Abstract
The beneficial effects of ammonium nitrate and potassium humate on carrots are well-documented. However, their impact on physiological and biochemical mechanisms under varying irrigation conditions still needs to be explored. Here, we investigated the effects of soil-applied ammonium nitrate and foliar-applied potassium humate on the physio-chemical characteristics and water use efficiency of carrot plants under three irrigation levels: 100%, 80%, and 60% of crop evapotranspiration (ETc). Carrot plants were treated with two rates of soil ammonium nitrate (200 and 250 kg N ha− 1), foliar potassium humate (200 and 400 g 100 L− 1), and four combinations of these treatments. Under 80% of ETc, the combined applications of soil ammonium nitrate and foliar potassium humate significantly influenced the leaf contents of chlorophyll a, nitrate, ammonium, catalase, carbohydrate, and soluble sugar patterns, enhancing osmotic regulation under water deficit conditions. Interestingly, when carrots were irrigated by 100% of ETc instead of 80 and 60% and sprayed with 400 g 100 L− 1 of potassium humate in combination with 250 kg N ha− 1 of ammonium nitrate, water use was decreased by 49.2 and 30.7%, respectively. We attributed that to: a), the observed increments in NH4 concentrations in the leaves under 100% ETc which caused negative physiological impacts on chlorophyll, and b) the change in C/N and N/P ratios. This highlights the importance of choosing a suitable irrigation pattern for carrot crops when the potassium humate in combination with ammonium nitrate is adapted. Overall, using foliar potassium humate at a rate of 200 g 100 L− 1 with soil ammonium nitrate applications at 250 kg N ha− 1 under 80% ETc attained the highest yield and water use efficiency.
Similar content being viewed by others
Introduction
Nowadays, the biggest challenge for carrot production is the frequency and severity of climate change1,2. Decreased soil fertility, rising temperatures, and water deficiency can stress many crops3,4. One of the factors reducing carrot yields is water deficiency5,6. It has been shown that carrot production decreases by 32.2% for a 20% decrease in water requirements of carrots7. However, the comprehensive impacts of soil moisture deficiency on carrots are still being determined5. Researchers emphasize that understanding the mechanisms underlying the plant’s response to water levels may be the key to developing strategies to enhance its tolerance and secure food demands in regions affected by water stress in the future5,8. Furthermore, it is essential to adopt innovative methods to improve water stress tolerance, especially for water-limited areas9. Another challenge is the constantly rising demand for water for purposes and sectors other than agriculture10. This challenge will be the main reason for irrigation water shortages in agriculture throughout the coming decades in Egypt11. Crop cultivation areas and water demands continually increase while available water resources remain constant4,12. Hence, it is essential to help sensitive crops with water deficits, like carrots, adapt to stressful conditions to secure food demand13. Adaptation and mitigation practices can be implemented to cope with water stress14,15,16. These include enhanced irrigation efficiency, suitable use of fertilizers17,18,19, and increased emphasis on finding the appropriate combination of both that is more efficient to resist unfavorable growth circumstances20.
Globally, carrots are one of the most important vegetable crops. Carrots cultivate more than 10% of arable land annually21. However, in Egypt, some problems are associated with increased carrot production, like the shortage of appropriate soil. Therefore, Egyptian farmers employed and promoted coping strategies, such as intensive agriculture practices, crop rotation management, and extension of agricultural lands to desert areas22, which are often either sandy or calcareous20,23,24. Sandy soils cover over 900 million ha; hence, they occur in many areas worldwide, especially in arid and semi-arid regions25. These soils generally have low water-retention capacity, weak soil structure aggregates development, high soil hydraulic conductivity and permeability conductivity, limited nutrient levels, and low organic matter levels25,26. Therefore, soil fertility often declines under intensive cultivation and depends on the soil’s organic carbon levels25. Despite Baumert et al.27 demonstrating that soil organic carbon doesn’t play an essential role in plant nutrient cycling, its role was pronounced in improving soil structure.
To deal with the above mentioned, enhancing the nutrient and water status of sandy soils is essential28. This can be achieved by applying organic applications as a source of carbon (e.g., humic acids) and inorganic applications such as ammonium nitrate29. In seek of the best agricultural practices, many researchers have sought to mitigate water deficit with the combined use of organic and inorganic components such as humic and calcium nitrate30, nitrogen (N) combined with humic acid and iron31, humic and urea32. Nitrogen fertilizers can be used as a complement to another antitranspirant like potassium humate, and in situations where these combinations are optimized, plant development is often maintained or even improved. Because of the benefits of organic applications were widely utilized to improve growth conditions in sandy soils. A study by Muhammad et al.33 has announced that applying humic acids to plants can provide rich nutrient sources and significantly increase plant development and productivity compared to no application. In addition, applying humic acids significantly enhances plant cell membrane permeability and root formation and increases soil water retention capacity, causing improvements in water use efficiency34,35. Also, humic applications can have various physiological and biochemical effects on plants, such as increasing the net photosynthetic rate, decreasing the accumulation of reactive oxygen species, enhancing proline synthesis, and increasing the activity of the antioxidant enzyme system36. However, others have found that humic applications may cause a non-significant impact on total phenols and total carbohydrates37 and proline38 or reduce antioxidant activity, resulting in negative impacts on plant growth, especially under higher concentration rates39. Consequently, Ampong et al.40 found that some study’s recommendations for using humic applications on different crops must be completed, adequate, and consistent. Therefore, it would be preferable for any study that recommends a general benefit of humic applications on soils, crops, or growth conditions to test their actual impacts on the field under specific growth conditions. On the other hand, ammonium nitrate has a significant role in promoting plant growth and development. The principal reasons contributing to the enhancements in plant development and associated physiochemical traits under various growth conditions encompass higher accumulation of carbon and N41, increased chlorophyll synthesis and photosynthetic rate42, and the generation of total chlorophyll and antioxidants43. However, Ma et al.44 found that when ammonium nitrate was applied in a different ratio, a significant difference in the examined osmoregulators (soluble protein and free proline contents) was observed, in contrast to the soluble sugars and chlorophyll contents.
Under water deficit circumstances, photosynthesis and transpiration are negatively affected45. Each of N and potassium are essential nutrients that significantly regulate physiological processes46. Hence, to mitigate the harmful impacts of water deficit conditions, supplying plants with ammonium nitrate and potassium humate is essential. Nonetheless, many plants have differential responses for nutrient combinations throughout their growth circle. Therefore, previous studies indicated that any combinations must be applied with the right proportions to maintain the stoichiometric pattern47.
Based on those mentioned above, it can be hypothesized that little data are available about the impact of ammonium nitrate and potassium humate on the physiochemical mechanisms of droughted stressed plants. Plants show many different responses under water deficit conditions. Therefore, considering the above facts, this paper was undertaken to further understand the reaction of carrots, in terms of plant physio-chemical characteristics and water use efficiency, to ammonium nitrate alone and in combination with potassium humate under different irrigation conditions.
Materials and methods
The description of the experimental site
This study was conducted at the experimental farm in the South of Egypt at the latitude of 22o, 24`0.10` N, longitude of 31o,35`0.41` E, and altitude of 188 m during the seasons of 2019/2020 and 2020/2021. The studied area lies in arid climatic conditions, and the weather data during the cropping seasons are shown in Fig. 1. The soil texture in the experimental station was loamy sand. Soil physical and chemical properties were determined by following Estefan et al.48. The soil chemical and physical properties at the experimental field and the qualitative characteristics of irrigation water are shown in Table 1. The farm met its water needs by drawing high-quality groundwater from an on-site deep, with no constraints for the study (Table 2).
The experimental details
This field experiment was performed under different irrigation levels to examine the efficiency of two rates of ammonium nitrate as a soil application (SAN) and two rates of foliar potassium humate (FKH) on carrot plants. The experiment layout was a split plot based on a randomized complete block design with three replications. Irrigation levels were determined at three levels as the main plots {100%, 80%, and 60% of carrot crop evapotranspiration (ETc)}. Concurrently, in the subplots carrot plants were treated with: T1 (tap water applications) (as control), T2 (SAN = 200 kg N ha− 1), T3 (SAN = 250 kg N ha− 1), T4 (FKH = 200 g 100 L− 1 water), T5 (FKH = 400 g 100 L− 1 water), T6 (SAN + FKH = 200 kg N ha− 1+ FKH = 200 g 100 L− 1 water), T7 (SAN + FKH = 200 kg N ha− 1+ FKH = 400 g 100 L− 1 water), T8 (SAN + FKH = 250 kg N ha− 1+ FKH = 200 g 100 L− 1 water), and T9 (SAN + FKH = 250 kg N ha− 1+ FKH = 400 g 100 L− 1 water). Irrigation in the current study was implemented through a set sprinkler irrigation system using a metal impact sprinkler 3/4” male (Rain Bird Sprinkler, U.S.A) with a discharge of 1.2–1.4 m3 h− 1 at 3 bars. Each plot had a manometer valve to control the operating pressure at 3 bar. Beside a flow emitter and valve to control the mounts of the targeted irrigation water level. Moreover, to avoid the potential impacts of water infiltration on the adjacent plots, a buffer zone of nine meters was meticulously maintained between the experimental treatments. The experimental net space was (9.0 m long × 9.0 m wide). Additionally, the experimental work involved 81 plots {3 irrigation levels × 9 fertilization treatments × 3 replicates}. Carrot seeds were purchased from Takii Seeds Co. This cultivar is recommended as a high-yielding commercial cultivar. Moreover, this cultivar and the implemented methods in the current study complied with international, national, and institutional guidelines and legislation: the carrot seeds (Daucus carota L.), cv. Kuroda Max was sown in 1st week of November during the first and second seasons of 2019/2020 and 2020/2021, respectively. The carrot plant density in the current study was established at a rate of approximately 0.8 million plants per hectare. The roots were harvested on March 10, 2020, and March 15, 2021. The experimental area was divided into two parts to examine the effects of the fertilizer treatments: one part was used in the first year, and the other was used in the second year. Commercially available fertilizers were applied, where phosphorus in calcium superphosphate (16% P2O5) form was applied at 40 kg P2O5 ha − 1 in two equaled portions during seedbed preparation and after 30 days of planting date. Both of soil amounts of N (200 and 250 kg N ha− 1) in the form of ammonium nitrate (33.5% N) and 125 kg K2O ha − 1 of potassium sulfate (48% K2O) were divided into equal portions, and applied once 30 days after emergence in the stem elongation stage (20–39, BBCH code) and after 60 days of emergence in the flowering stage (40–89, BBCH code). The FKH product was purchased from Farma Egypt Co, and it had 80.0% humic substances involving 68.0% humic acid, 5% fulvic acid, pH 9.5, and 10.0% potassium. Both FKH doses (200 and 400 g 100 L− 1 water) were sprayed and applied twice in the same concentrations after 60 and 75 days from the emergence in the flowering growth stage (40–89, BBCH code). Throughout the growing seasons, the district’s commercial carrot production regularly conducted all relevant agricultural practices, including plant protection practices against disease, weeds, and pests.
The irrigation calculation procedures
Determining the irrigation water requirement involved downloading the actual weather data obtained from the Toshka agrometeorological station and calculating the crop evapotranspiration of carrots to establish the exact amount for subsequent irrigation events for each treatment. The quantity of irrigation water for each treatment was calculated using the following equations:
Reference evapotranspiration
The daily reference evapotranspiration (ETo) was calculated by entering weather data in the CROPWAT package, version 8.0. Then, ETo was determined by Penman-Monteith equation as indicated by Allen et al.49:
Where:
ETo = Reference evapotranspiration (mm day− 1), Rn = Net radiation (MJm− 2d− 1), G = Soil heat flux (MJm− 2d− 1), Δ = Slope vapor pressure and temperature curve (kPa oC−1), γ = Psychrometric constant (kPa °C− 1), U2 = Wind speed at 2 m height (ms− 1), es-ea = Vapor pressure deficit (kPa), T = Mean daily air temperature at 2 m height (°C).
Crop evapotranspiration
The crop evapotranspiration of carrots (ETc) was calculated based on Allen et al.46 as the following equation:
Were ETc = Crop evapotranspiration (mm), ETo = Reference evapotranspiration (mm), Kc = Crop coefficient.
Before the experiment started, soil moisture parameters were determined, and a reduction in soil moisture was allowed to 60% of the available water, which was the critical limit on carrot development based on a previous study7. Consequently, the irrigation water intervals were carried out every two days. Moreover, in the two winter growing seasons of 2019/2020 and 2020/2021, the applied irrigation water for carrot plants was determined using the reference plots where 100% of ETc was maintained. Subsequently, the stressful irrigation treatments were calculated by applying pre-determined irrigation levels. Specifically, stressful irrigation levels were established at 80% and 60% of the ETc. Accordingly, the average two-season quantities of applied irrigation water were 4241, 3393, and 2545 m3 ha− 1 for 100, 80, and 60%, respectively. Irrigation regime levels were started in the stem elongation stage (20–39, BBCH code) after 45 days of emergence, namely when the carrot plants had reached the end of the true leaf growth stage, characterized by the complete formatting and opening of the third genuine leaf50.
Biochemical measurements
The youngest fully expanded carrot leaves (fourth or fifth leaf from the top) were chosen at the flowering growth stage (40–89, BBCH code) after 65 days of emergence (DAE) and before the physiological maturity stage (120 DAE).
Estimation of chlorophyll a, b, and total carotenoids
At 65 and 120 DAE, carrot chlorophyll a and b contents in the leaves were measured via spectrophotometer based on51. 100 mg of fresh carrot leaves were collected, crushed, pulverized, and dissolved in 10 ml acetone 80%, then left in the dark for 48 h to extract the chlorophyll. For five minutes, the obtained extract was centrifuged at 3000 rpm. After that, chlorophyll a, chlorophyll b, and total carotenoids were measured using a spectrophotometer at a wavelength of 645, 663, and 470 nm, respectively.
Estimation of soluble sugars and carbohydrate
Carrot leaf samples were dried and crushed into a fine powder. Carbohydrate extraction and estimation in the leaves were based on the Anthrone method52. The 0.5 g of the powdered material was boiled with 5 ml of 80% ethanol and incubated for 30 min at 80 °C for soluble sugars extraction. The mixture was decanted after centrifugation for 10 min, followed by the extraction. The residual was resuspended in 80% ethanol. Then, the same procedure was repeated twice. After that, the supernatant was thoroughly mixed with anthrone (8.6 mM anthrone in 80% v/v H2SO4) as a reagent at 620 nm. Finally, the absorbance was quantified using the same method as soluble sugars.
Estimation of proline
Proline concentration was determined as described by Bates et al.53. The 0.5 g of fresh leaf samples were extracted from carrot leaf tissues by 0.6 ml of 80% hot ethanol. After that, the obtained extract was mixed with 10 ml of 3% sulfosalicylic acid, and the solution was homogenized in a water bath at 99 °C. After centrifugation at 23 000 × g for 10 min, 2 ml of the supernatant was taken and added to 2 ml of glacial acetic acid and 3 ml of 2.5% ninhydrin reagent. In boiling water, the reaction mixture was incubated for one hour. After adding 4 ml of toluene, the absorbance was measured using a spectrophotometer at 520 nm, and the pellet was re-extracted three times. The mean value of proline contents was expressed as µ mol g− 1 FW.
Estimation of ammonium (NH4)
To measure NH4 in carrot leaves, 0.5 g was taken, homogenized under liquid nitrogen with 6 ml of 10 mM formic acid, and left for five minutes. After repeating centrifugation at 4 °C and 12,000 rpm × g for 10 min 3 times, the supernatant previously described by Shi et al.54 was diluted with 2.5 ml o-phthalaldehyde (OPA) solution. Finally, by spectrophotometer, the absorbance of the sample was measured at 410 nm. To calculate NH4 + concentrations in plant leaves, the standard formula of Li et al.55 was used.
Estimation of Nitrate (NO3)
Based on Zhao and Wang’s56 method, frozen (0.1 g) leaf samples were added to 1 ml deionized water and boiled at 100 °C for 20 min at least. After centrifugation at 15,871 x g for 10 min, 0.1 ml of the supernatant was transferred into a new 12-ml tube, and then 0.1 ml of deionized water was used as a control. 0.4 ml salicylic acid and sulphuric acid (H2SO4) were added in each tube. Then, the sample was vortexed well and incubated for 20 min at room temperature. After that, in each tube, 9.5 ml of 8% (w/v) NaOH solution was added and cooled down for 20–30 min at room temperature. Then, the nitrate contents of each sample, with the control for reference, were measured using a spectrophotometer at 410 nm.
Estimation of hydrogen peroxide (H2O2)
For the H2O2 assay, frozen leaf materials (0.5 g) were homogenized in KH2PO4-KOH buffer (pH 7.8). Then, using titanium chloride (TiCl2) and a spectrophotometer, the optical density was measured at 410 nm. The same procedure was repeated on the standards, which contained no plant material in the range of 0.75 to 5.35 mM H2O2 [Estefan et al.48.
Estimation of antioxidant enzymes
The antioxidant activity determinations were estimated based on the methods of Azevedo Neto et al.57 and Ahsan et al.58. To confirm the results, three replicates for each treatment were used. The carrot leaves in each experimental unit were randomly selected from ten different plants. And antioxidants results were expressed in µ mol g− 1 FW.
The activity of catalase (CAT)
A spectrophotometer was used to determine CAT activity at a wavelength of 240 nm. The reaction mixture consisted of 3 ml of 50 mM potassium phosphate buffer added to 1 g of fresh carrot leaves, then mixed with 30% H2O2 (10 µl) and 50 µl of protein extract. For 5 min, the CAT activity was monitored based on Aebi’s59, and the readings were recorded at 20-s intervals. And by monitoring H2O2 degradation in the absorbance range of one minute at 240 nm via spectrophotometer.
The activity of superoxide dismutase (SOD)
To estimate the activity of SOD enzyme in carrot leaves, 1 g of fresh leaves was crushed into fine powder and homogenized for five minutes with a reaction mixture consisting of (K-phosphate buffer pH 6.8 + 0.1 mm EDTA). After filtration, the mixture was centrifuged at 16,000 × g for fifteen minutes, then 50 µl of the enzymatic extract was added to 75 μm nitro blue tetrazolium chloride (NBT) + 13 mm l-methionine + 100 μm EDTA + 2 μm riboflavin in a 50 mm potassium phosphate buffer pH 7.8. Then SOD enzyme activity was measured by a spectrophotometer at a wavelength of 560 nm.
The activity of peroxidase (POD)
To determine POD enzyme activity, a reaction mixture consisted of 50 ml + 3 ml phosphate buffer (0.1 M, pH 7.0) + 30 ml 2% H2O2 + 50 ml guaiacol (20 mM). After adding 2.0 ml of 20% chloroacetic acid, an increase in absorbance at 470 nm was recorded.
The activity of ascorbate peroxidase (APX)
Regarding the evaluation of APX activity, a reaction mixture was composed of 1500 µl of 50 mM potassium phosphate buffer (pH 6.0) + 600 µl of 0.1 mM EDTA + 10 µl of 30% H2O2 + 400 µl of 0.5 mM ascorbic acid + 50 µl of protein extract. The reaction was initiated by monitoring H2O2 degradation every 20 s for two minutes at 290 nm using a spectrophotometer60.
Dry matter determination
Based on the method described by Shipley & Vu61, five grams of fresh carrot samples (leaves - roots) were washed in deionized water, oven-dried to a constant weight at 70 °C at least 48 h, and then weighed again (dry weight). After that, total dry matter values were obtained by summing the relevant carrot organs.
Yield
At the harvest, one m− 2 of root samples were taken from each sub-plot, weighted then carrot yield was converted to kg ha− 1, and each sampling comprised 3 replicates.
Water use efficiency (WUE) calculation
The WUE was determined according to62
where WUE = Water use efficiency (kg m− 3), Y = Root yield (kg ha− 1) and ETc = Crop evapotranspiration.
The statistical analysis
Combined data of the two growing seasons were performed to analyze the individual and interactive impacts of the differences among the examined application and irrigation levels on the examined variables through split plot design (two-way ANOVA) in the statistical package Costat version 6.303. The significant differences were maintained at 0.05 to compare treatment means with the post hoc Tukey test, as per Casella et al.63. Lowercase letters above error bars indicate statistically significant differences (p < 0.05).
Results
The impacts of SAN and FKH under various irrigation levels
Chlorophyll a, b, and total carotenoids
Based on the results of variance analysis (Supplementary Table S1), the impacts of various irrigation levels and fertilized treatments (T1:T9) on chlorophyll a, b, and total carotenoid contents at 65 DAE and 120 DAE were significant (p < 0.05). The results in (supplementary Table S2) show the individual effects of adopting ammonium nitrate and potassium humate under different irrigation levels on (chlorophyll a, chlorophyll b, and carotenoids) in carrot leaves. While Fig. 2 shows the interactive impacts on (chlorophyll a, chlorophyll b, and carotenoids) in carrot leaves. As can be seen in (Fig. 2A, B, C, and D), in contrast to 100% ETc; irrigated carrot plants with 80 or 60% ETc under control treatment at 65 and 120 DAE showed significant reductions in average chlorophyll a and b contents. While executing 80% ETc irrigation level was pronounced in terms of yielding higher carotenoid contents at 65 DAE compared to 100 or 60% ETc irrigation levels (Fig. 2E). However, at 120 DAE, carotenoid contents were significantly increased by adopting 100% ETc and decreased by adopting 80 or 60% ETc irrigation levels (Fig. 2F).
Data in Fig. 2C showed that by adopting 100% of ETc at 65 DAE, the highest chlorophyll b contents were observed by applying combined applications of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8). Likewise, the maximum chlorophyll b contents after 120 DAE were obtained by applying combined applications of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8) under 100 and 80% of ETc, which were significantly equaled by applying combined applications of (200 kg N ha− 1+ 400 g FKH 100 L− 1 water- T7).
On the other hand, in contrast to the short period, when it comes to maximizing carotenoid contents in the long term (at 120 DAE), the higher application rates and irrigation levels remain preferable. According to the results in (Fig. 2A), executing 100% of ETc irrigation level and applying the combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) were statistically yielding greater carotenoid contents.
H2O2, NO3, and NH4
According to the statistical analysis, the impacts of various irrigation levels and examined applications on H2O2, NO3, and NH4 contents at 65 and 120 DAE were significant (p < 0.05). By comparing irrigation water levels in the control treatments in (Fig. 3), adopting 80 and 60% of ETc resulted in significant increases in H2O2 and NO3 compared to 100% of ETc at 65 DAE and 120 DAE, as can be seen in (Fig. 3A, B, C, and D); in contrast to NH4 (Fig. 3E and F).
By comparing the individual applications of SAN and FKH at 65 DAE, applying both individual applications of FKH under 100 and 80% of ETc and the higher rate of SAN under 100% of ETc; led to significant increases in H2O2 contents compared to the same irrigation levels in the control treatment. At the same time, there was no significant effect in the remaining treatments. At 120 DAE, the obtained results in (supplementary Table S3) showed that any individual applications of SAN and FKH under 80 and 60% of ETc, didn’t attain significant impacts on H2O2 contents compared to the same irrigation levels in the control treatment. However, when adopting 100% of ETc and applying the individual applications of FKH at a rate of (200 g 100 L− 1 water- T4), H2O2 content attained a lower significant value. On the other hand, collectively adopting (60% of ETc) with or without applying any individual and combined applications of SAN and FKH achieved the maximum increase of H2O2 contents at 65 and 120 DAE.
Generally, the results in (Fig. 3C and D) showed that applying the combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9 under 100% of ETc) after 65 and 120 DAE resulted in the highest NO3 concentration compared to the other treatments.
On the other hand, it was shown that the maximum significant NH4 accumulations could be observed by applying the individual applications of (SAN at the rate of 250 kg N – T3 under 100% of ETc) and applying the combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9 under 100% of ETc) after 65 and 120 DAE, respectively (Fig. 3E and F).
The mean interactive impacts of irrigation water levels and different applications and rates of [soil ammonium nitrate (SAN) and foliar potassium humate (FKH)] as individual or combined applications during the growing seasons (2019/2020 and 2020/2021) on (A) Chlorophyll a- 65 DAE, (B) Chlorophyll a- 120 DAE, (C) Chlorophyll b- 65 DAE, (D) Chlorophyll b- 120 DAE, (E) Carotenoids − 65 DAE, and (F) Carotenoids − 120 DAE. Error bars indicate standard errors from the mean. Bars with different letters are statistically significant at p ≤ 0.05. Abbreviations: T1 (tap water applications) (as control), T2 (SAN = 200 kg N ha− 1), T3 (SAN = 250 kg N ha− 1), T4 (FKH = 200 g 100 L− 1 water), T5 (FKH = 400 g 100 L− 1 water), T6 (SAN + FKH = 200 kg N ha− 1+ FKH = 200 g 100 L− 1 water), T7 (SAN + FKH = 200 kg N ha− 1+ FKH = 400 g 100 L− 1 water), T8 (SAN + FKH = 250 kg N ha− 1+ FKH = 200 g 100 L− 1 water), and T9 (SAN + FKH = 250 kg N ha− 1+ FKH = 400 g 100 L− 1 water).
The mean interactive impacts of irrigation water levels and different applications and rates of [soil ammonium nitrate (SAN) and foliar potassium humate (FKH)] as individual or combined applications during the growing seasons (2019/2020 and 2020/2021) on (A) H2O2- 65 DAE, (B) H2O2- 120 DAE, (C) NO3- 65 DAE, (D) NO3 − 120 DAE, (E) NH4 − 65 DAE, (F) NH4 − 120 DAE, (G) Proline − 65 DAE, and (H) Proline − 120 DAE. Error bars indicate standard errors from the mean. Bars with different letters are statistically significant at p ≤ 0.05. Abbreviations: T1 (tap water applications) (as control), T2 (SAN = 200 kg N ha− 1), T3 (SAN = 250 kg N ha− 1), T4 (FKH = 200 g 100 L− 1 water), T5 (FKH = 400 g 100 L− 1 water), T6 (SAN + FKH = 200 kg N ha− 1+ FKH = 200 g 100 L− 1 water), T7 (SAN + FKH = 200 kg N ha− 1+ FKH = 400 g 100 L− 1 water), T8 (SAN + FKH = 250 kg N ha− 1+ FKH = 200 g 100 L− 1 water), and T9 (SAN + FKH = 250 kg N ha− 1+ FKH = 400 g 100 L− 1 water).
Proline and antioxidant enzymes
The obtained results in (supplementary Tables S3 & S4) and (Fig. 3G and H) indicated that adopting stressful irrigation levels 80 and 60% of ETc led to increased proline content after 65 DAE. Interestingly, after 120 DAE, irrigated carrot plants with 100 and 80% ETc irrigation levels showed the highest average proline contents. After 65 DAE, treatment carrot plants with the individual applications of FKH showed higher proline contents under 80 and 60% of ETc irrigation levels. However, applying any individual application of SAN and FKH under 100% of ETc irrigation level improved proline contents better than control 100% of ETc. Overall, after 65 DAE, the highest proline content was obtained by adopting 60% of ETc and applying combined applications of combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9). However, that significantly equaled adopting the same irrigation level and applying the combined application of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8). After 120 DAE, the highest proline content was seen using combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) and adopting 80% of ETc irrigation level.
Concerning the antioxidant enzymes, the results in (Fig. 4A, B, C, D, E, F, G, and H) showed that executing control 60% of ETc after 65 DAE was statistically significant in terms of yielding higher CAT, SOD, POD, and APX contents, compared to control treatments for 100 and 80% of ETc. Conversely, after 120 DAE, it was shown that adopting 100 or 80% ETc irrigation levels could achieve higher antioxidant accumulations.
On the other hand, the findings indicated that by adopting 80% of ETc, the maximum CAT contents were observed by applying combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) after 65 and 120 DAE. On the other side, when it comes to maximizing SOD contents, the maximum contents were observed by applying combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) under 60% of ETc after 65 DAE. While after 120 DAE, applying combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) under 80% of ETc was pronounced in terms of yielding greater SOD contents, although that significantly equaled the adoption same irrigation level and applying the combined application of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8).
A similar finding was observed for POD and APX contents. In contrast, the results indicated that the maximum contents were observed by applying combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) under 60% of ETc irrigation level after 65 DAE. And by applying combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) under 80% of ETc after 120 DAE.
The mean interactive impacts of irrigation water levels and different applications and rates of [soil ammonium nitrate (SAN) and foliar potassium humate (FKH)] as individual or combined applications during the growing seasons (2019/2020 and 2020/2021) on antioxidant enzymes (A) CAT- 65 DAE, (B) CAT- 120 DAE, (C) SOD- 65 DAE, (D) SOD- 120 DAE, (E) POD − 65 DAE, (F) POD- 120 DAE, (G) APX − 65 DAE, and (H) APX- 120 DAE. Error bars indicate standard errors from the mean. Bars with different letters are statistically significant at p ≤ 0.05. Abbreviations: T1 (tap water applications) (as control), T2 (SAN = 200 kg N ha− 1), T3 (SAN = 250 kg N ha− 1), T4 (FKH = 200 g 100 L− 1 water), T5 (FKH = 400 g 100 L− 1 water), T6 (SAN + FKH = 200 kg N ha− 1+ FKH = 200 g 100 L− 1 water), T7 (SAN + FKH = 200 kg N ha− 1+ FKH = 400 g 100 L− 1 water), T8 (SAN + FKH = 250 kg N ha− 1+ FKH = 200 g 100 L− 1 water), and T9 (SAN + FKH = 250 kg N ha− 1+ FKH = 400 g 100 L− 1 water).
Dry matter, carbohydrate, and soluble sugars
To compare the irrigation levels differences in dry matter contents after 65 and 120 DAE, the results in (supplementary Table S5) and (Fig. 5A and B) showed that dry matter contents were decreased when adopting 80 and 60% of ETc compared to control 100% of ETc after 65 DAE. After that, in contrast to 60% of ETc, adopting 80% of ETc significantly equaled and attained the same improvements in dry matter contents.
Collectively, applying the combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9 under 100% of ETc) after 65 DAE resulted in the highest dry matter content compared to the other treatments. After 120 DAE, the maximum dry matter contents were obtained by applying combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9 under 100% of ETc), although that was significantly equaled by applying combined applications of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8 under 100% of ETc).
Based on the results of variance analysis (Supplementary Table S1) and (supplementary Table S5), the individual and interaction effects of examined irrigation levels and various applications significantly affected the carbohydrate and soluble sugar concentrations.
As can be seen in (Fig. 5C), it was shown that the maximum significant carbohydrate accumulations after 65 DAE could be observed by applying the combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9 under 80% of ETc), although that were significantly equaled by applying combined applications of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8 under 100% of ETc). After 120 DAE, the maximum significant carbohydrate accumulations could be observed by adopting 80% of ETc and applying either the combined applications of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8) or (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9).
The results in (Fig. 5D) indicated that by comparing irrigation levels in control treatment after 120 DAE, carbohydrate contents enhanced by (240.2 and 73.7%) for 100 and 80% of ETc irrigation levels compared to 60% of ETc, respectively. Meanwhile, relative to control 60% of ETc after 120 DAE in (Fig. 5F), leaves soluble sugars attained increases by 5.4 and 11.4% for 100 and 80% of ETc irrigation levels, respectively.
Concerning soluble sugars, the results in (Fig. 5E) showed that the maximum significant soluble sugars after 65 DAE could be obtained by applying the combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9 under 60% of ETc), although that were significantly equaled by applying combined applications of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8 under 60% of ETc). After 120 DAE (Fig. 5F), the maximum significant soluble sugar accumulations could be observed by adopting 100% ETc and applying the combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9).
The mean interactive impacts of irrigation water levels and different applications and rates of [soil ammonium nitrate (SAN) and foliar potassium humate (FKH)] as individual or combined applications during the growing seasons (2019/2020 and 2020/2021) on (A) Dry matter − 65 DAE, (B) Dry matter − 120 DAE, (C) Carbohydrate − 65 DAE, (D) Carbohydrate − 120 DAE, (E) Soluble sugars − 65 DAE, and (F) Soluble sugars − 120 DAE. Error bars indicate standard errors from the mean. Bars with different letters are statistically significant at p ≤ 0.05. Abbreviations: T1 (tap water applications) (as control), T2 (SAN = 200 kg N ha− 1), T3 (SAN = 250 kg N ha− 1), T4 (FKH = 200 g 100 L− 1 water), T5 (FKH = 400 g 100 L− 1 water), T6 (SAN + FKH = 200 kg N ha− 1+ FKH = 200 g 100 L− 1 water), T7 (SAN + FKH = 200 kg N ha− 1+ FKH = 400 g 100 L− 1 water), T8 (SAN + FKH = 250 kg N ha− 1+ FKH = 200 g 100 L− 1 water), and T9 (SAN + FKH = 250 kg N ha− 1+ FKH = 400 g 100 L− 1 water).
Carrot yield and WUE
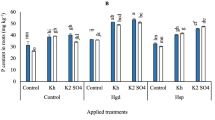
According to the statistical analysis in (Supplementary Table S1) and (supplementary Table S6), the impacts of various irrigation levels and fertilized treatments (T1:T9) on carrot yield and WUE contents at 65 and 120 DAE were significant (p < 0.05). As can be observed in (Fig. 6A), by comparing the impacts of examined irrigation levels in the control treatment, the results demonstrated that the carrot yield was decreased by adopting 60% ETc irrigation level compared to 100% ETc by 54.2%, but, no marked significant differences were observed between 100 and 80% ETc. Hence, treating stressed carrot plants with SAN and FKH applications could help the plants overcome the negative impacts of water deficit and enhance carrot root yield under these circumstances. The results showed that by adopting 80% of ETc irrigation level, the highest carrot root yield was observed by applying combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9). However, that was significantly equaled by adopting the same irrigation level and applying a combined application of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8). On the other hand, it is worth noting that there was an antagonistic relationship between the obtained carrot root yield and higher application rates of SAN and FKH, and the intensity of this relationship was found to depend on the irrigation level implemented. In this sense, there was a gradual increase in irrigation amounts and application rates. Under (100% of ETc) irrigation level, the combined applications of (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) were significantly decreased compared to T6, T7, and T8 by 29.8, 33.1, and 36.1%, respectively.
Data depicted in (Fig. 6B) showed that the maximum rise of WUE was observed by adopting combined applications of (250 kg N ha− 1+ 200 g FKH 100 L− 1 water- T8) or by applying (250 kg N ha− 1+ 400 g FKH 100 L− 1 water- T9) under 80% of ETc irrigation level.
The mean interactive impacts of irrigation water levels and different applications and rates of [soil ammonium nitrate (SAN) and foliar potassium humate (FKH)] as individual or combined applications during the growing seasons (2019/2020 and 2020/2021) on (A) Carrot yield and (B) Water use efficiency (WUE). Error bars indicate standard errors from the mean. Bars with different letters are statistically significant at p ≤ 0.05. Abbreviations: T1 (tap water applications) (as control), T2 (SAN = 200 kg N ha− 1), T3 (SAN = 250 kg N ha− 1), T4 (FKH = 200 g 100 L− 1 water), T5 (FKH = 400 g 100 L− 1 water), T6 (SAN + FKH = 200 kg N ha− 1+ FKH = 200 g 100 L− 1 water), T7 (SAN + FKH = 200 kg N ha− 1+ FKH = 400 g 100 L− 1 water), T8 (SAN + FKH = 250 kg N ha− 1+ FKH = 200 g 100 L− 1 water), and T9 (SAN + FKH = 250 kg N ha− 1+ FKH = 400 g 100 L− 1 water).
Discussion
The primary agronomic practices in crop management revolve around the supply of water and nutrients through irrigation and fertilizer applications. These practices are particularly crucial under stressful conditions64,65. When addressing the management of proper irrigation and fertigation to enhance the growth of drought-sensitive crops such as carrots, it is imperative to conduct a comprehensive study on the impact of water deficit on crop growth in the short and long term, as well as to assess the potential yield if SAN and FKH are utilized. These applications have the potential to bolster plant resistance against the adverse effects of water deficit and represent a significant strategy for increasing WUE66.
Carrot physiological and biochemical responses under full and limited irrigation in a short-term duration
Abiotic stresses such as water stress confine crop production and change their physiological and biochemical processes67,68. The current study investigated the impact of various water levels on carrots based on different physiological and biochemical characteristics. Based on the conclusions obtained in the short water deficit term (after 65 DAE), in the presence of inadequate water quantities (80 and 60% ETc), carrot plants exhibit low [pigment contents (except for carotenoids in plants irrigated by 80% of ETc), NH4, dry matter, and carbohydrate contents], alongside with high contents of [proline, H2O2, antioxidants, soluble sugar, and NO3 levels]. These influences on carrot traits by water deficit have also been reported by69. When carrot plants are subjected to water deficit, a lot of the physiological processes are directly or indirectly affected by chlorophyll contents. Previous studies have found that the pigment contents in the leaves decreased sharply with increasing water deficit7,70. Regarding the reduction in chlorophyll a and b content under water deficit, an explanation was represented by Mibei et al.71 attributed that due to stoma closure, leaf area reduction, gas exchange restriction, destruction of the pigment-protein complexes, and chloroplast lipids induced by oxidative; which ultimately declining photosynthetic pigments and activity. However, other studies have found mild water stress increases chlorophyll contents72. This could be associated with plants’ water stress tolerance, which can vary widely depending on plant ability, species, genotype, and developmental stage73. Nonetheless, carotenoids, among many metabolites, have been reported to have the ability to enhance within plant tissues under certain levels of water stress. In this concern, Zhang et al.74 showed that water deficit is a vital factor influencing the biosynthesis and metabolism of carotenoids. Hence, under unfavorable conditions, some plants tend to regulate the accumulation of carotenoids, which play significant roles in plant stress resistance. The authors further clarified carotenoids’ role as antioxidants, previously known to eliminate free radicals and maintain the redox balance, and better plant response to water deficit circumstances75. Overall, based on study findings, in contrast to 60% of ETc, this strategy (carotenoids enhancement) at 80% of ETc was adopted effectively for mitigating ROS accumulations (H2O2), possibly due to the effects of drought stress intensity. Moderate stress situations improve the amount of secondary metabolites in vegetables.
On the other hand, severe water stress causes negative changes in dry matter accumulation by affecting photosynthates and reductions in phosphate absorption since dry matter content is related to phosphate availability and uptake76. Plants experience reduced nutrient absorption and synthesis due to inadequate water supply77. On the other hand, it was reported that the synthesis, hydrolysis, and translocation of carbohydrates (polysaccharides) within leaf tissues are adversely affected by water deficit78. Under stress environments, plants adapt to these conditions, usually by physiological and biochemical modifications via stimulation of osmoprotectants and defensive antioxidants to confront the drought-generated hazard molecules79. In this experiment, carrot plants in the short water deficit term employed mechanisms to protect leaf tissues from the higher levels of ROS, including antioxidants and proline accumulations, sufficient accumulation of soluble sugar amounts in cytoplasm tissues, enhanced NO3 accumulation, and maintained carotenoid levels as much as possible, which matched with69. In this concern, Mibei et al.71 indicated that when plants are subjected to water deficiency, they produce huge amounts of antioxidants to adapt to the stress circumstances. A greater antioxidant production under these conditions helps carrot plants provide multiple benefits, including enhancing osmotic potential, balancing cell redox status, and maintaining cell membranes80,81. These changes ultimately resulted in increases in water uptake and cellular molecules preservation. Similarly, sustaining water status within plant tissues is vital to pass these conditions under unfavorable water deficit conditions. Hence, sufficient accumulations of soluble sugar and proline under stressful conditions decreased the water potential of the cells, promoted water retention in plants, and sustained adequate metabolic C/N ratios, in agreement with previous studies73,82,83. On the other hand, significant improvements were observed in leaf NO3 accumulations in plants subjected to limited irrigation and leaf NH4 accumulations under full irrigation levels. These results are in contrast with previous studies that demonstrated water deficit significantly reduced NO3 absorption and accumulation compared to full-watered irrigation conditions, whereas NH4 absorption and accumulation significantly enhanced under water-stressed irrigation conditions84. On the contrary, Tian et al.85 found increases in leaf NH4 contents and increases in leaf NO3 contents only when water deficit was accompanied by N deficiency. They added that NO2 contents in leaves were significantly increased under water deficit conditions. Also, Xia et al.86 mentioned that, in opposite trends to the roots, NO3 content in sweet potato leaves was significantly increased under water deficit (the same as in this study). In contrast, N-metabolism enzymes and nitrate reductase (NR) activities were significantly decreased. These conflicting findings between the previous studies could be due to the differences in the soil type, crop, and water stress intensity–duration–and frequency. On the other side, based on a study by Zia et al.87, plants under water stress conditions spend considerable energy dealing with stress tolerance, making this process a cost-intensive phenomenon. Urlić et al.88 showed that plants mostly use both N forms (NO3 and NH4); NO3 assimilation into plant metabolites requires more energy than NH4 because it has to be reduced. Therefore, under well-watered growth conditions, NH4 accumulations were expected to dominate, opposite to stress conditions. The reasons for the increase in NO3 accumulations under water deficit levels can be explained by (i) enhancement in the NO3 accumulations compensated osmotically for reductions in carbohydrate concentration when the photosynthesis rate was decreased. In this concern, similar to previous studies, a significant negative correlation was found between carbon (carbohydrate) and NO3 in plant tissues, which indicated that the plants used organic solutes instead of NO3 as part of iso-osmotic control88,89. Additionally, (ii) the reductions in NR activities under stressful conditions, which is considered the key enzyme of the transformation of NO3 into organic N compounds90,91. Finally, (iii) less availability and absorption of NH4 due to the alkalinity conditions and increased N losses via volatilization. In contrast to NO3 availability in the soil, NH4 availability and uptake are more tightly linked to soil pH and soil moisture alterations. In this respect, Jones et al.92 reported that when the soil pH exceeded 7.5, ammonia (NH3) began to volatilize, and the rate of this process doubled with increasing temperature. This increase in volatilization is due to the reduction in NH3 solubility in the soil solution as temperature rises93. Additionally, Stremińska and Błaszczyk94 investigated the impact of pH on NH3 loss through denitrification and found that the loss was most pronounced within a pH range of 6 to 8.
Carrot physiological-biochemical responses under full and limited irrigation in the long-term duration
As Reid and Gillespie95 and Khalid et al.96 mention, carrots are very sensitive to water deficits. Nonetheless, they can tolerate moderate deficit levels97,98.
Under prolonged water stress, the equilibrium between ROS production and scavenging by antioxidants (including carotenoids) is disrupted, resulting in an imbalance in cellular redox homeostasis and more membrane damage99,100. In this concern, increased carotenoid production was effective at 80% of ETc irrigation level during the short-term period of water deficit, as evidenced by the illustrated figures and tables. Nevertheless, the findings of this study recorded a marked reduction in the impacts of water deficit that appeared during the long-term period on the carotene accumulations of carrots in both irrigation levels 80 and 60% of ETc. Water deficit sharply affected carotenoid production and accumulation at the end stage of plant growth5,100. An explanation was represented by Mibei et al.71 in this regard; they mentioned that although carotenes are a crucial part of the plant antioxidant defense system, they are very susceptible to oxidative destruction. Hence, in severe stress or prolonged water deficit, β-carotene could be rapidly destroyed. Consequently, they are no longer available to protect plants against oxidative damage.
The results obtained in this study showed that in the long term, carrot plants responded dynamically by enhancing their tolerant strategy by controlling soluble sugar synesis and carbohydrate hydrolysis and scavenging the ROS by proline and enzymatic components.
The increment of ROS species under stressed conditions accompanied other reactive nitrogen species (RNS) increments. Consequently, more damage is expected to disrupt the functioning of the plant cells, especially under 60% of ETc. In this regard, Deng et al.101 showed that higher RNS accumulations could exacerbate more damage for plants compared to ROS accumulations by triggering free radical peroxidation. In the current study, NO3− contents were lower under fully-watered conditions (ETc 100%), as reported in lettuce102. They also added that ETc 80% was similar to ETc 100 for recording the same lower NO3 values, in contrast to ETc 60%. The results showed that in stressed carrot plants, a continuous enhancement in NO3 contents was noted before the harvest, representing a conservative plant strategy for water reduction. However, compared to stressful irrigation levels during the short term, NO3 contents in plant leaves tend to be lower. In agreement with this result, Sørensen et al.103 indicated that while high NO3 levels were found when water stress occurred during the early growth of carrots, the opposite was found when water stress occurred just before harvest. Similarly, Schlering et al.76 found a similar result on radish; they attributed these reductions in NO3 at harvest under water stress to the high radiation and evapotranspiration rates that might have resulted in balanced conditions between the uptake and assimilation of NO3. Although NO3 accumulations in leaves are considered lower RNS harmful, their presence was considered an indicator of increasing the accumulation of the other RNS species, especially NO, which is the end product of the NO3-NO2-NO pathway104. The presence of these components has conflicting impacts depending on their concentrations. It is beneficial under low levels or can cause oxidative damage to plant tissues when their production is enhanced more than cellular scavenging105,106. A study by Zhang et al.107 reported that the highest NO3 content in plant organs is unfavorable and even unacceptable in food nutrition. Obidiegwu et al.108 showed that leaf NO3 is vital in enhancing plant growth, improving osmotic regulation, and controlling stomatal closing by affecting guard cell depolarization. Perlikowski et al.109 reported that even lower RNS levels might decrease plants tolerant to water stress and their ability to recover after stress cessation. On the other hand, Umar and Iqbal110 have reported that NO3 level is negatively correlated with soluble carbon compounds, mainly sugars. The previous compounds (NO3 and soluble sugars) play complementary roles in cell turgor maintenance111. In the same concern, Seginer et al.112 hypothesized that plants regulate a mechanism to adjust the NO3 content to the soluble, non-structural carbon compound level for better osmotic turgor adjustments.
As stated in the short term, carbohydrate hydrolysis benefits carrot plants during irrigation-limited conditions due to the water-balancing mechanism. In keeping with this trend, the data from the study showed that at ETc 80% level, carbohydrate amounts such as (starch, glycogen, and cellulose) significantly decreased than ETc 100%. In contrast, soluble sugar amounts such as sucrose, glucose, and fructose increased. While at ETc 60% irrigation level, both carbohydrates and soluble sugars were decreased during the long-term compared to those observed at ETc 100%. In this experiment with irrigation at ETc 80%, the root activity rate, including absorption and exudates, probably increased more than the shoot activity rate, including the assimilation process and respiration, leading to a better plant tolerance against water deficiency. This situation attained better access to water and nutrients in soil layers for carrot roots. Also, the ability of the plant to transport the products of synthesis and hydrolysis processes from the source (leaves) to the sink (roots) is decreased slightly because of a higher increase in sap viscosity113. Similar results were reported by Lemoine et al.114 under mild water deficit levels, where plant shoot growth and photosynthetic productivity are restricted while both (root growth and development) continue and, consequently, the carbon flow to various sink organs (roots) is decreased. In this case, the accumulation of soluble sugar in leaves is increased, causing a significant reduction in water potential and enhancements in plants tolerant to water stress. Following this finding, studies by Lemoine et al.114 and Burke115 indicated that in high-respiration conditions (water stress conditions), soluble sugar accumulation in the plant leaves (source) increased, which has been assumed to (A) provide an energy source to maintain cell survival. (B) potential role in osmotic adjustment to sustain metabolic activity in plant leaves. Another hypothesis was represented by Hummel et al.116 that the sugar accumulation amounts in leaves may also be attributed to a reduction in carbon demands due to growth limitation. They suggested that water deficit conditions did not lead to carbon depletion, whereas they consistently observed a more favorable carbon balance.
When water stress intensity is increased more to 60% of ETc, the reductions of soluble sugar accumulation exceed those of unaffected plants at 100% of ETc. This study attributed these results to the impacts of adopting this irrigation level, which remarkably decreased root efficiency and increased nutrient fixation, leading to a decrease in P absorption, nutrient transport, respiration rate, and a decrease in new photoassimilates, resulting in notable reductions in the soluble sugar accumulation in leaves. This is supported by previous studies117,118.
Apart from the plant mechanisms discussion under short and long-water deficit terms, a significant reduction in carrot yield subjected to limited irrigation at 80& 60% of ETc was observed. However, in contrast to 60% ETc, these reductions in yield weren’t reflected in attaining significant decreases in WUE under 80% ETc irrigation level. This indicates that at 60% ETc, the available water for the plants is critically decreased, resulting in exceeded plant ability to control the excessive production of ROS species. Consequently, these impacts result in decreased root development and reduced WUE. This aligns with the findings of Ali119, which showed the sensitivity of carrot roots to water deficit.
Interestingly, when carrot plants were exposed to prolonged water stress at 80% ETc, positive impacts on carrot yield and WUE were observed. This highlights the importance of determining the suitable water stress pattern for carrot crops. It is concluded that at 80% ETc, although the carrot plants have to deal with prolonged water stress, the reduction in water application amounts stimulated root activities and efficiency. Moreover, this reduction in irrigation amounts promoted root penetration more profoundly into the soil layers, enabling increased root elongation. Consequently, water, nutrient uptake, assimilation process in the leaves, and accumulation of photoassimilates in the roots improved, resulting in enhanced yield and WUE under these circumstances.
Overall, the previous findings have clarified that the plant’s relative success in growing and developing well under water deficit conditions depends on water stress intensity and the plant’s ability to combine multiple adaptation strategies to survive where water stress happens. This could explain the beneficial impacts yielded 80% ETc compared to 60% ETc.
Carrot responses to SAN and FKH applications under various irrigation levels in the short-term duration
Under stressful conditions, to improve plant tolerance to water deficit, it is essential to (i) adjust osmolyte accumulations, such as proline and sugar, to enhance osmoprotectants that help maintain cell turgor and water status and (ii) increase the level of antioxidants while controlling H2O2 production to improve tolerance efficiency. In this respect, SAN and FKH applications have the potential to attain that of plants affected by water stress. In the current study, significant enhancements were observed in the antioxidants, carbohydrates, and proline accompanied by substantial changes in NO3 and soluble sugar contents of carrot plants under short water stress-term conditions × high applications of SAN and FKH.
Comparing the changes in the various physiological and biochemical characteristics as a result of SAN and FKH applications with the control treatments indicated that after 65 DAE, the contents of (chlorophyll b, carotenoids except for 80% ETc, and dry matter) significantly increased by increasing the applied water and the examined application rates, and irrigation level bars took the same gradual increases patterns. Likewise, (H2O2 except for 60% ETc, proline, SOD, POD, and APX) significantly increased by decreasing the applied water and increasing application rates of SAN and FKH, and irrigation level bars took the same gradual patterns. Conversely, modifications in the obtained patterns were found in the contents of (chlorophyll a, NO3, NH4, CAT, carbohydrates, and soluble sugars), especially at 80% of the ETc irrigation level in the combination treatments. This may account for the impact of SAN and FKH applications in attaining better stress tolerance by these modifications under this irrigation level. These modifications are supposed to play a vital role in osmotic regulation in water stress conditions. They can affect various physio-biochemical processes related to plant defense functions, plant metabolism, and synthesis, either directly or indirectly.
The modifications in carbohydrate and soluble sugar contents highlight the potential role in osmotic adjustment under water deficit conditions. Increased accumulation of chlorophyll a in leaves under ETc 80% resulted in enhanced light harvesting, decreased chlorophyll fluorescence, and helped to convert the absorbed photons energy into chemical energy120. Similarly, increased CAT production in carrot leaves at 80% of the ETc protects plant cells from rising oxidative levels under these conditions, promoting the antioxidant capacity and improving plants’ ability to resist water deficiency; these findings agree with121. Among antioxidant enzymes, CAT is the most efficient and has the potential to neutralize ROS species, including H2O2, into H2O and O2122. In this respect, studies by Alam and Ghosh123 and Poli et al.124 have highlighted the efficient role of higher CAT activities and contents in scavenging the ROS at the early plant stages compared to the other antioxidant enzymes. Also, previous studies observed that sensitive crops like carrots were associated with decreased activities and contents of CAT under water deficit conditions125,126. These noteworthy results underscore the potential of SAN and FKH applications as valuable tools for bolstering carrot resistance to water deficiency by modifying and increasing CAT contents.
By adopting an ETc 80% irrigation level, the combinations of SAN and FKH applications in a short water deficit period effectively strengthen carrot plants’ ability to withstand irrigation water deficiency and protect them against the harmful effects of high levels of NH4 in leaves. In this respect, de Castro et al.127 concluded that a high concentration of NH4 in the plant leaves increased chloroplast degradation, decreased photosynthesis rate, and intensified NH4 toxicity. They attributed NH4 toxicity to (i) the lack of balance between N absorption and assimilation and (ii) the acidic stress induced by the higher proton amounts in the chloroplasts released during NH4 assimilation. Hence, reductions in NH4 concentrations have been previously observed by SAN and FKH application combinations under ETc 80% irrigation level, as opposed to (ETc 100 and 60%) and the individual SAN applications. This study, therefore, concluded that NH4 regulation in plants is linked to (i) the obtained irrigation level and (ii) appropriate application combined with SAN, which is essential in higher plants to balance NH4 and NO3 in N assimilation107,128. Conversely, controlling NO3 accumulation by SAN and FKH combinations at ETc 80% irrigation level and equalizing or reducing their concentration in leaves compared to ETc 100% helps adapt carrot plants to abiotic stress.
These modifications show carrot plants’ dynamic responses to short-water deficits and highlight the fundamental roles of chlorophyll a, NO3, NH4, carbohydrates, soluble sugars, and CAT in regulating and improving different physiological processes that allow plants to adapt and survive in diverse conditions.
Carrot responses to SAN and FKH applications under various irrigation levels in the long-term duration
As indicated in the short-water deficit term, the simultaneous application of SAN and FKH improved soluble sugar contents compared to the control treatment, especially in the limited irrigation levels, compensating for the declined water stress tolerance efficiency. This resulted in higher levels of osmoprotectants in carrot leaves under these conditions, leading to enhanced physiological processes and water potential, ultimately alleviating water stress’s harmful impacts. The results showed that these adaptive responses continued, and the converted amounts of soluble sugar exceeded or equaled those obtained under complete irrigation treatment by applying SAN and FKH under ETc 80 and 60% of ETc, except for T9. The reductions in soluble sugars under ETc 80 and 60% of ETc as a consequence of SAN and FKH applications are due to the effective antioxidant system activation, led to a decreased need for soluble sugar accumulations at this point, which is ultimately contributed to raising the carbohydrate synthesis processes.
Another differential plant response examined in this study was the impact of SAN and FKH applications on pigment contents under various irrigation levels. To mitigate the detrimental effects of water deficit on chlorophyll contents, individual and combined applications of SAN and FKH were applied. The findings of the current study indicated that these applications under water deficit levels had a significant positive impact on chlorophyll contents in carrots, which is consistent with the findings of other studies on the positive effects of SAN and FKH applications under mild water deficiency on similar plants43,129,130. However, under ETc 80%, the impact of these applications was found to be more significant on chlorophyll a and chlorophyll b content, which equaled or passed ETc 100%. In this concern, Khayatnezhad & Gholamin131 and Chowdhury et al.132 reported plants that can sustain higher chlorophyll contents under water deficit conditions can contribute to avoiding negative influences and be more resistant to stress. Under study conditions, this has been achieved by using these applications. These increases in chlorophyll concentration under ETc 80% are attributed to (SAN and FKH) ability to promote chlorophyll formation while concomitantly preventing its degradation, which aligns with previous research conducted by133. Under biotic or abiotic stresses, the application of both N and humic substances showed significant improvements in plant pigments134,135. Additionally, humic materials containing potassium have dual positive effects on plant growth, as both humate and potassium are stress inducers136. Moreover, this positive impact on chlorophyll concentrations can be attributed to the irrigation level it adopts, which improves root uptake efficiency and facilitates plant assimilation processes. Understanding this plant response to SAN and FKH applications under mild water deficiency can help scientists and farmers develop innovative practices for alleviating the detrimental impacts and enhancing the tolerance of carrot plants under challenging water deficit conditions. On the other hand, a notable increase in carotenoid content was observed when SAN and FKH applications were applied in water-stressed carrot plants compared to the control treatment. These elevated levels of carotenoids indicate the presence of oxidative damage in carrot plants experiencing water stress. The elevated levels of ROS/RNS (NO3 and H2O2) under water stress resulted in harmful conditions termed cell redox status, which exceeded the ability of carotenoids to deal with loneliness.
Regarding NO3 and H2O2 levels under water scarcity, the results indicate that the different water levels and the SAN and FKH applications highly influenced NO3 concentrations in carrot leaves. Based on the measured data during the long-term period, under 80&60% of ETc, high NO3 concentration in carrot leaves was observed with the individual applications and rates of SAN and KH compared to ETc100%. In contrast, the combination of SAN and FKH significantly decreased NO3 concentrations under ETc 80%, compared to ETc100%. While under ETc 60%, there was a marked increment in NO3 by applying the combined applications. However, these increments didn’t exceed or equal those obtained in ETc100% irrigation levels. Zhang et al.107 suggested that NO3 increases under full irrigation conditions could happen mainly due to the imbalance between N supply and demand, which is required for optimal growth. Moreover, Anjana & Iqbal137 found that externally higher applied K rates help to enhance NO3 absorption and transport toward the plant leaves under normal conditions. Additionally, numerous studies have highlighted the beneficial impacts of foliar applications of SAN and humic components on various crops El-Nasr138 and Tavares et al.139. They found a strong association between these examined applications and NO3, resulting in the enhancements of NO3 accumulation. However, in this regard, Nazaryuk et al.140, Fawzy141, and Shahein et al.142 found that applying humic acid as a foliar spray gave lower NO3 concentrations in head lettuce. At the same time, Haghighi et al.143 indicated no significant impact of humic acid on NO3 accumulation in tomatoes.
On the other hand, we need to advance our understanding of the mechanisms by which NO3 production is regulated under water deficit conditions based on stress intensity and duration. It is necessary to remember the significant role that NO3 plays in enhancing plant development and osmotic regulation144. Consequently, under severe water stress (ETc 60%), higher NO3 amounts could effectively improve plant tolerance, which, in turn, is promoted by the combined application of SAN and FKH applications. Meng et al.145 concluded another possible reason for improved NO3 content in leaves under water deficit levels was closely related to increased K uptake and transport under these conditions. Besides that, under water deficit conditions, the oxidative process was amplified by reducing CO2 assimilation, which induces excess excitation energy and electron flux to O2146.
These reductions can be achieved by applying SAN and FKH under ETc 80%. This study postulated that under the ETc 80% level, carrot plants experience a certain degree of water stress, which triggers some physiological and biochemical responses in higher NO3 amounts. Stressed carrot plants can benefit from applying SAN and FKH, as these can help activate their defense mechanisms. This can improve the plant’s water status and synthesis processes by increasing nutrient uptake while reducing the harmful impacts of ROS (H2O2 levels). Consequently, this result concluded that the assimilation of NH4 was promoted under these conditions accompanied by decreasing NO3 amounts. Therefore, Bian et al.91 highlighted the potential role of the optimal irrigation regime in decreasing NO3 content and concomitantly attaining water-saving, which could provide an effective strategy under water deficit conditions.
On the other hand, under such growth conditions, the adoption of SAN and FKH applications at ETc 100 & ETc 80 irrigation levels increased antioxidant accumulation of (CAT, POD and, APX) and proline, which is due to improved nutrient uptake levels like N and iron. At the same time, Wang et al.147 demonstrated that iron is considered the main component in the formation of CAT, POD, and APX. Previous studies have indicated that N is involved in proline biosynthesis and differentiation across the plant growth cycle148,149,150. In the same context, Meena et al.150 stated that phytohormones regulate proline metabolism by controlling the accumulation of N2 or calcium (Ca2+). Nonetheless, the current study observed significant changes in carrot leaves’ antioxidants and proline contents under (SAN and FKH) applications × long water stress-term conditions. In contrast to the short water stress term, which attained high content, the collective antioxidants and proline values were lower in the long water stress period. However, ETc 100 & ETc 80 irrigation levels were taking the same gradual pattern as the short water stress term (ETc 80 > ETc 100); the ETc, 60% level, was the exception. These collective reductions in antioxidants and proline levels highlight the dynamic nature of plant responses to water deficit and the positive impacts of applications like SAN and FKH in improving growth conditions, as mentioned by a previous study151. Meena et al.150 illustrated that the catabolic activity and proline contents start to decrease within plant tissues when there is no need or their contents are in excess amounts. Nevertheless, this study attributed these declines in the long-term period to the reductions in water and nutrient uptake capacity152, which decline with root aging. Simultaneously, the biosynthesis of antioxidants and proline decreases under these conditions, especially under the ETc 60% level.
Impacts of SAN and FKH applications under various irrigation levels on carrot yield and WUE
Conclusively, the findings in the current study showed that the adoption of combined applications like T8 or T9 under ETc by 80% increased the carrots yield and achieved a higher WUE. In this respect, this study concluded that by applying these combined applications and rates under this limited irrigation level, many effective mechanisms discussed earlier, including regulation of NH4 and NO3, enhanced pigment contents, improved antioxidant defense systems, and sufficient proline concentrations in the cytoplasm, seem to have yielded beneficial effects throughout the short and prolonged water stress until harvest, which is consistent with findings from43. These mechanisms, accompanied by other improvements in nutrient homeostasis, plant growth, and development, enhance their drought tolerance and overall yield under such growth conditions.
Under stressful conditions at ETc 60%, plants produce ROS species, which help maintain carrot growth and enhance stress tolerance. Nonetheless, by increasing stress intensity, the ROS production rate increases exponentially, exceeding the ability of antioxidant scavengers. Hence, the plants, especially those sensitive to water deficit, cannot control and detoxify reactive oxygen species within plant cells, leading to detrimental oxidative consequences in plant development, yield, and WUE. Therefore, the results indicated that irrespective of adopting individual and combined applications of SAN + FKH, the lowest carrot yield and WUE were achieved under ETc 60%.
On the other hand, one interesting finding in this study was the sudden reduction in yield and WUE by applying the higher rate of SAN and FKH in treatment T9 under the ETc 100% level. Such a finding is significant as adopting this rate under ETc 100% is supposed to attain the highest improvements in carrot yield relative to the low applications. In this respect, this study hypothesized two reasons: A) that can be attributed to the change in C/N and N/P ratios. This could be confirmed through the nutritional analysis. This led to an increase in the vegetative growth rate processes (aerial parts) rather than the reproductive processes, which is reflected in decreased or delayed accumulation processes and led to unfavorable outcomes on yield. In this regard, a study by153 found a similar result in sweet potatoes by applying a higher application rate under well-watered irrigation conditions. The other reason, B), can be attributed to the observed increments in NH4 concentrations in the leaves. That triggered a series of negative physiological impacts on chlorophyll contents. As demonstrated previously, a high concentration of NH4 has deleterious consequences on chloroplasts and photosynthesis rate127. Also, in support of these findings, Xia et al.86 addressed the possible reasons behind the superiority of mild irrigation level compared to whole irrigation level in terms of groundnut yield to: (i) improve nitrification intensity under mild irrigation level, resulting in increased N available contents, maintained photosynthetic components, and enhanced nutrient transport and accumulate to sink organs; (ii) the presence of some reductions in chlorophyll content and plant tolerance under excessive applications of irrigation and N.
Conclusion
The findings indicate that in the short water stress term duration, by irrigating plants at 80% of crop evapotranspiration, carrot plants tend to increase the synthesis of (carotenoids except for 60% of crop evapotranspiration, nitrate, proline, antioxidants, and soluble sugar) and decrease (ammonium and carbohydrate contents) in response to the production of reactive oxygen species. In the prolonged water stress term duration, carrot plants tend to modify their defense mechanisms by increasing (nitrate, proline except for 60% of crop evapotranspiration, antioxidants except for 60% of crop evapotranspiration, and soluble sugar) and decreasing ammonium and carbohydrate contents. These adaptive responses at 80% of crop evapotranspiration improve their resilience to water deficiency and allow carrot plants to maintain physiological processes and yield. By rearranging chlorophyll a, nitrate, ammonium, catalase, carbohydrate, and soluble sugars accumulations at 80% of crop evapotranspiration, the combined application of ammonium nitrate and potassium humate helps plants to optimize their adaptive responses and minimize the detrimental impacts of water deficit. Overall, the findings indicate that ammonium nitrate and potassium humate applications and rates positively affected carrot physiological and biochemical processes and yield under various irrigation levels despite observed negative impacts at the high rates of these applications under full irrigation level. Therefore, it is recommended to apply combined applications of ammonium nitrate at 250 kg N ha− 1 in combination with foliar potassium humate at 200 g 100 L− 1 water under 80% of the crop evapotranspiration for carrots to improve chlorophyll content, antioxidant enzymes, proline, carbohydrate, yield and water use efficiency of the carrot plants. This approach highlights the potential benefits of combining nutrient applications and irrigation management strategies to enhance carrot performance and address the challenges of water scarcity in agriculture.
Data availability
All the data related to this work can be sourced from the corresponding authors.
Change history
18 July 2025
This article has been updated to amend the license information.
References
Blando, F. et al. Bioactive compounds and antioxidant capacity in anthocyanin-rich carrots: a comparison between the black carrot and the Apulian landrace Polignano carrot. Plants 10, 564. (2021).
Kowalczyk, Z. & Kuboń, M. Assessing the impact of water use in conventional and organic Carrot production in Poland. Sci. Rep. 12, 1–11 (2022).
Abaza, A. S. D., Elshamly, A. M. S. & Alwahibi, M. S. Impact of different sowing dates and irrigation levels on NPK absorption, yield and water use efficiency of maize. Sci. Rep. 13, 12956 (2023).
Gruda, N. S., Dong, J. & Li, X. From salinity to Nutrient-Rich vegetables: strategies for quality enhancement in protected cultivation. Crit. Rev. Plant. Sci. 45. (2024).
Junaid, M. D., Öztürk, Z. N. & Gökçe, A. F. Exploitation of tolerance to drought stress in Carrot (Daucus Carota L.): An overview. Stress Biology. 3, 55 (2023).
Murtaza, G., Usman, M. & Iqbal The impact of Biochar addition on morpho-physiological characteristics, yield, and water use efficiency of tomato plants under drought and salinity stress. BMC Plant. Biol. 24, 356 (2024).
Elshamly, A. M. S. & Nassar, S. M. A. Stimulating growth, root quality, and yield of carrots cultivated under full and limited irrigation levels by humic and potassium applications. Sci. Rep. 13, 14260 (2023).
El-Tohamy, W. A., Dasgan, H. Y. & Gruda, N. S. Impact of gibberellic acid on water status, growth, and development of cape gooseberry in newly reclaimed sandy lands within arid regions. Horticulturae 9, 1283 (2023).
Badr, M. A., El-Tohamy, W. A., Salmanb, S. R. & Gruda, N. Yield and water use relationships of potato under different timing and severity of water stress. Agric. Water Manag. 271, 107793 (2022).
Abd Ellah, R. G. Water resources in Egypt and their challenges, lake Nasser case study. Egypt. J. Aquat. Res. 46, 1–12 (2020).
Nikiel, C. A. & Eltahir, E. A. B. Past and future trends of Egypt’s water consumption and its sources. Nat. Commun. 12, 4508 (2021).
Ma, C., Yang, Z. & Xia, R. Rising water pressure from global crop production—a 26-yr multiscale analysis. Resour. Conserv. Recycl 172, 105665 (2021).
Badr, M. A., El-Tohamy, W. A., Abu Hussein, S. D. & Gruda, N. Tomato yield, physiological response, water and nitrogen use efficiency under deficit and partial root-zone drying irrigation in an arid region. J. Appl. Bot. Food Qual. 91, 332–340 (2018).
El-Bially, M. A., Saudy, H. S., El-Metwally, I. M. & Shahin, M. G. Efficacy of ascorbic acid as a cofactor for alleviating water deficit impacts and enhancing sunflower yield and irrigation water–use efficiency. Agric. Water Manage. 208, 132–139 (2018).
El-Tohamy, W. A., El-Abagy, H. M., Badr, M. A. & Gruda, N. Drought tolerance and water status of bean plants (Phaseolus vulgaris L.) as affected by citric acid application. J. Appl. Bot. Food Qual. 86, 212–216 (2013).
Raza, M. A. S., Aslam, M. U. & Valipour, M. Seed priming with selenium improves growth and yield of Quinoa plants suffering drought. Sci. Rep. 14, 886 (2024).
Elshamly, A. M. S., Gameh, M. A. & Rushdi, M. K. Effect of irrigation systems, pulse irrigation technique and Cobalt application on productivity and water use efficiency of tomato plants grown in new reclaimed soil. Assiut J. Agric. Sci. 47 (2016). ,6 – 2.
Saudy, H. S., El-Bially, M. A., Hashem, F. A. & Shahin, M. G. El–Gabry, Y. A. The changes in yield response factor, water use efficiency, and physiology of sunflower owing to ascorbic and citric acids application under mild deficit irrigation. Gesunde Pflanzen. 75, 899–909 (2023).
Badr, M. A., Abou Hussein, S. D., El-Tohamy, W. A. & Gruda, N. Efficiency of subsurface drip irrigation for potato production under different dry stress conditions. Healthy Plants 62, 63–70 (2010).
El-Bially, M. A., Saudy, H. S., Hashem, F. A., El–Gabry, Y. A. & Shahin, M. G. Salicylic acid as a tolerance inducer of drought stress on sunflower grown in sandy soil. Gesunde Pflanzen 74, 603–613 (2022).
Ayupov, D. et al. Improving technology elements in multi-purpose Carrot cultivation. Bulgarian J. Agric. Sci. 25, 21–29 (2019).
Lago-Olveira, S., El-Areed, S. R., Moreira, M. T. & González-García, S. Improving environmental sustainability of agriculture in Egypt through a life-cycle perspective. Sci. Total Environ. 890, 164335 (2023).
Badr, M. A., Abou Hussein, S. D., El-Tohamy, W. A. & Gruda, N. Nutrient uptake and yield of tomato under various methods of fertilizer application and levels of fertigation in arid lands. Healthy Plants 62, 11–19 (2010).
Haider, M. W. et al. Rejuvenating potato growth and yield in challenging semiarid and saline sandy Cholistan: Harnessing PGPB-coated N and P application strategies. BMC Plant. Biol. 24, 386. https://doi.org/10.1186/s12870-024-05056-x (2024).
Lipiec, J. & Usowicz, B. Quantifying cereal productivity on sandy soil in response to some soil-improving cropping systems. Land 10, 1199 (2021).
Osman, K. T. Management of soil problems. In Sandy Soils; Osman, K. T., Ed.; Springer International Publishing: Cham, Switzerland 37–65. (2018).
Baumert, S., Khamzina, A. & Vlek, P. L. G. Soil organic carbon sequestration in Jatropha curcas systems in Burkina Faso. Land. Degrad. Dev. 27, 1813–1819 (2014).
Manzoor, S., Habib-ur-Rahman, M., Haider, G., Ghafoor, I., Ahmad, S., Afzal, M., Nawaz, F., Iqbal, R., Yasin, M., Danish, S. & Ghaffar, A. Biochar and slow-releasing nitrogen fertilizers improved growth, nitrogen use, yield, and fiber quality of cotton under arid climatic conditions. Environ. Sci. Pollut. Res. 1–14 (2022).
Iqbal, R., Raza, M. A., Saleem, M. F., Khan, I. H., Ahmad, S., Zaheer, M. S., Aslam, M. U. & Haider, I. Physiological and biochemical appraisal for mulching and partial rhizosphere drying of cotton. J. Arid Land 11, 785–794 (2019).
Akladious, S. A. & Mohamed, H. I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 236, 244–250 (2018).
Ali, Q., Shabaan, M., Ashraf, S., Kamran, M., Zulfiqar, U., Ahmad, M., Zahir, Z. A., Sarwar, M. J., Iqbal, R., Ali, B. & Ali, M. A. Comparative efficacy of different salt tolerant rhizobial inoculants in improving growth and productivity of Vigna radiata L. under salt stress. Sci. Rep. 13, 17442 (2023).
Leite, J. M. et al. J. Co-addition of humic substances and humic acids with urea enhances foliar nitrogen use efficiency in sugarcane (saccharum officinarum L). Heliyon 6, e5100. (2020).
Muhammad, S. et al. Compost and humic acid amendments are a practicable solution to rehabilitate weak arid soil for higher winter field pea production. Sci. Rep. 13, 17519 (2023).
Anwar, Z. et al. Biofortification of maize with zinc and iron not only enhances crop growth but also improves grain quality. Asian J. Agric. Biol. 202102079. (2022).
Yildiztekin, M., Tuna, A. L. & Kaya, C. Physiological effects of the brown seaweed (Ascophyllum nodosum) and humic substances on plant growth, enzyme activities of certain pepper plants grown under salt stress. Acta Biol. Hung. 69, 325–335 (2018).
Abd El-Fattah, D. A., Hashem, F. A. & Abd-Elrahman, S. H. Impact of applying organic fertilizers on nutrient content of soil and lettuce plants, yield quality and benefit-cost ratio under water stress conditions. Asian J. Agric. Biol. 202102086. (2022).
Aminifard, M. H., Aroiee, H., Azizi, M., Nemati, H. & Jaafar, H. Z. E. Effect of humic acid on antioxidant activities and fruit quality of hot pepper (Capsicum annuum L). Herbs Spices Med. Plants. 18, 360–369 (2012).
Rashedy, A. A., Abd-El Nafea, M. & Khedr, E. H. Co-application of proline or calcium and humic acid enhances productivity of salt stressed pomegranate by improving nutritional status and osmoregulation mechanisms. Sci. Rep. 12, 1–10 (2022).
Ghasemi, K. et al. Antioxidant properties of Garlic as affected by selenium and humic acid treatments. New. Z. J. Crop Hort Sci. 43, 173–181 (2015).
Ampong, K., Thilakaranthna, M. S. & Gorim, L. Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 4, 848621 (2022).
Chen, W., Luo, J. K. & Shen, Q. R. Effect of NH4–N/NO3–N ratios on growth and some physiological parameters of Chinese cabbage cultivars. Pedosphere 15, 310–318 (2005).
Chafai, W., Gabardi, E. L., Douira, S. & Khalid, A. A. Diversity and mycorrhizal potential of arbuscular mycorrhizal fungi in two natural soils in the Eastern region of Morocco. Asian J. Agric. Biol. 202102101. (2022).
Naseri, A., Alirezalu, A. & Noruzi, P. The effect of different ammonium to nitrate ratios on antioxidant activity, morpho-physiological and phytochemical traits of Moldavian balm (Dracocephalum moldavica). Sci. Rep. 12, 16841 (2022).
Ma, D. et al. Effects of the nitrate and ammonium ratio on plant characteristics and erythropalum scandens Bl. substrates. Plos One. 18, e0289659 (2023).
Raza, M. A. S., Ahmad, S., Saleem, M. F., Khan, I. H., Iqbal, R., Zaheer, M. S., Haider, I. & Ali, M. Physiological and biochemical assisted screening of wheat varieties under partial rhizosphere drying. Plant Physiol. Biochem. 116, 150–166 (2017).
Hou, W., Tränkner, M. & Lu, J. Interactive effects of nitrogen and potassium on photosynthesis and photosynthetic nitrogen allocation of rice leaves. BMC Plant. Biol. 19, 302 (2019).
Albano, X., Whitmore, A. P. & Sakrabani, R. Effect of different organic amendments on actual and achievable yields in a cereal-based cropping system. J. Soil. Sci. Plant. Nutr. 23, 2122–2137 (2023).
Estefan, G., Sommer, R. & Ryan, J. Methods of soil, plant, and water analysis: a manual for the west, Asia and North Africa region. ICARDA, Beirut, Lebanon. (2013).
Allen, R. G., Pereira, L. S., Raes, D. & Smith, M. Crop evapotranspiration: guidelines for computing crop water requirements. Irrigation and drainage paper no 56. Food and agriculture Organization of the United Nations (FAO), Rome, Italy. (1998).
Morrow, M., Sharma, V., Hochmuth, R., Barrett, C. & Burani-Arouca, M. Carrot (Daucus carota) production in the sandy soils of North Florida. Nitrogen Fertilization Guidelines (2023).
Ahmadi, S. Z., Zahedi, B. & Ghorbanpour, M. Comparative morpho-physiological and biochemical responses of Capsicum annuum L. plants to multi-walled carbon nanotubes, fullerene C60 and graphene nanoplatelets exposure under water deficit stress. BMC Plant. Biol. 24, 116 (2024).
Mo, Q. et al. Leaf nonstructural carbohydrate concentrations of understory Woody species regulated by soil phosphorus availability in a tropical forest. Ecol. Evol. 10, 8429–8438 (2020).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207 (1973).
Shi, S. et al. & NH4+ toxicity, which is mainly determined by the high NH4+/K+ ratio, is alleviated by CIPK23 in arabidopsis. Plants. 9, 501. (2020).
Li, C. et al. Determination of optimal NH4+/K+ concentration and corresponding ratio critical for growth of tobacco seedlings in a hydroponic system. Front. Plant. Sci. 14, 1152817 (2023).
Zhao, L. & Wang, Y. Nitrate assay for plant tissues. Bio Protoc 7, e (2017) (2029).
Azevedo Neto, A. D., Nogueira, R. J. M. C., Melo Filho, P. A. & Santos, R. C. Physiological and biochemical responses of peanut genotypes to water deficit. J. Plant. Interact. 5, 1–10 (2010).
Ahsan, A. F. M. S. et al. Assessment and assortment of tomato genotypes against salinity at vegetative stage. Asian J. Agric. Biol. 202108321. (2022).
Aebi, H. Catalase in vitro. Methods in enzymology. Acad. Press. 105, 121–126 (1984).
Ranieri, A., Castagna, A. & Soldatini, G. F. Differential stimulation of ascorbate peroxidase isoforms by Ozone exposure in sunflower plants. J. Plant. Physiol. 156, 266–271 (2000).
Shipley, B. & Vu, T. T. Dry matter content as a measure of dry matter concentration in plants and their parts. New. Phytol. 153, 359–364 (2002).
Elshamly, A. M. S. Interaction effects of sowing date, irrigation levels, Chitosan, and potassium silicate on yield and water use efficiency for maize grown under arid climate. Gesunde Pflanzen. 75, 1601–1613 (2023).
Casella, G. Statistical Design, 1st edn. Springer. (2008).
Saudy, H. S. & El–Momen, A. El–khouly, N.S. Diversified nitrogen rates influence nitrogen agronomic efficiency and seed yield response index of Sesame (Sesamum indicum, L.) cultivars. Comm. Soil. Sci. Plant. Anal. 49, 2387–2395 (2018).
Elshamly, A. M. S. Cobalt combined with potassium humate as beneficial applications in alleviating water stress impacts on groundnut during sensitive growth stages. J. Soil. Sci. Plant. Nutr. 23, 4505–4520 (2023).
Man Hong, Y., Lei, Z., Sheng-Tao, X. & McLaughlin, N. B. Jing-Hui, L. Effect of water soluble humic acid applied to potato foliage on plant growth, photosynthesis characteristics and fresh tuber yield under different water deficits. Sci. Rep. 10, 1–10 (2020).
Sindesi, O. A., Ncube, B., Lewu, M. N., Mulidzi, A. R. & Lewu, F. B. Cabbage and Swiss Chard yield, irrigation requirement and soil chemical responses in zeolite-amended sandy soil. Asian J. Agric. Biol. 202111387. (2023).
Mohammed, K. A. S., Hussein, H. M. & Elshamly, A. M. S. Monitoring plant responses in field-grown peanuts exposed to exogenously applied Chitosan under full and limited irrigation levels. Sci. Rep. 14, 6244 (2024).
Du, Y. et al. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant. Physiol. Biochem. 146, 1–12 (2020).
Elshamly, A. M. S. & Abaza, A. S. Precise partial root-zone irrigation technique and potassium-zinc fertigation management improve maize physio-biochemical responses, yield, and water use in arid climate. BMC Plant. Biol. 24, 775 (2024).
Mibei, E. K., Ambuko, J., Giovannoni, J. J., Onyango, A. N. & Owino, W. O. Carotenoid profiling of the leaves of selected African eggplant accessions subjected to drought stress. Food Sci. Nutr. 5, 113–122 (2017).
Elshamly, A. M. et al. Zinc and amino acids improve the growth, physiological, and biochemical attributes of corn under different irrigation levels. Rhizosphere 29, 100820 (2024).
Basal, O., Zargar, T. B. & Veres, S. Elevated tolerance of both short-term and continuous drought stress during reproductive stages by exogenous application of hydrogen peroxide on soybean. Sci. Rep. 14, 2200 (2024).
Fatemi, R., Yarnia, M., Mohammadi, S., Vand, E. K. & Mirashkari, B. Screening barley genotypes in terms of some quantitative and qualitative characteristics under normal and water deficit stress conditions. Asian J. Agric. Biol. 2022071. (2023).
Hou, X., Rivers, J., León, P., Mcquinn, R. P. & Pogson, B. J. Synthesis and function of apocarotenoid signals in plants. Trends Plant. Sci. 21, 792–803 (2016).
Schlering, C. et al. Chemical composition of field grown radish (Raphanus sativus L. Var. sativus) as influenced by season and moderately reduced water supply. J. Appl. Bot. Food Qual. 92, 343–354 (2019).
Saudy, H. S., El-Metwally, I. M. & Abd El-Samad, G. A. Physio–biochemical and nutrient constituents of peanut plants under Bentazone herbicide for broad–leaved weed control and water regimes in dry land areas. J. Arid Land. 12, 630–639 (2020).
Shokat, S., Großkinsky, D. K. & Roitsch, T. Activities of leaf and Spike carbohydrate-metabolic and antioxidant enzymes are linked with yield performance in three spring wheat genotypes grown under well-watered and drought conditions. BMC Plant. Biol. 20, 400 (2020).
Hadid, M. L. et al. Modulating the antioxidant defense systems and nutrients content by proline for higher yielding of wheat under water deficit. Not Bot. Horti Agrobo. 51, 13291 (2023).
Abeed, A. H. A. et al. S. Ameliorative effects of exogenous potassium nitrate on antioxidant defence system and mineral nutrient uptake in radish (Raphanus sativus L.) under salinity stress. ACS Omega (2023).
Elshamly, A. M. S., Parrey, Z. A., Gaafar, A. R. Z., Siddiqui, M. H. & Hussain, S. Potassium humate and Cobalt enhance peanut tolerance to water stress through regulation of proline, antioxidants, and maintenance of nutrient homeostasis. Sci. Rep. 14, 1625 (2024).
Khan, N. et al. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak J. Bot. 52, 355–363 (2020).
Taratima, W., Kunpratum, N. & Maneerattanarungroj, P. Effect of salinity stress on physiological aspects of pumpkin (Cucurbita moschata Duchesne. ‘Laikaotok’) under hydroponic condition. Asian J. Agric. Biol. 202101050. (2023).
Ullah, A. et al. & Phosphorous supplementation alleviates drought-induced physio-biochemical damages in calligonum mongolicum. Plants. 11,3054. (2022).
Tian, J., Pang, Y. & Zhao, Z. Drought, salinity, and low nitrogen differentially affect the growth and nitrogen metabolism of Sophora japonica (L.) in a semi-hydroponic phenotyping platform. Front. Plant. Sci. 12, 715456 (2021).
Xia, G., Wu, Q., Chi, D., Chen, J. & Wang, S. Enhancing water productivity while improving peanut kernel quality by water regulation under different nitrogen levels. Irrig. Drain. 69, 86–94 (2020).
Zia, R., Nawaz, M. S., Siddique, M. J., Hakim, S. & Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 242, 126626 (2021).
Urlić, B. et al. Effect of NO3 and NH4 concentrations in nutrient solution on yield and nitrate concentration in seasonally grown leaf lettuce. Acta Agric. Scand. Sect. B 67, 748–757 (2017).
Burns, I. G., Zhang, K. F., Turner, M. K. & Edmondson, R. Iso-osmotic regulation of nitrate accumulation in lettuce. J. Plant. Nutr. 34, 283–313 (2010).
Schiattone, M. I. et al. Impact of irrigation regime and nitrogen rate on yield, quality and water use efficiency of wild rocket under greenhouse conditions. Sci. Hort. 229, 182–192 (2018).
Bian, Z. et al. A review of environment effects on nitrate accumulation in leafy vegetables grown in controlled environments. Foods 9, 732 (2020).
Victoria, O. et al. Seed treatment with 24-epibrassinolide improves wheat germination under salinity stress. Asian J. Agric. Biol. (2023).
Grzyb, A., Wolna-Maruwka, A. & Niewiadomska, A. The significance of microbial transformation of nitrogen compounds in the light of integrated crop management. Agron 11, 1415 (2021).
Stremińska, M. A. & Błaszczyk, M. The biogeochemical cycle of nitrogen in soils of coniferous forest ecosystems. Post. Mikrobiol. 43, 235–250 (2004).
Reid, J. B. & Gillespie, R. N. Yield and quality responses of carrots (Daucus Carota L.) to water deficits. New. Zeal J. Crop Hort. 45, 299–312 (2017).
Khalid, M. F., Huda, S. & Yong, M. Alleviation of drought and salt stress in vegetables: Crop responses and mitigation strategies. Plant. Growth Regul. 99, 177–194 (2023).
Schmidhalter, U. & Oertli, J. J. Germination and seedling growth of carrots under salinity and moisture stress. Plant. Soil. 132, 243–251 (1991).
Baba, L. Y. & Simon, G. The influence of irrigation frequency on yield and water use efficiency of Carrot. Agric. Water Res. 5, 47–55 (2015).
De Cruz, M. H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant. Signal. Behav. 3, 156–165 (2008).
Sachdev, S., Ansari, S. A., Ansari, M. I., Fujita, M. & Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 10, 277 (2021).
Sarkar, M., Akter, S. & Bakhashwain, A. A. Influence of effective irrigation water usage on Carrot root productivity and quality properties in soilless culture. J. Soil. Sci. Plant. Nutr. 24, 1042–1058 (2024).
Deng, Y. Y. et al. Nitric oxide as an all-rounder for enhanced photodynamic therapy: Hypoxia relief, glutathione depletion and reactive nitrogen species generation. Biomaterials 187, 55–65 (2018).
Abd–Elrahman, S. H., Saudy, H. S. & El–Fattah, D. A. A. Effect of irrigation water and organic fertilizer on reducing nitrate accumulation and boosting lettuce productivity. J. Soil. Sci. Plant. Nutr. 22, 2144–2155 (2022).
Sørensen, J. N., Jørgensen, U. & Kühn, B. F. Drought effects on the marketable and nutritional quality of carrots. J. Sci. Food Agric. 74, 379–391 (1997).
Timilsina, A., Dong, W., Hasanuzzaman, M., Liu, B. & Hu, C. Nitrate–nitrite–nitric oxide pathway: A mechanism of hypoxia and anoxia tolerance in plants. Int. J. Mol. Sci. 23, 11522 (2022).
Di Meo, S., Reed, T. T., Venditti, P. & Victor, V. M. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med. Cell. Longev. 1–44. (2016).
Zhang, J. L. V. J. et al. Appropriate ammonium-nitrate ratio improves nutrient accumulation and fruit quality in pepper (capsicum annuum L). Int. J. Agron. 9, 683 (2019).
Obidiegwu, J., Bryan, G., Jones, H. & Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant. Sci. 6, 542 (2015).
Perlikowski, D., Lechowicz, K. & Pawłowicz, I. Scavenging of nitric oxide up-regulates photosynthesis under drought in Festuca arundinacea and F. glaucescens but reduces their drought tolerance. Sci. Rep. 12, 6500 (2022).
Umar, A. S. & Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications: A review. Agron. Sustain. Dev. 27, 45–57 (2007).
Veen, B. W. & Kleinendorst, A. Nitrate accumulation and osmotic regulation in Italian ryegrass (Lolium multiflorum Lam). J. Exp. Bot. 36, 211–218 (1985).
Seginer, I., Buwalda, F. & van Straten, G. Nitrate concentration in greenhouse lettuce: A modeling study. Acta Hortic. 456, 189–197 (1998).
Sevanto, S. Drought impacts on phloem transport. Curr. Opin. Plant. Biol. 43, 76–81 (2018).
Lemoine, R., La Camera, S. & Atanassova, R. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant. Sci. 4, 272 (2013).
Burke, J. J. Evaluation of source leaf responses to water-deficit stresses in cotton using a novel stress bioassay. Plant. Physiol. 143, 108–121 (2007).
Hummel, I. et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant. Physiol. 154, 357–372 (2010).
Hartmann, H., Ziegler, W., Kolle, O. & Trumbore, S. Thirst beats hunger–declining hydration during drought prevents carbon starvation in Norway Spruce saplings. New. Phytol. 200, 340–349 (2013).
Dien, D. C., Mochizuki, T. & Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in rice (Oryza sativa L.) varieties. Plant. Prod. Sci. 22, 530–545 (2019).
Ali, A. A. Effect of deficit irrigation during growth stages on water use efficiency of Carrot under El-Ismailia conditions. Egypt. J. Soil. Sci. 57, 393–406 (2017).
Papageorgiou, G. C., Tsimilli-Michael, M. & Stamatakis, K. The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: A viewpoint. Photosynth Res. 94, 275–290 (2007).
Hasanuzzaman, M. et al. Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 9, 681 (2020).
Alam, M. M., Nahar, K., Hasanuzzaman, M. & Fujita, M. Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant. Omics J. 7, 271–283 (2014).
Alam, N. B. & Ghosh, A. Comprehensive analysis and transcript profiling of Arabidopsis thaliana and Oryza sativa catalase gene family suggests their specific roles in development and stress responses. Plant. Physiol. Biochem. 123, 54–64 (2018).
Poli, Y. et al. Increased catalase activity and maintenance of photosystem II distinguishes high-yield mutants from low-yield mutants of rice Var. Nagina22 under low-phosphorus stress. Front. Plant. Sci. 9, 1543 (2018).
Sekmen, A. H., Ozgur, R., Uzilday, B. & Turkan, I. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 99, 141–149 (2014).
Sarker & Oba, U. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 8, 16496 (2018).
de Castro, S. A. Q., Kichey, T., Persson, D. P. & Schjoerring, J. K. Leaf scorching following foliar fertilization of wheat with Urea or Urea–Ammonium nitrate is caused by ammonium toxicity. Agron 12. (2022).
Yoneyama, T., Matsumaru, T., Usui, K. & Engelaar, W. M. H. G. Discrimination of nitrogen isotopes during absorption of ammonium and nitrate at different nitrogen concentrations by rice (Oryza sativa L.) plants. Plant. Cell. Environ. 24, 133–139 (2001).
Zayed, B. A., Salem, A. K., Bassiouni, S. M. A. & Gad, K. I. Response of wheat crop to nitrogen sources and application times under saline sodic soil conditions. J. Plant. Prod. 5, 1403–1414 (2014).
Alharbi, K. et al. Potassium humate and plant growth-promoting microbes jointly mitigate water deficit stress in soybean cultivated in salt-affected soil. Plants 11, 3016 (2022).
Khayatnezhad, M. & Gholamin, R. The effect of drought stress on the superoxide dismutase and chlorophyll content in durum wheat genotypes. Adv. Life Sci. 8, 119–123 (2021).
Chowdhury, M. S. N., Sani, M. N. H., Siddique, A. B., Hossain, M. S. & Yong, J. W. H. Synergistic effects of Biochar and potassium co-application on growth, physiological attributes, and antioxidant defense mechanisms of wheat under water deficit conditions. Plant. Stress 100452. (2024).
Gholami, F., Amerian, M. R. & Asghari, H. R. Assessing the effects of 24-epibrassinolide and yeast extract at various levels on Cowpea’s morphophysiological and biochemical responses under water deficit stress. BMC Plant. Biol. 23, 593 (2023).
Saudy, H. S. Maize–cowpea intercropping as an ecological approach for nitrogen-use rationalization and weed suppression. Archiv Agron. Soil. Sci. 61, 1–14 (2015).
Hadid, M. L. et al. Al-Elway OAAI Pyridoxine-HCl plus gypsum and humic acid reinforce salinity tolerance of coriander plants with boosting yield and modifying oil fractionations. Russ J. Plant. Physiol. 71. (2024).
Rizk, T. Y., kholousy, A. S. O., Saudy, H. S., Sultan, S. H. S. & Abd Alwahed, S. H. A. Breaking dormancy and enhancing germination of Avena sterilis L. and Amaranthus retroflexus L. weeds by gibberellic acid and potassium nitrate to keep soil and crops healthy. Gesunde Pflanzen. 75, 757–763 (2023).
Anjana, S. U. & Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 27, 45–57 (2007).
El-Nasr, A. Effect of some nitrogen sources and potassium levels on yield, quality and nitrate accumulation in lettuce leaves. J. Plant. Prod. 27, 3401–3411 (2002).
Tavares, O. C. H. et al. Humic acid as a biotechnological alternative to increase N-NO3- or N-NH4 + uptake in rice plants. Biocatal. Agric. Biotechnol. 20, 101226 (2019).
Nazaryuk, V. M., Klenova, M. I. & Kalimullina, F. R. Ecoagrochemical approaches to the problem of nitrate pollution in agroecosystems. Russ J. Ecol. 33, 392–397 (2002).
Fawzy, Z. F. Increasing productivity of head lettuce by foliar spraying of some bio and organic compounds. Mesop. J. Agric. 38, 20–28 (2010).
Shahein, M. M., Afifi, M. M. & Algharib, A. M. Study the effects of humic S.bstances on growth, chemical constituents, yield and quality of two lettuce cultivars (cv. S. Dark green and big Bell). J. Mater. Environ. Sci. 6, 473–486 (2015).
Haghighi, M. & Da Teixeira, J. A. Amendment of hydroponic nutrient solution with humic acid and glutamic acid in tomato (Lycopersicon esculentum mill.) culture. Soil. Sci. Plant. Nutr. 59, 642–648 (2013).
Cao, X. et al. Ammonium uptake and metabolism alleviate PEG-induced water stress in rice seedlings. Plant. Physiol. Biochem. 132, 128–137 (2018).
Meng, S., Zhang, C., Su, L., Li, Y. & Zhao, Z. Nitrogen uptake and metabolism of Populus simonii in response to PEG-induced drought stress. Environ. Exp. Bot. 123, 78–87 (2016).
Talbi, S. et al. Effect of drought on growth, photosynthesis and total antioxidant capacity of the saharan plant Oudeneya Africana. Environ. Exp. Bot. 176, 104099 (2020).
Wang, Y. P. et al. Effects of iron deficiency stress on plant growth and quality in flowering Chinese cabbage and its adaptive response. Agrono 12, 875 (2022).
Monreal, J. A. et al. Proline content of sugar beet. Storage roots: response to water deficit and nitrogen fertilization at field conditions. Environ. Exp. Bot. 60, 257–267 (2007).
Kaur, G. & Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 59, 609–619 (2015).
Meena, M., Divyanshu, K. & Kumar, S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 5, e02952 (2019).
Elshamly, A. M. S. Minimizing the adverse impact of drought on corn by applying foliar potassium humate combined with Chitosan. J. Soil. Sci. Plant. Nutr. 23, 1913–1929 (2023).
Lo Gullo, M. A., Nardini, A., Salleo, S. & Tyree, M. T. Changes in root hydraulic conductance (K-R) of Olea oleaster seedlings following drought stress and irrigation. New. Phytol. 140, 25–31 (1998).
Elshamly, A. M. S., Iqbal, R. & Elshikh, M. S. Chitosan combined with humic applications during sensitive growth stages to drought improves nutritional status and water relations of sweet potato. Sci. Rep. 14, 6351 (2024).
Acknowledgements
The author would like to thank the Water Studies and Research Complex (WSRC) Station and National Water Research Center (NWRC) for their financial support in conducting this work. Thanks to the support of the University of Bonn, Germany, the manuscript is published based on the DEAL agreement. The authors also extend their appreciation the Researchers Supporting Project number (RSPD2025R941), King Saud University, Riyadh, Saudi Arabia.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL. Open Access Funding enabled and organized by Projekt DEAL. The authors also extend their appreciation the Researchers Supporting Project number (RSPD2025R941), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
A.M.S.E, A.D.S.A, designed the sampling strategy. A.M.S.E, A.D.S.A, designed the experiments. A.M.S.E, performed the experiment. A.M.A.M, H.R, and R.I, provided materials and supervision. A.M.S.E wrote the manuscript. J.I, S.A, R.I, and N.S.G. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The field and laboratory studies were carried out in accordance with all applicable institutional, national, and international laws.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elshamly, A.M.S., Abaza, A.D.S., Mustafa, A.EZ.M.A. et al. Synergistic effect of ammonium and potassium on carrot growth, physio-biochemical mechanisms, and water use efficiency under varying irrigation regimes. Sci Rep 15, 16151 (2025). https://doi.org/10.1038/s41598-025-00690-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00690-3