and vegetables, and also contribute to the agronomic, industrial and nutritional value of plant products. It affects the quality of seeds and fruits, the astringency of plant products, and the health value of food products2. Flavonoids play an important role in the growth, development and defense of plants, and these substances have a better scavenging of free radicals so that they can resist the attack of pathogens, which is also important for human health3.
Flavonoids are generated from phenylalanine through the phenylpropanoid pathway4. In this pathway, phenylalanine, an aromatic amino acid, is converted to p-coumaroyl-CoA through the activity of phenylalanine ammonia lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumarate: CoA ligase (4 CL). PAL catalyzes the first committed step in the general phenylpropanoid pathway, namely, the deamination of phenylalanine to trans-cinnamic acid5. The second step in the general phenylpropanoid pathway involves the activity of C4H, a cytochrome P450 monooxy-genase in plants, which catalyzes the hydroxylation of trans-cinnamic acid to generate p-coumaric acid. This is also the first oxidation reaction in the flavonoid synthesis pathway6. In the third step of the general phenylpropanoid pathway, 4 CL catalyzes the formation of p-coumaroyl-CoA by the addition of a co-enzyme A (CoA) unit to p-coumaric acid. PAL and 4 CL are encoded by gene families with multiple members, e.g., up to 20 members of the Solanum tuberosum PAL gene family7. In plants, the activity of 4 CL is positively correlated with the anthocyanin and flavonol content in response to stress8, while PAL, C4H, and 4 CL are often coordinately expressed9. Chalcone synthase (CHS) is the first key enzyme in the next process, leading the phenylpropanoid pathway to flavonoid synthesis. This enzyme catalyzes the reaction between p-coumaroyl-CoA and malonyl-CoA to synthesize chalcone10. CHS is one of the most abundant enzymes in the phenylpropanoid pathway, but its catalytic efficiency is low. The transcription of CHS in plants was inhibited by high concentrations of cinnamic acid and promoted by high concentrations of coumaric acid11. The key enzyme in the next step is chalcone isomerase (CHI), which catalyzes the further synthesis of chalcone into flavanone, the precursor of isoflavone, and thus enters the isoflavone metabolic branch10. Naringenin is a flavanone. In the analysis of the genes related to the synthesis of flavonoids in Trollius chinensis, Wang et al. found that chalcone was isomerized to naringenin under the catalytic effect of CHI, and naringenin, as a major metabolite, could be generated under the catalytic effect of different enzymes to produce a variety of products, which could be generated under the effect of flavanone-3-hydroxylase (F3H) and flavonol synthase (FLS) to produce Kaempferol, and Kaempferol could be generated under the catalytic effect of flavonoid 3’,5’-hydroxylase (F3’5’H) to produce Quercetin, and so on12.
Whole genome resequencing has been widely used in animal, plant and microbial fields13, and with the reduction of the cost of second-generation sequencing technology, the scale of whole genome resequencing in plants has been expanding, and 187 species of plants have been reported to have been subjected to whole genome resequencing14, and these large-scale whole-genome resequencing has promoted the construction of a complete map, and accelerated the improvement of crops. GWAS based on whole-genome resequencing is a technique that uses variation among individuals in a target group to determine molecular markers associated with complex trait variation, and then unearths genes related to the target traits, and has been widely used in various fields. Xia et al.15 determined the total flavonoid content in brown rice using core rice germplasm containing 633 copies from 32 countries, and a total of 53 quantitative trait loci (QTLs) were detected and eight candidate genes were identified through a genome-wide association study. Zhao16 investigated 208 Xanthoceras sorbifolia germplasm resources for important agronomic traits, and explored SNP loci and candidate genes associated with important agronomic traits such as leaf water content and leaf hairiness through GWAS, and screened out transcription factors related to the target traits, mainly bHLH85 and R2R3-MYB, in addition to a number of genetically encoded proteins.
There is a lack of research on the synthesis pathways of flavonoids and their related gene mining in Xanthoceras sorbifolia plants. In order to promote the basic research and comprehensive development and utilisation of Xanthoceras sorbifolia, and to promote its better growth and development, so that people can make fuller use of the value of Xanthoceras sorbifolia, the present study, through further isolation and analysis, identified the differences in flavonoid content of Xanthoceras sorbifolia leaves from different seed sources, and used this characteristic law to select the excellent single plants with higher flavonoid quality, so as to provide the basis for the development of Xanthoceras sorbifolia related to the flavonoids of the new varieties. Whole genome resequencing and genome-wide association analyses will be used to explore the genetic factors affecting the flavonoid content of Xanthoceras sorbifolia and the molecular mechanism of its synthesis, so as to provide a basis for molecular breeding and genetic improvement of Xanthoceras sorbifolia.
Materials and methods
Plant materials
In 2021, 226 seeds of Xanthoceras sorbifolia were collected from eight provinces and sown in Zaoyuan Conservation Bank of the Shandong Provincial Center of Forest and Grass Germplasm Resources, Jinan, Shandong Province. Xanthoceras sorbifolia resource centre is managed with regular watering and irrigation. Leaf collection was carried out in May 2023, and the plants averaged 0.58 m in height, with sturdy branches, lush foliage, and good growth potential. For each material, well-grown Xanthoceras sorbifolia seedlings were randomly selected, and leaves were collected from the upper, middle, and lower parts of the plant, as well as from the outer and inner parts of the crown, using good growth conditions and the absence of pests and diseases as the criteria for leaf collection. The samples were rapidly placed in liquid nitrogen for cryogenic transport after collection, and stored in a −80 °C refrigerator for backup. Information on the distribution of sampling locations is shown in Fig. 1.
Distribution map of seed source areas for 226 Xanthoceras sorbifolia materials. Mapping with ArcGIS (v10.8, https://www.esri.com/zh-cn/arcgis/).
Determination of flavonoid content
Take the standard and dissolve it with methanol to make 5000 ng/ml standard stock solution. Take a certain volume of configured standard reserve solution, methanol dilution to different concentrations and then mixed to obtain different concentrations of mixed standards. The leaves of Xanthoceras sorbifolia were rapidly ground with liquid nitrogen, 0.5 g of the ground powder was taken in 3 repetitions, weighed precisely and dissolved in 75% ethanol solution by volume. Extraction was performed with an ultrasonic cleaner (250 W, room temperature 25 °C) for 20 min and centrifuged (20 min, 10000 rpm, 4 °C). Dilute 5 times. Take 1 ml of the diluted solution and pass it through a 0.22 μm microporous organic filter membrane for on-line detection.
A Thermo Fisher UltiMate 3000 liquid phase system was used, and the chromatographic column: Agela Technologies-Innoval ODS-2-C18 column (2.1 mm × 75 mm, 5 μm); Mobile phase A: methanol, mobile phase B: water (containing 0.1% formic acid by volume)17,18. Gradient elution with the following gradient procedure: 0 ~ 2 min: 97% B ~ 97% B, 2 ~ 4 min: 97% B ~ 95% B, 4 ~ 6 min, 95% B ~ 80% B, 6 ~ 9 min: 80% B ~ 50% B, 9 ~ 11 min: 50% B ~ 40% B, 11 ~ 13 min: 40% B ~ 5% B, 13 ~ 20 min: 5% B ~ 5% B, 20 ~ 20.1 min: 5% B ~ 60% B, 20.1 ~ 22 min: 60% B ~ 97% B, 22 ~ 25 min: 97% B ~ 97% B; Column temperature: 40 °C, injection volume: 5 µL, equilibrated for 10 min before injection, flow rate: 0.3 ml/min. An AB Sciex Triple Quad 3200 mass spectrometry detection system was used. The ion source is an electrospray ion (ESI) source, negative ion scanning mode; inlet voltage (EP): −10 V; ion spray voltage: −4500 V. Nitrogen was passed through the entire process, with each gas pressure set as follows, curtain gas: 30 psi; atomising gas (Gas 1): 50 psi; and heating gas (Gas 2): 55 psi. Interface heater: open state; turbo spray temperature: 550 °C. In the present study, multiple reaction monitoring (MRM) mode was used for the quantification of flavonoids in the leaves of Xanthoceras sorbifolia. The monitored ion pairs19 and other parameters of the tested components are shown in Table 1. The peak times of the standards and samples were the same for each compound, the parameters of the method were set reasonably, and the compounds identified were the target compounds (Fig. S1, S2).
Whole genome resequencing
In this study, the Illumina platform was used for sequencing, and the QC-qualified libraries were up-sequenced with a fragment size of 150 bp bipartite sequence. The raw image data files were obtained and converted into sequenced reads by CASAVA Base Calling analysis. Raw Data is filtered for artificial joint sequences and low-quality sequences. Specifically, splice-contaminated reads (reads with splice-contaminated bases greater than 5 bp), low-quality reads (more than 50% of reads with base quality values below 19), and reads with an N-content ratio greater than 5% are removed.
Population variation detection
The superior strain of Xanthoceras sorbifolia “WF18”20 was used as the reference genome, and the filtered Clean reads were compared to the reference genome using genome comparison software Burrows-Wheeler Aligner (BWA, v0.7.9a, https://bio-bwa.sourceforge.net/)21 in mem mode. The sequences were sequenced after comparison using Samtools software (v0.1.19, https://samtools.sourceforge.net/)22 and low quality (MQ < 4) reads were removed, after which PCR repeats were removed by Picard-tools (v1.13, http://broadinstitute.github.io/picard/) software.
SNP sites and Insertion and Deletion (InDel) sites were detected using the mutation analysis software Genome Analysis Toolkit (GATK, v3.3-0, http://www.broadinstitute.org/gsa/wiki/index.php/The_Genome_Analysis_Toolkit)23 using Haplotype Caller mode and joint calling methods. Further filtering and screening was done based on quality value, depth and repeatability (SNP: QD < 2.0, ReadPosRankSum < −8.0, FS > 60.0, QUAL < 30.0, DP < 4.0, MQ < 40.0, MappingQualityRankSum < −12.5; InDel: QD < 2.0, ReadPosRankSum < −20.0, FS > 200.0, QUAL < 30.0, DP < 4.0), and the detected variant sites were annotated accordingly using ANNOVAR software (v20160423, http://www.openbioinformatics.org/annovar/)24 and the resulting genome annotation files (gff/gtf) of Xanthoceras sorbifolia.
The vcf files of SNPs were converted to ped format by VCFtools software (v0.1.15, https://vcftools.github.io/index.html)25 or the ped files were filtered directly to retain only the valid loci on the main chromosome (removing contig as well as scaffold). Filtering was performed using PLINK software (v1.9, https://www.cog-genomics.org/plink/1.9/)26, loci with a minimum allele frequency of less than 0.05 were removed, loci and individuals with deletion rates greater than 10% were removed, and loci with Hardy-Weinberg equilibrium less than 10E-6 were removed. The number of valid SNPs was also calculated by the Genetic Type I error calculator software (GEC, v0.2, http://statgenpro.psychiatry.hku.hk/gec/)27 to obtain the Significant P Value value, which was used as a threshold for subsequent GWAS results after Bonferroni correction.
Population genetic analysis
Population structure analysis was carried out using Admixture (v1.3.0, http://www.genetics.ucla.edu/software)28, with K taking the values of 2 ~ 10, and the K value with the smallest Cross-Validation (CV) error was taken as the optimal K value according to the CV cross validation method. According to the Neighbour-Joining (NJ) algorithm, the evolutionary relationships between the samples were calculated using the PHYLogeny Inference Package software (Phylip, v3.696, https://phylipweb.github.io/phylip/)29 and the resultant graphs of the evolutionary trees were plotted using Newick utils (v1.6, http://cegg.unige.ch/newick_utils)30. The linkage disequilibrium (LD) was obtained by calculating r2 by the software PopLDdecay (v1.29, https://github.com/BGI-shenzhen/PopLDdecay)31, while the distance corresponding to half of the maximum value of r2 was taken as the half-loss distance.
GWAS
In this study, Genome-wide Efficient Mixed Model Association software (GEMMA, v0.94, http://stephenslab.uchicago.edu/software.html)32 was used for association analysis using linear mixed model (LMM). The results were corrected for multiple testing using the Bonferroni method, and compared with the threshold value (Significant P Value) calculated by the GEC v0.2 software, if the specified threshold line was exceeded it was used as a candidate locus associated with the target gene, if the threshold line was not exceeded the top 10 loci with the smallest arrangement of p-values were used as candidate loci. A range of 50000 bp upstream and downstream of the candidate site was selected as the candidate interval. Manhattan and Quantile-quantile (QQ) plots were drawn with R (v3.3.1, https://cran-archive.r-project.org/bin/windows/base/old/3.3.1/) to demonstrate the results of GWAS. Functional annotation of the genes in the candidate region was carried out, and the mRNA sequences of the genes were extracted from the fa and gff files, and then the sequences were blasted with the Uniprot database, and the optimal comparison results with an e value of less than 1e−5 were selected to extract the corresponding annotation information in the database.
Results
Analysis of variance of flavonoid content in Xanthoceras sorbifolia
The results of ANOVA of flavonoid composition of different groups of Xanthoceras sorbifolia are shown in Table 2, except for Myricitrin, L-Epicatechin and Kaempferol, there were highly significant and significant differences in other indexes, indicating that the content of most of the flavonoids in Xanthoceras sorbifolia leaves was widely varied and the diversity was high.
The results of intergroup analysis of flavonoid content in different groups of Xanthoceras sorbifolia are shown in Table 3, and the contents of seven components of flavonoids and total flavonoids in Xanthoceras sorbifolia leaves showed a certain range of variability among different individuals. The range of variation of L-Epicatechin was the largest, with the maximum value of 15726.89 mg/kg for the plants in Gansu, the minimum value of 41.17 mg/kg for the plants in Inner Mongolia, the maximum value was about 382 times of the minimum value, and the mean value was 1649.82 mg/kg. Myricitrin had the smallest range of variation from 7954.35 mg/kg (Inner Mongolia) to 50275.6 mg/kg (Inner Mongolia), and the maximum value was 6.32 times of the minimum value. Rutin, Quercetin, Kaempferol and total flavonoids also showed some range of variation, but the coefficients of variation were lower compared to L-Epicatechin. Among the seven flavonoids, Rutin and Myricitrin had the largest mean values, and both of them were more abundant in the leaves of Xanthoceras sorbifolia than other flavonoids.
Principal component analysis of flavonoid content in Xanthoceras sorbifolia
The principal component analyses of seven flavonoid components and total flavonoids content of different seed sources were shown in Table 4, and the eigenvalues of the four principal components were all greater than 0.7, with their cumulative contribution rate of 85.47%, so the flavonoid quality of Xanthoceras sorbifolia leaves could be judged according to the four principal components.
Calculating the composite scores of the principal components can screen out the good single plants with high quality of flavonoids in Xanthoceras sorbifolia leaves, and can evaluate the quality of flavonoids in Xanthoceras sorbifolia leaves from different sources, the higher the composite score, the better the quality of flavonoids in Xanthoceras sorbifolia leaves. The top 10 single plants ranked by the comprehensive score were screened as shown in Table 5, which had high flavonoid quality, and the total flavonoid content of the Inner Mongolia group ZYJ030, Shaanxi group 2021ZYJ004, Gansu group 2021 WL088, Shanxi group 2021 WD032, Inner Mongolia group 2021 WL034, and Gansu group 2021 WL065 plants was more than 100000 mg/kg, and these materials can be used as good single plants with high flavonoid content for the selection of good varieties. The comprehensive evaluation of flavonoid quality of Xanthoceras sorbifolia leaves from different seed sources is shown in Table 6. It was found that the flavonoid quality of Xanthoceras sorbifolia leaves from Gansu seed source area was higher, with an average total flavonoid content of 71655.75 mg/kg, while the other seed sources were in the order of Shanxi (67810.62 mg/kg), Ningxia (65235.65 mg/kg), Shaanxi (62710.42 mg/kg), Shandong (59650.85 mg/kg), Inner Mongolia (44186.68 mg/kg), Liaoning (41283.00 mg/kg), Hebei (32809.33 mg/kg).
Whole genome resequencing and population variation detection
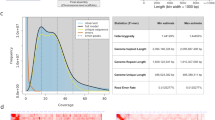
High-throughput sequencing was performed on 104 samples of Xanthoceras sorbifolia, resulting in 255 Gb of raw data. After filtering, 250 Gb of clean data was obtained. The quality of library construction was better and the sequencing quality was relatively high, with an average sequencing depth of 12.4 (Fig. S3). The comparison rate to the reference gene averaged 90.8% (Fig. S4), which is a high comparison rate. A total of 23540497 population SNPs and 3984005 InDel sites were detected after the comparison (Fig. 2A), and the SNP sites mainly occurred in the intergenic region, accounting for 81.77% (Fig. 2B). The SNPs were subjected to quality control (Fig. S5), and 4556824 SNPs were obtained after quality control (Tab. S1), and 2329521.8 SNPs that could be used for GWAS were finally obtained (Tab. S2).
Group structure analysis
Due to the non-random distribution of genetic variation in a population, which leads to the division of the whole population into specific subgroups, the dynamic pattern of change in population differentiation due to genetic variation in population evolution can be demonstrated by the structure of the population, which determines the subgroup to which an individual belongs. The results of this study showed that the CV error was the smallest when K = 3 (Fig. 3A), so it was most appropriate to divide this Xanthoceras sorbifolia population into three subgroups.
As shown in Fig. 3C, the K = 2, K = 3, and K = 4 results are displayed to show the proportion of genetic background of each subpopulation that each individual has, and the three subpopulations into which they are divided are labelled as subpopulation 1, subpopulation 2, and subpopulation 3 based on different ancestries in the K = 3 stacked plot, which are indicated in red, blue, and cyan, respectively. The fact that the various source plants are distributed in different subpopulations suggests that the differences brought about by the geography of the seed source do not create a degree of geographic isolation, and that there is genetic exchange between the regions.
Phylogenetic tree construction
The evolutionary tree was constructed based on the proximity of kinship between different samples, and the filtered SNPs were used to construct Fig. 3D using the NJ method using PHYLIP v3.696 software. The more closely related samples were pooled together and divided into three branches, which were marked using different colours to form three subgroups, which was in good agreement with the population structure and the results of the principal component analysis.
Analysis of LD results
As shown in Fig. 3B, r2 decays from 0.44 to 0.026, and the distance is 100 bp when r2 falls to half of its maximum value (0.22), so the population has a decay distance of 100 bp. A larger LD decay distance indicates a slower rate of decay, suggesting that the population has been subject to selection and that the population is less genetically diverse. The results showed that when r2 decreased to 0.2, the distance was 200 bp; when r2 decreased to 0.15, the distance was 1.7 kb; and when r2 decreased to 0.1, the distance was 14.5 kb, so the LD decayed faster and the genetic diversity of this population was higher.
Group Structure of 226 Xanthoceras sorbifolia samples. (A) Diagram showing the value of 226 samples based on clustering from 1 to 18, X-axis is K value 1–18, Y-axis is cross-validation errors; (B) LD decay plot. The LD decay distance is defined as the distance at which the LD coefficient r2 reaches half of its maximum value; (C) Clustering analysis when the number of subgroups is in the range 2–4, the colours represent separate groups; (D) Phylogenetic tree of 226 Xanthoceras sorbifolia samples.
Results of GWAS
The L-Epicatechin content was correlated with the SNP locus. As shown in Fig. 4A, the tail end of the QQ plot is warped, indicating that a locus associated with L-Epicatechin content exists and that the data are relatively reliable. As shown in Fig. 5 A Manhattan plot, there were 39 SNPs associated with L-Epicatechin content, two SNPs were found on chromosome 2, nine on chromosome 3, two on chromosome 4, three on chromosome 5, one on chromosome 6, one on chromosome 7, five on chromosome 8, two on chromosome 9, four on chromosome 10, four on chromosome 11, one on chromosome 12, two on chromosome 13, two on chromosome 14, and one on chromosome 15. Associating the SNPs with (−)-Epigallocatechin, the QQ and Manhattan diagrams are shown in Figs. 4B and 5B, respectively, there are 12 SNPs associated with (−)-Epigallocatechin, of which 1 was screened located on chromosome 4, 1 was screened located on chromosome 5, 4 were screened on chromosome 6, 7 were screened on chromosome 2, 1 on chromosome 13 and 3 on chromosome 14. Associating the SNPs with (+)-Gallocatechin, the QQ and Manhattan diagrams are shown in Figs. 4C and 5C, respectively, with 12 SNPs associated with the (+)-Gallocatechin, one on chromosome 4, one on chromosome 5, three on chromosome 6, two on chromosome 7, one on chromosome 13, and four on chromosome 14. Associating the SNPs with Kaempferol, the QQ and Manhattan diagrams are shown in Figs. 4D and 5D, respectively. There is only one locus associated with Kaempferol, located at Chr13:15192478, with a p-value of 7.57E-9. Associating SNPs with the Myricitrin, the QQ and Manhattan diagrams are shown in Figs. 4E and 5E, respectively. There are nine SNPs associated with Myricitrin, and one SNP locus was screened on chromosome 3, one on chromosome 7, two on chromosome 9, three on chromosome 15, and two on chromosome 11, respectively. Associating SNPs with Quercetin, the QQ and Manhattan diagrams are shown in Figs. 4F and 5F, respectively, under the significance threshold condition of a = 0.05, no SNP associated with Quercetin were found. Associating SNPs with Rutin, the QQ and Manhattan diagrams are shown in Figs. 4G and 5G, respectively, under the significance threshold condition of a = 0.05, no SNP associated with Rutin was found, but the 10 SNPs with the smallest p-value were selected as candidate SNP. Correlating the SNPs with Total flavonoids, the QQ and Manhattan diagrams are shown in Figs. 4H and 5H, respectively, one SNP associated with Total flavonoids content existed, located at Chr1:23886516.
Quantile-quantile (Q-Q) plots illustrating the distribution of observed p-values compared to expected p-values for flavonoids in Xanthoceras sorbifolia leaves. (A) L-Epicatechin; (B) (−)-Epigalocatechin; (C) (+)-Gallocatechin; (D) Kaempferol; (E) Myricitrin; (F) Quercetin; (G) Rutin; (H) Total flavonoids.
Manhattan plots of association results of LMM model for flavonoid content traits, the X-axis indicates the SNPs along each chromosome, the Y-axis is the -log 10 for the association, the horizontal straight line indicates the threshold of significant association. Each dot represents a SNP. (A) L-Epicatechin; (B) (−)-Epigallocatechin; (C) (+)-Gallocatechin; (D) Kaempferol; (E) Myricitrin; (F) Quercetin; (G) Rutin; (H) Total flavonoids.
Discussion
This study showed that the flavonoid qualities of plants from eight seed source locations were in the following order from high to low: Gansu, Shanxi, Ningxia, Shaanxi, Shandong, Inner Mongolia, Liaoning, and Hebei, and that the flavonoid content increased sequentially from east to west in terms of the geographic location of the seed sources. The general trend of annual precipitation distribution from east to west in China is decreasing from south-east to north-west, and the area with the strongest light is the Qinghai-Tibet Plateau, with the lowest in the Sichuan Basin, and the radiation and light gradually increase from east to west. Some studies have shown that altitude and Ultraviolet radiation b (UV-B) radiation affect the flavonoid content33,34,35, for example, a positive correlation between Ultraviolet (UV) and Quercetin concentration in Betula platyphylla36, and a positive correlation between Quercetin and Luteolin concentration and UV intensity in Ligustrum lucidum leaves37. Azuma et al.38 showed that temperature and light could regulate the synthesis of flavonoids in grape berry skin by modulating the expression of three MYB-related genes (VlMYBA1-3, VlMYBA1-2 and VlMYBA2) and other genes in the flavonoid synthesis pathway. Lv et al.39 showed that the contents of flavonoids, and the expression levels of genes involved in flavonoid biosynthesis (PAL, C4H, 4 CL, CHS1 and DTX41) were enhanced in response to UV-B compared to CK. Wang et al.40 studied the effect of climatic conditions on flavonoid content in Fagopyrum tataricum, light intensity can promote the accumulation of flavonoids and the expression of key enzyme genes, and the expression levels of PAL and 4 CL were positively correlated with the flavonoid content of Fagopyrum tataricum. This suggests that the high flavonoid content in the leaves of the plants from the Gansu seed source in this study may be due to the suitable environmental factors in Gansu that promoted the expression of genes related to flavonoid synthesis, which in turn increased the flavonoid content. In addition, there were associations between changes in drought conditions41,42, rainfall and temperature43 and flavonoid content, with drought-resistant plants up-regulating relevant structural genes and promoting flavonoid biosynthesis in response to the negative effects of water deficit conditions the drier the environment, while non-drought-resistant plants were found to have lower flavonoid content the more they were deprived of water44,45. However, Xanthoceras sorbifolia is a typically drought-resistant plant, suggesting that less precipitation may promote the accumulation of flavonoid content within Xanthoceras sorbifolia plants. Among the materials of this study, Inner Mongolia plant ZYJ030, Shaanxi plant 2021ZYJ004, Gansu plant 2021 WL088, Shanxi plant 2021 WD032, Inner Mongolia plant 2021 WL034 and Gansu plant 2021 WL065 scored high composite scores. The leaves had the best flavonoid quality and had a total flavonoid content of more than 100,000 mg/kg. These materials can be used as target plants with high flavonoid content for selection of superior varieties.
Candidate intervals were identified by the selected significant sites, genes present within the intervals were searched for, and these genes were annotated separately, and a total of 11 protein-coding genes were located. The analyses revealed the identification of six genes that may be involved in flavonoid synthesis. There are four genes related to L-Epicatechin, evm.TU.Chr12.585, evm.TU.Chr2.1108, evm.TU.Chr9.385, evm.TU.Chr9.420. The main proteins encoded by genes with SNP loci closely associated with L-Epicatechin are WAP (Fragment), PRL1, PMK, and Phenylacetaldehyde reductase (PME53). The WAP is located on the Golgi apparatus, and it has been found that in tomato it binds specifically to MAF1 via a convoluted helical structural domain46, which binds to and inhibits RNA polymerase (pol) III, thereby inhibiting the synthesis of tRNAs and other non-coding RNAs47. However, Sharma et al.48 reported that a miRNA (miR858) primary product in Arabidopsis thaliana encoding the peptide priPEP858a can regulate the process of flavonoid biosynthesis. Elevated miR858a gene expression levels and reduced transcript levels of the corresponding target genes are accompanied by reduced levels of total anthocyanins and flavonoid hormones, which in turn affects important functions in plant development, thus WAP may have some indirect effects on flavonoid synthesis. Flores-Pérez et al.49 showed that PRL1 regulates sugar-responsive and hormone metabolism in Arabidopsis thaliana through inhibition of SNF1-associated protein kinase, interacts with ATHKAP2, an α-import protein nuclear import receptor50, and signals through PRL1 to cell wall changes to alter gene expression and sugar-responsive metabolism51. However, L-Epicatechin belongs to the flavanol group of flavonoids, often in the form of sugar compounds (glycosides)52. Studies have shown that C-glycosylated flavonoid synthesis requires enzymes encoding flavonoid synthase, C-glycosyltransferase, glucose oxidase, rhamnosyltransferase, and glutathione S-transferase for transport to the vacuole53,54, suggesting the possibility that PRL1 may regulate L-Epicatechin biosynthesis by regulating or transferring glycosides in L-Epicatechin. Li et al. investigated Tetrastigma hemsleyanum. and found that the expression of p38/PMK-1 was associated with the synthesis of flavonoid components, which in turn ameliorated inflammation-induced damage55, thus it appears that the PMK gene encodes proteins that regulate the synthesis of flavonoids. PAR catalyses the reduction of 2-phenylethylamine to produce 2-phenylethanol, which is a component of the floral scent in petals56. Zhang et al.57 found that phenylalanine (Phe) of the mangiferic acid pathway is a precursor of flavonoids in Camellia sinensis. In addition to this, another pathway for phenylalanine is through phenylalanine metabolism catalysed by PAR to produce 2-phenylethanol, When PAR-related genes are up-regulated, the pathway is enhanced and competes with the pathway for flavonoid synthesis for phenylalanine, which inhibits flavonoid synthesis, so PAR may have an indirect effect on flavonoid synthesis.
The genes related to Kaempferol are evm.TU.Chr14.669, evm.TU.Chr2.1357 and the encoded proteins are Amino acid transporter (ANT1), Sugar transport protein 8 (STP8). Ant1 and Ant2 determine anthocyanin biosynthesis and activate the synthesis of anthocyanins by affecting the expression of flavonoid 3’hydroxylase gene (F3’H) and Ans structural genes58. F3’H is a key enzyme gene for the synthesis of various other flavonoids from the flavonoid precursor dihydroflavonoids59, and F3’H increased the concentration of Kaempferol in Saccharomyces cerevisiae yeast60, suggesting that ANT1 may regulate the biosynthesis of Kaempferol by interacting with the structural gene F3’H in turn. A related study demonstrated that the transcriptome-analysed differential gene Ans regulates the flavonoid synthesis pathway61, so ANT1 may regulate flavonoid biosynthesis by acting with the Ans structural gene. STP8 may be acquired through active indirect uptake of hexoses via sugar/hydrogen co-genation, catalyses the high-affinity proton-dependent uptake of glucose and the acquisition of galactose and mannose, and is a homodimeric carrier of hexoses that is strongly expressed in plant reproductive organs. Their protein products may contribute to the uptake of sugars into pollen tubes and embryo sacs62. STP8 may modulate Kaempferol synthesis by regulating transport sugars.
In this study, the highest flavonoid content was found in the leaves of Xanthoceras sorbifolia plants from Gansu. Since previous studies have shown that environmental factors can regulate the expression of flavonoid-related genes, it was hypothesized that the suitable temperature and altitude, sufficient precipitation and light in Gansu promoted the expression of flavonoid compounds in Xanthoceras sorbifolia evm.TU.Chr12.585, evm.TU.Chr2.1108, evm.TU.Chr9.385, evm.TU.Chr9.420, evm.TU.Chr14.669, evm.TU. Chr2.1357 were expressed, which promoted the process of flavonoid synthesis, and consequently increased the content of flavonoids within the leaves of Xanthoceras sorbifolia.
Data availability
The datasets generated and/or analysed during the current study are available in the National Center for Biotechnology Information (NCBI) repository, https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1224959 and https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA905673.
References
Li, K. R. & Feng, C. H. Effects of Brassinolide on drought resistance of Xanthoceras sorbifolia seedlings under water stress. Acta Physiol. Plant 33, 1293–1300. https://doi.org/10.1007/s11738-010-0661-0 (2011).
Xu, W. J., Dubos, C. & Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 20, 176–185. https://doi.org/10.1016/j.tplants.2014.12.001 (2015).
Li, X. et al. Antioxidant activity of Xanthoceras sorbifolia leaf extract. For. Sci. Technol. 2, 48–52 (2024).
Wang, Z. L. et al. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 56, 465–484. https://doi.org/10.1080/13880209.2018.1492620 (2018).
Williams, J. S., Thomas, M. & Clarke, D. J. The gene StlA encodes a phenylalanine ammonia-lyase that is involved in the production of a Stilbene antibiotic in Photorhabdus luminescens TT01. Microbiology 151, 2543–2550. https://doi.org/10.1099/mic.0.28136-0 (2005).
Wohl, J. & Petersen, M. Functional expression and characterization of cinnamic acid 4-hydroxylase from the hornwort Anthoceros agrestis in Physcomitrella patens. Plant Cell. Rep. 39, 597–607. https://doi.org/10.1007/s00299-020-02517-z (2020).
Chen, X. Y. & Liu, P. Molecular biology and genetic engineering of plant secondary metabolism. Chin. Bull. Life Sci. 2, 8–11. https://doi.org/CNKI:SUN:SMKX.0.1996-02-003 (1996).
Pietrowska-Borek, M., Chadzinikolau, T. & Kozłowska, M. Effect of urban pollution on 4-coumarate: CoA ligase and flavonoid accumulation in Berberis thunbergii. Dendrobiology 64, 79–85. https://doi.org/10.1080/07352689.2010.524518 (2010).
Mizutani, M., Ohta, D. & Sato, R. Isolation of a cDNA and a genomic clone encoding cinnamate 4-Hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiol. 113, 755–763. https://doi.org/10.1104/pp.113.3.755 (1997).
Doxin, R. A. & Paiva, N. L. Stress-induced phenylpropanoid metabolism. Plant Cell 7, 1085–1097. https://doi.org/10.1105/tpc.7.7.1085 (1995).
Loake, G. J. et al. Phenylpropanoid pathway intermediates regulate transient expression of a chalcone synthase gene promoter. Plant Cell 3, 829–840. https://doi.org/10.1105/tpc.3.8.829 (1991).
Wang, H. W. et al. Transcriptome analysis and mining of flavonoid synthesis-related genes in Lonicera japonica based on high-throughput sequencing. Mol. Plant Breed. 20, 5974–5983. https://doi.org/10.13271/j.mpb.020.005974 (2022).
Ye, W., Sun, D. & Han, B. Progress of whole genome resequencing in livestock and poultry research. China Anim. Husb. Vet. Med. 50, 4125–4132 (2023).
Song, B. et al. Plant genome resequencing and population genomics: Current status and future prospects. Mol. Plant 16, 1252–1268. https://doi.org/10.1016/j.molp.2023.07.009 (2023).
Xia, H. J. et al. Genome-wide association study reveals the genetic basis of total flavonoid content in brown rice. Genes 14, 1684. https://doi.org/10.3390/genes14091684 (2023).
Zhao, Y. Genome-wide Association Analysis and Candidate Gene Mining for Important Agronomic Traits in Xanthoceras sorbifolia (Chinese Academy of Forestry Sciences, 2020).
Miao, M. et al. Extraction, composition, and antioxidant activity of flavonoids from Xanthoceras sorbifolium bunge leaves. J. AOAC Int. 106, 769–777. https://doi.org/10.1093/jaoacint/qsac148 (2022).
Zanatta, A. C. et al. Understanding the seasonal effect of metabolite production in Terminalia catappa L. leaves through a concatenated MS- and NMR-based metabolomics approach. Metabolites 13, 349. https://doi.org/10.3390/metabo13030349 (2023).
Sidhic, J. et al. Phytochemical composition and antioxidant and anti-inflammatory activities of Humboldtia sanjappae Sasidh. & Sujanapal, an endemic medicinal plant to the Western Ghats. Molecules 28, 6875. https://doi.org/10.3390/molecules28196875 (2023).
Liang, Q. et al. The genome assembly and annotation of Yellowhorn (Xanthoceras sorbifolium Bunge). Giga Sci. 8, 71. https://doi.org/10.1093/gigascience/giz071 (2019).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. https://doi.org/10.1093/bioinformatics/btp324 (2009).
Li, H. et al. The sequence alignment/map (SAM) format and samtools. Bioinformatics 25, 2078–2079. https://doi.org/10.1093/bioinformatics/btp352 (2009).
McKenna, A. et al. The genome analysis toolkit: A mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. https://doi.org/10.1101/gr.107524.110 (2010).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, 164. https://doi.org/10.1093/nar/gkq603 (2010).
Danecek, P. et al. The variant call format and vcftools. Bioinformatics 27, 2156–2158. https://doi.org/10.1093/bioinformatics/btr330 (2011).
Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. https://doi.org/10.1086/519795 (2007).
Li, M. X., Yeung, J. M., Cherny, C. C. & Sham, P. C. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 131, 747–756. https://doi.org/10.1007/s00439-011-1118-2 (2012).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 9, 19. https://doi.org/10.1161/01.ATV.0000137190.63214.c5 (2009).
Plotree, D. & Plotgram, D. PHYLIP-phylogeny inference package (version 3.2). Cladistics 5, 163–166. https://doi.org/10.1111/j.1096-0031.1989.tb00562.x (1989).
Junier, T. & Zdobnov, E. M. The Newick utilities: High-throughput phylogenetic tree processing in the Unix shell RID A-6748-2011. Bioinformatics 26, 1669–1670. https://doi.org/10.1093/bioinformatics/btq243 (2010).
Zhang, C., Dong, S. S., Xu, J. Y., He, W. M. & Yang, T. L. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 35, 1786–1788. https://doi.org/10.1093/bioinformatics/bty875 (2019).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824. https://doi.org/10.1038/ng.2310 (2012).
Casati, P. & Walbot, V. Differential accumulation of maysin and rhamnosylisoorientin in leaves of high-altitude landraces of maize after UV-B exposure. Plant Cell Environ. 28, 788–799. https://doi.org/10.1111/j.1365-3040.2005.01329.x (2010).
Bakhshi, D. & Arakawa, O. Effects of UV-B irradiation on phenolic compound accumulation and antioxidant activity in ‘Jonathan’ apple influenced by bagging, temperature and maturation. J. Food Agric. Environ. 4, 75–79. https://doi.org/10.1016/j.fbio.2019.04.010 (2006).
Berardi, A. E., Fields, P. D., Abbate, J. L. & Taylor, D. R. Elevational divergence and clinal variation in floral color and leaf chemistry in Silene vulgaris. Am. J. Bot. 103, 1508–1523. https://doi.org/10.3732/ajb.1600106 (2016).
Stark, S., Julkunen-Tiitto, R., Holappa, E., Mikkola, K. & Nikula, A. Concentrations of foliar Quercetin in natural populations of white Birch (Betula pubescens) increase with latitude. J. Chem. Ecol. 34, 1382–1391. https://doi.org/10.1007/s10886-008-9554-8 (2008).
Tattini, M., Galardi, C., Pinelli, P., Massai, R. & Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New. Phytol. 163, 547–561. https://doi.org/10.1111/j.1469-8137.2004.01126.x (2004).
Azuma, A., Yakushiji, H., Koshita, Y. & Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 236 (4), 1067–1080. https://doi.org/10.1007/s00425-012-1650-x (2012).
Lv, M. et al. Effect of UV-B radiation on growth, flavonoid and Podophyllotoxin accumulation, and related gene expression in Sinopodophyllum hexandrum. Plant Biol. 23 (Suppl 1), 202–209. https://doi.org/10.1111/plb.13226 (2021).
Wang, D. et al. Light intensity-a key factor affecting flavonoid content and expression of key enzyme genes of flavonoid synthesis in Tartary buckwheat. Plants 11, 2165. https://doi.org/10.3390/plants11162165 (2022).
Agati, G. et al. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 72, 35–45. https://doi.org/10.1016/j.plaphy.2013.03.014 (2013).
Hughes, M. N., Carpenter, L. K. & Cannon, G. J. Estimating contribution of anthocyanin pigments to osmotic adjustment during winter leaf reddening. J. Plant Physiol. 170, 230–233. https://doi.org/10.1016/j.jplph.2012.09.006 (2013).
Martinidou, E. et al. Assessment of the chemical and genetic variability among accessions of Cicerbita alpina (L.) Wallr., an alpine plant with anthelmintic properties. Front. Plant Sci. 14, 1269613. https://doi.org/10.3389/fpls.2023.1269613 (2023).
Rao, M. J. et al. LC-MS/MS-based metabolomics approach identified novel antioxidant flavonoids associated with drought tolerance in citrus species. Front. Plant Sci. 14, 1150854. https://doi.org/10.3389/fpls.2023.1150854 (2023).
Hu, H. C. et al. Integrated analysis of metabolome and transcriptome data for Uncovering flavonoid components of Zanthoxylum bungeanum Maxim. leaves under drought stress. Front. Nutr. 8, 801244. https://doi.org/10.3389/fnut.2021.801244 (2022).
Patel, S., Brkljacic, J., Gindullis, F., Rose, A. & Meier, I. The plant nuclear envelope protein MAF1 has an additional location at the golgi and binds to a novel golgi-associated coiled-coil protein. Planta 222, 1028–1040. https://doi.org/10.1007/s00425-005-0076-0 (2005).
Blayney, J. et al. Impact on Arabidopsis growth and stress resistance of depleting the Maf1 repressor of RNA polymerase III. Gene 815, 146130. https://doi.org/10.1016/j.gene.2021.146130 (2022).
Sharma, A., Badola, P. K., Bhatia, C., Sharma, D. & Trivedi, P. K. Primary transcript of miR858 encodes regulatory peptide and controls flavonoid biosynthesis and development in Arabidopsis. Nat. Plants. 6, 1262–1274. https://doi.org/10.1038/s41477-020-00769-x (2020).
Flores-Pérez, U. et al. Pleiotropic regulatory locus 1 (PRL1) integrates the regulation of sugar responses with isoprenoid metabolism in Arabidopsis. Mol. Plant 3, 101–112. https://doi.org/10.1093/mp/ssp100 (2010).
Nemeth, K. et al. Pleiotropic control of glucose and hormone responses by PRL1, a nuclear WD protein, in Arabidopsis. Genes Dev. 12, 3059–3073. https://doi.org/10.1101/gad.12.19.3059 (1998).
Li, Y. et al. Signaling from an altered cell wall to the nucleus mediates sugar-responsive growth and development in Arabidopsis thaliana. Plant Cell. 19, 2500–2515. https://doi.org/10.1105/tpc.106.049965 (2007).
Borovaya, A. S. & Klykov, G. A. Some aspects of flavonoid biosynthesis and accumulation in buckwheat plants. Plant Biotechnol. Rep. 14, 1–13. https://doi.org/10.1007/s11816-020-00614-9 (2020).
Harborne, J. B. The flavonoids: Advances in research since 1986. New Phytol. 111, 559–565. https://doi.org/10.1007/978-1-4899-2911-2_5 (1989).
Marrs, K. A., Alfenito, M. R., Lloyd, A. M. & Walbot, V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature 375, 397–400. https://doi.org/10.1038/375397a0 (1995).
Li, Y. L., Chu, Q., Liu, Y. Y., Ye, X. & Zheng, X. D. Radix tetrastigma flavonoid ameliorates inflammation and prolongs the lifespan of Caenorhabditis elegans through JNK, p38 and Nrf2 pathways. Free Radic.Res. 53, 562–573. https://doi.org/10.1080/10715762.2019.1613534 (2019).
Chen, X. M. et al. Functional characterization of rose phenylacetaldehyde reductase (PAR), an enzyme involved in the biosynthesis of the scent compound 2-phenylethanol. J. Plant Physiol. 168, 88–95. https://doi.org/10.1016/j.jplph.2010.06.011 (2010).
Zhang, L., Mei, X., Wan, S. & Deng, C. Integration of metabolome and transcriptome reveals the relationship of benzenoid-phenylpropanoid pigment and aroma in purple tea flowers. Front. Plant Sci. 12, 762330. https://doi.org/10.3389/fpls.2021.762330 (2021).
Gordeeva, I. E., Glagoleva, Y. A., Kukoeva, V. T., Khlestkina, K. E. & Shoeva, Y. O. Purple-grained barley (Hordeum vulgare L.): Marker-assisted development of NILs for investigating peculiarities of the anthocyanin biosynthesis regulatory network. BMC Plant Biol. 19, 49–57. https://doi.org/10.1186/s12870-019-1638-9 (2019).
Tian, J., Zhang, C. B. & Sun, Y. Z. Progress of research on genetic engineering and chemical synthesis of flavonoids and their structural modification. Food Ferment. Ind. 47, 252–258. https://doi.org/10.13995/j.cnki.11-1802/ts.024835 (2021).
Rodriguez, A. et al. Metabolic engineering of yeast for fermentative production of flavonoids. Bioresour. Technol. 245, 1645. https://doi.org/10.1016/j.biortech.2017.06.043 (2017).
Wang, Y. et al. Unraveling the molecular basis of color variation in Dioscorea alata tubers: Integrated transcriptome and metabolomics analysis. Int. J. Mol. Sci. 25, 2057. https://doi.org/10.3390/ijms25042057 (2024).
Rottmann, T. et al. Sugar transporter STP7 specificity for 1-arabinose and d-xylose contrasts with the typical hexose transporters STP8 and STP12. Plant Physiol. 176, 2330–2350. https://doi.org/10.1104/pp.17.01493 (2018).
Acknowledgements
The authors would like to thank the members of the group for their collection of germplasm material as well as their continued advice and helpful discussions.
Funding
This research was funded by the Shandong Provincial Key Research and Development Program (Agricultural Seed Project) (2020LZGC0904) and the Open Subject of Science and Technology Innovation Platform of Natural Resources Department of Shandong Province (SDFGR-PT2025-01).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.H., X.X. and Y.Z.; methodology, Y.H. and L.W.; software, Y.H.; validation, Y.H., L.L. and L.W.; formal analysis, Y.H.; investigation, L.W., L.L., D.W. and Y.Z.; resources, X.X. and Y.Z.; data curation, Y.H.; writing -original draft preparation, Y.H.; writing-review and editing, Y.H., X.X., L.L., D.W. and Y.Z.; visualization, Y.H. and L.L.; supervision, X.X.; project administration, Y.Z.; funding acquisition, X.X. and Y.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huo, Y., Wang, L., Lu, L. et al. Genome-wide association analysis and gene mining of flavonoids in Xanthoceras sorbifolia. Sci Rep 15, 20808 (2025). https://doi.org/10.1038/s41598-025-00514-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00514-4